Abstract
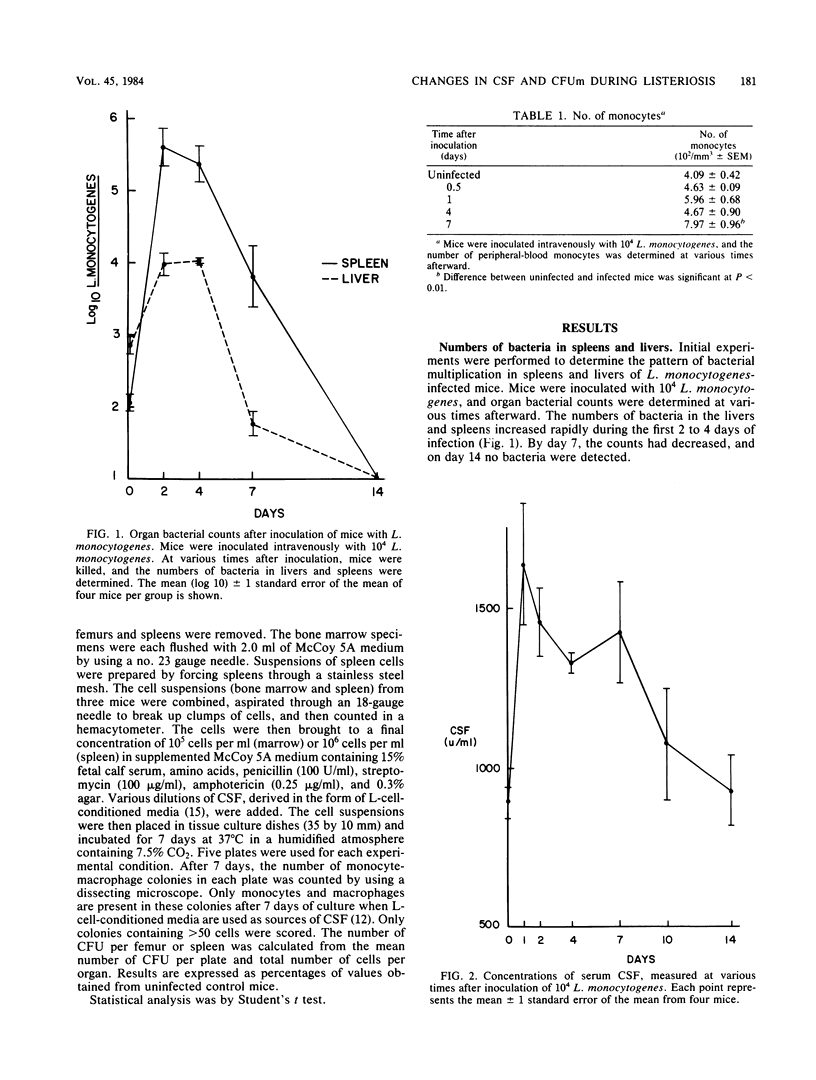
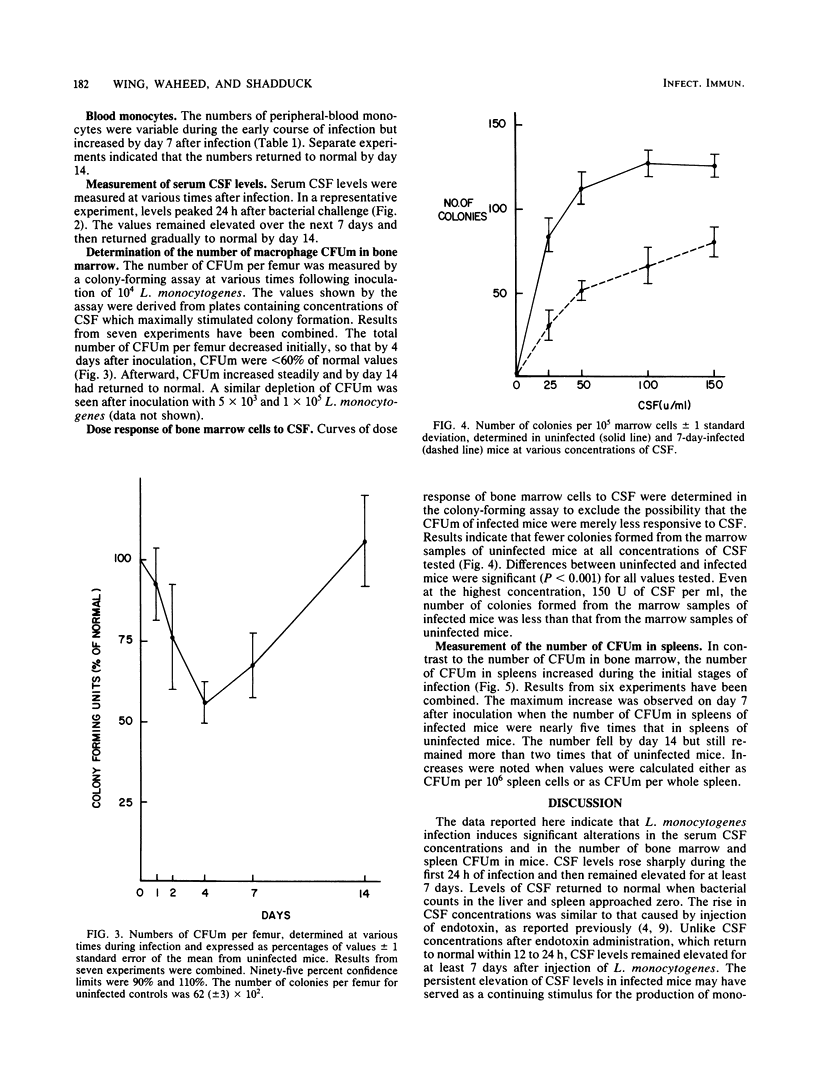
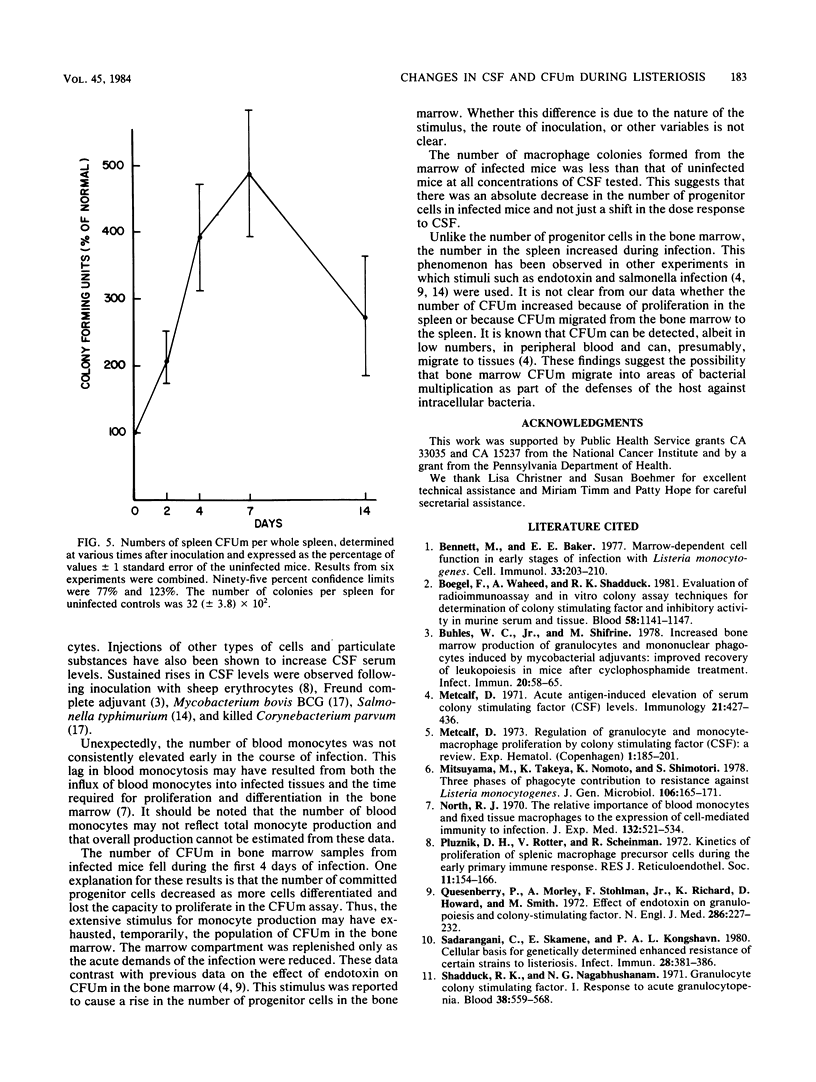
The capacity of a host to produce and mobilize monocytes is an essential component of host defenses during the early phases of infection caused by Listeria monocytogenes. In this study, the concentrations of colony stimulating factor (CSF) and the numbers of monocyte progenitor cells (CFUm) were measured in mice during infection with L. monocytogenes. The concentration of CSF in serum increased sharply during the first 24 h of infection and remained elevated for the next 7 days. The number of CFUm in the bone marrow, however, decreased during the first 4 days after injection of L. monocytogenes. Thereafter, the number increased slowly, returning to normal on day 14. The decrease in marrow progenitor cells did not appear to result from a reduced sensitivity to CSF. In contrast to bone marrow changes, spleen progenitor cells increased greater than 400%, reaching a peak 7 days after bacterial challenge. These data indicate that monocyte production during L. monocytogenes infection is correlated with a rise in serum CSF concentration, depletion of bone marrow CFUm, and an increase in the number of spleen CFUm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M., Baker E. E. Marrow-dependent cell function in early stages of infection with Listeria monocytogenes. Cell Immunol. 1977 Sep;33(1):203–210. doi: 10.1016/0008-8749(77)90147-2. [DOI] [PubMed] [Google Scholar]
- Boegel F., Waheed A., Shadduck R. K. Evaluation of radioimmunoassay and in vitro colony assay techniques for determination of colony-stimulating factor and inhibitory activity in murine serum and tissue. Blood. 1981 Dec;58(6):1141–1147. [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Increased bone marrow production of granulocytes and mononuclear phagocytes induced by mycobacterial adjuvants: improved recovery of leukopoiesis in mice after cyclophosphamide treatment. Infect Immun. 1978 Apr;20(1):58–65. doi: 10.1128/iai.20.1.58-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Regulation of granulocyte and monocyte-macrophage proliferation by colony stimulating factor (CSF): a review. Exp Hematol. 1973;1(4):185–201. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznik D. H., Rotter V., Scheinman R. Kinetics of proliferation of splenic macrophage precursor cells during the early primary immune response. J Reticuloendothel Soc. 1972 Feb;11(2):154–166. [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Sadarangani C., Skamene E., Kongshavn P. A. Cellular basis for genetically determined enhanced resistance of certain mouse strains to listeriosis. Infect Immun. 1980 May;28(2):381–386. doi: 10.1128/iai.28.2.381-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadduck R. K., Nagabhushanam N. G. Granulocyte colony stimulating factor. I. Response to acute granulocytopenia. Blood. 1971 Nov;38(5):559–568. [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Pigoli G., Boegel F., Higgins L. Fractionation of antibodies to L-cell colony-stimulating factor by affinity chromatography. Blood. 1979 Jun;53(6):1182–1190. [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A. Phenotypic expression of genetically controlled host resistance to Listeria monocytogenes. Infect Immun. 1979 Jul;25(1):345–351. doi: 10.1128/iai.25.1.345-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudgett A., McNeill T. A., Killen M. Granulocyte-macrophage precursor cell and colony-stimulating factor responses of mice infected with Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):450–455. doi: 10.1128/iai.8.3.450-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification and properties of L cell-derived colony-stimulating factor. J Lab Clin Med. 1979 Jul;94(1):180–193. [PubMed] [Google Scholar]
- Wing E. J., Kresefsky-Friedman D. Y. Decreased resistance to Listeria monocytogenes in mice injected with killed corynebacterium parvum: association with suppression of cell-mediated immunity. J Infect Dis. 1980 Feb;141(2):203–211. doi: 10.1093/infdis/141.2.203. [DOI] [PubMed] [Google Scholar]
- Wolmark N., Levine M., Fisher B. The effect of a single and repeated administration of Corynebacterium parvum on bone marrow macrophage colony production in normal mice. J Reticuloendothel Soc. 1974 Oct;16(4):252–257. [PubMed] [Google Scholar]



