Abstract
Background
Breastfeeding clearly protects against early wheezing, but recent data suggest that it may increase later risk of atopic disease and asthma.
Objective
To examine the relationship between breastfeeding and later asthma and allergy outcomes using data from the Avon Longitudinal Study of Parents and Children, a large birth cohort in the United Kingdom.
Methods
We used adjusted logistic regression models to evaluate the association between breastfeeding and atopy at age 7 years, bronchial responsiveness to methacholine at age 8 years, and wheeze at ages 3 and 7½ years. Bayesian methods were used to assess the possibility of bias due to an influence of early wheezing on duration of breastfeeding as well as selection bias.
Results
Breastfeeding was protective for wheeze in the first 3 years of life (OR =0.80, 95% CI 0.70-0.90 for 6+ months relative to never) but not wheeze (OR=0.98, 95% CI 0.79-1.22), atopy (OR=1.12, 95% CI 0.92-1.35) or BHR (1.07, 95% CI 0.82-1.40) at ages 7-8 years. Bayesian models adjusting for the longer duration of breastfeeding among children with wheezing in early infancy gave virtually identical results.
Conclusions
We did not find consistent evidence for either a deleterious effect or a protective effect of breastfeeding on later risk of allergic disease in a large prospective birth cohort of children with objective outcome measures and extensive data on potential confounders and effect modifiers. Neither reverse causation nor loss to follow-up appears to have materially biased our results.
Clinical implications
Breastfeeding does not increase the risk of asthma or atopy in children, even when their mothers are asthmatic.
Capsule Summary
This study supports the evidence that breastfeeding does not adversely affect children with regard to asthma and other allergic outcomes. Breastfeeding’s protective role against early wheezing is a benefit for respiratory health.
Keywords: asthma, atopy, breastfeeding, bronchial hyperresponsiveness, allergy
Introduction
Breastfeeding protects the offspring from a variety of adverse health conditions1-3 but controversy remains regarding its role in the development of asthma and allergy. A substantial body of prospective studies supports a modest protective effect of breastfeeding against wheeze and asthma in infancy and early childhood4,5. However, the protective effect of breastfeeding on asthma in later childhood (age six years and above) is less evident5 and some recent studies suggest that breastfeeding actually increases the risk of asthma, wheeze or atopy to aeroallergens at older ages6-11. In one study, breastfeeding was associated with increased risk of asthma only in children with a family history of atopic illness6. Recent data suggesting that breastfeeding might enhance the risk of asthma phenotypes conflict with public health guidelines encouraging breastfeeding1 and have raised concern among pregnant women and their physicians12, 13.
Systematic reviews have highlighted methodological issues that contribute to the complexity of studying the association between breastfeeding and allergic disease 2-5,13,14. A key issue is recall bias in feeding history; this is eliminated by prospective data collection. Other important issues include lack of data on breastfeeding duration and degree of exclusivity, measurement and control for potential confounding factors including family allergic history, and the lack of objective or uniform classification of allergic outcomes 15. More recently it has been suggested that measuring the effects of breastfeeding on allergic disease may also be complicated by reverse causation, if mothers prolong breastfeeding because they note early symptoms of asthma or allergy in their children 16-18. Only recently have studies attempted to examine this possible source of bias in a quantitative manner7,17.
Given the recent controversy generated by new data on adverse effects of breastfeeding related to asthma phenotypes, we examined prospective data from the Avon Longitudinal Study of Parents and Children (ALSPAC), a population-based birth cohort study that enrolled over 14,000 pregnant women and followed their children through late childhood. We also evaluated potential reverse causation using prospective information on breastfeeding and early wheeze, analyzed with Bayesian methods 19. These methods also account for the possibility of selection bias due to loss to follow-up.
Methods
ALSPAC Study
We used data from the ALSPAC study (http://www.alspac.bris.ac.uk) which has been described in detail elsewhere 14. Additional information is available in the online repository. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees. The core ALSPAC sample consists of 14,541 pregnancies resulting in a cohort of 14,062 live births. Of these, 13,988 children were alive at one year of age. For reasons of confidentiality, data on the triplet and quadruplet children were not available, leaving 13,978 study children alive at age one year.
Breastfeeding
Data on breastfeeding were obtained from questions about dietary and feeding habits included in six questionnaires sent to mothers during the first four years of the child’s life. (Please see online repository for detailed information on breastfeeding.) We used data on breastfeeding from all questionnaires on which it was queried to minimize exclusion of subjects for missing data. We were able to assign breastfeeding duration (never, <1 month, 1 to <3 months, 3 to <6 months and 6+ months) to 90.3% (n = 12,623) of the cohort and exclusive breastfeeding to 90.9% (n = 12,706) of the cohort.
Health outcomes
Self-reported outcomes
Our primary self-reported outcomes were wheeze in the first 3 years of life and wheeze at 7½ years, between the measurements of the two objective outcomes (atopy and bronchial hyperresponsiveness). (See online repository for additional detail on wheeze.) We were able to classify 78.9% of the cohort with respect to wheezing status in the first 3 years.
We also considered wheezing at the questionnaire administered at 7½ years, between the measurements of the two objective outcomes (atopy and bronchial hyperresponsiveness). We had data on wheeze at 7½ years on 8,200 children.
Although asthma is subject to diagnostic bias based on factors that may be related to breast feeding, whereas wheeze, a symptom, is not, we examined asthma at the 7½ year questionnaire for comparison with stratified analyses similar to those of Wright et al.6 We defined current asthma as a report on the questionnaire of doctor-diagnosis of asthma, plus either wheeze or the use of asthma medications within the previous 12 months. The question about doctor diagnosis of asthma read “Has a doctor ever actually said that your study child has asthma?”. We had questionnaire data on asthma at 7½ years on 8,131 children.
Objective outcome measures
Atopy
At the age of approximately seven years, 7,245 ALSPAC participants (of 12,715 invited children) underwent skin prick testing to a core panel of common allergens (cat, mixed grass pollen, peanut, egg white, Dermatophagoides pteronyssinus (Der p), and mixed nuts (ALK Abelló, Horshølm, Denmark). (See online repository for more detail on skin prick tests.) A positive skin test for a given allergen was defined as a mean wheal diameter of ≥2 mm, with a zero response to the negative control.
We defined atopy as a positive test to at least one of the aeroallergens included in the core panel (cat, mixed grass and house dust mite). We have previously reported that positivity to these three core aeroallergens predicted 95% of reactivity to any of 29 allergens that were tested in three panels on separate thirds of the children20. We were able to classify atopy to aeroallergens for 6,512 children.
Lung function and bronchial hyper-responsiveness
At the age of approximately eight years, 7,081 ALSPAC participants (56.7% of 12,478 children invited) visited the research clinic (“Focus @ 8”) for tests of lung function. We used American Thoracic Society 21 criteria to select lung function measurements acceptable for analysis. (See online repository for more detail on lung function and bronchial hyper-responsiveness.)
Of the 6,997 children with acceptable lung function measurements, 6.4% (n = 446) had FEV1 <70% and thus were not invited to participate in assessments of bronchial hyper-responsiveness (BHR) using the rapid methacholine inhalation test 22. Approximately 67% (n = 4,364) of the 6,551 children with FEV1 FEV1 ≥70% completed bronchial challenge. The challenge was stopped when the FEV1 fell by more than 20% from the post-saline value, with a cumulative dose of ≥1.2 mg methacholine.
Confounders
Variables were considered as potential confounders if they were known to be associated with breastfeeding and each outcome of interest. Questionnaire data were used for all variables evaluated as confounders. For variables with the potential to change over time (e.g., environmental tobacco smoke exposure, crowding), we used the highest value reported on questionnaires administered during the first four years of life.
Statistical Analyses
We used logistic regression to evaluate the relationship between each measure of breastfeeding and each health outcome. Breastfeeding measures included ever vs. never breastfed, duration of breastfeeding (never, <1 month, 1 to <3 months, 3 to <6 months, 6+ months), and duration of exclusive breastfeeding (never breastfed, exclusive breastfeeding < 4 months, exclusive breastfeeding ≥ 4 months). Sample sizes for analyses of different outcomes varied, due to differences in available information (Table E1). As expected, more children were included in analyses of wheezing because this was ascertained by questionnaire rather than clinic visits.
We used a change-in-estimate method to evaluate variables as confounders of the relationship between breastfeeding (ever vs. never) and each outcome 23. For each logistic model, our cutoff criterion was a 10% change in the beta coefficient for breastfeeding in relation to the outcome. (See online repository for detail on potential confounders.) Final models were adjusted for sex, maternal age (continuous), maternal smoking in last 2 months of pregnancy (yes, no), environmental tobacco smoke in the first 4 years of life (none, low, moderate/high), maternal asthma, maternal allergy, and older siblings (yes/no). We also evaluated each of the variables included as confounders as potential effect modifiers in stratified analyses.
To examine the potential for reverse causation, we used life table analysis and Kaplan Meyer survival curves 24 to describe the temporal relationship between wheezing and cessation of breastfeeding. We examined breastfeeding duration stratified by early wheezing status, including children who never wheezed and those who wheezed before cessation of breastfeeding. There were 9,166 children with information on both early wheezing and breastfeeding. (See online repository for additional information on breastfeeding and wheeze among these children.)
To model the potential for reverse causation in more detail, we used Bayesian analysis to fit a joint model designed to incorporate the potential for early wheezing to modify the duration of breastfeeding in assessing the relationship between breastfeeding and the later health outcomes 19. (See online repository for information about Bayesian analysis.) The Bayesian analysis also adjusts for possible selection bias due to loss to follow-up for the ascertainment of outcomes at ages seven and eight. Models were adjusted for the confounders identified in previous logistic regression models.
We used SAS version 9.1 (SAS Institute, Cary, NC) to conduct all analyses except for joint modeling. Models were fitted jointly using WinBUGS (Windows version of Bayesian inference Using Gibbs Sampling), a freely available software package for Bayesian inference (available at http://www.mrc-bsu.cam.ac.uk/bugs/).
Results
Approximately 25% of the children in the ALSPAC cohort were never breastfed, 25% were breastfed for at least 6 months, and approximately 8.5% were exclusively breastfed for at least 4 months (Online Repository Table E1). Participation in the clinic visits at age seven years for skin prick testing and eight years for bronchial responsiveness testing was higher among children who had been breastfed, had older siblings, less postnatal exposure to environmental tobacco smoke, or who had mothers who were older, had asthma, allergy, or had not smoked during the last two months of pregnancy (Online Repository Table E1).
Wheezing in the first three years of life was reported for 4,721 children (51.9% of questionnaire respondents with breastfeeding and covariate data) and wheezing at 7½ years by 781 children (10.5%). Skin prick tests at age seven years revealed atopy in 1,235 children (21.3% of those tested with breastfeeding and covariate data) and methacholine challenge indicated BHR for 615 children at age eight years (15.7%) (Table 1).
Table 1.
Breastfeeding in relation to wheeze, atopy and bronchial hyperresponsiveness in the ALSPAC cohort
| Outcome Present | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Prev1 | OR2 Crude | 95% CI | OR Adj.3 | 95% CI | |||||
| N | % | N | % | Lower | Upper | Lower | Upper | ||||
| Wheezing in the first 3 years | |||||||||||
| Ever breastfed | |||||||||||
| No | 907 | 20.7 | 1,254 | 26.6 | 58.0 | 1.00 | 1.00 | ||||
| Yes | 3,472 | 79.3 | 3,467 | 73.4 | 50.0 | 0.72 | 0.66 | 0.80 | 0.88 | 0.79 | 0.97 |
| Breastfeeding duration | |||||||||||
| Never | 907 | 20.7 | 1,254 | 26.6 | 58.0 | 1.00 | 1.00 | ||||
| < 3 months | 1,356 | 31.0 | 1,534 | 32.6 | 53.1 | 0.82 | 0.73 | 0.92 | 0.94 | 0.83 | 1.05 |
| 3 - < 6 months | 596 | 13.6 | 591 | 12.5 | 49.8 | 0.72 | 0.62 | 0.83 | 0.88 | 0.75 | 1.02 |
| ≥6 months | 1,519 | 34.7 | 1,334 | 28.3 | 46.8 | 0.64 | 0.57 | 0.71 | 0.80 | 0.70 | 0.90 |
| Exclusive breastfeeding | |||||||||||
| Never breastfed | 907 | 20.7 | 1,254 | 26.6 | 58.0 | 1.00 | 1.00 | ||||
| < 4 months | 2,995 | 68.4 | 3,035 | 64.4 | 50.3 | 0.73 | 0.66 | 0.81 | 0.88 | 0.79 | 0.97 |
| ≥ 4 months | 475 | 10.9 | 421 | 8.9 | 47.0 | 0.64 | 0.55 | 0.75 | 0.83 | 0.70 | 0.98 |
| Wheeze at 7.5 years | |||||||||||
| Ever breastfed | |||||||||||
| No | 1,420 | 21.4 | 173 | 22.2 | 10.9 | 1.00 | 1.00 | ||||
| Yes | 5,231 | 78.7 | 608 | 77.9 | 10.4 | 0.95 | 0.80 | 1.14 | 0.96 | 0.79 | 1.16 |
| Breastfeeding duration | |||||||||||
| Never | 1,420 | 21.4 | 173 | 22.2 | 10.9 | 1.00 | 1.00 | ||||
| < 3 months | 2,084 | 31.4 | 231 | 29.6 | 10.0 | 0.91 | 0.74 | 1.12 | 0.93 | 0.75 | 1.15 |
| 3 - < 6 months | 906 | 13.7 | 107 | 13.7 | 10.6 | 0.97 | 0.75 | 1.25 | 0.99 | 0.76 | 1.29 |
| ≥ 6 months | 2,226 | 33.5 | 269 | 34.5 | 10.8 | 0.99 | 0.81 | 1.21 | 0.98 | 0.79 | 1.22 |
| Exclusive breastfeeding | |||||||||||
| Never | 1,420 | 21.4 | 173 | 22.2 | 10.9 | 1.00 | 1.00 | ||||
| < 4 months | 4,491 | 67.7 | 534 | 68.5 | 10.6 | 0.98 | 0.81 | 1.17 | 0.98 | 0.81 | 1.18 |
| ≥ 4 months | 726 | 10.9 | 73 | 9.4 | 9.1 | 0.83 | 0.62 | 1.10 | 0.83 | 0.61 | 1.12 |
| Atopy at 7 years (7,834 attended clinic visit, of which 7,245 entered allergy session) | |||||||||||
| Ever breastfed | |||||||||||
| No | 888 | 19.5 | 217 | 17.6 | 19.6 | 1.00 | 1.00 | ||||
| Yes | 3,656 | 80.4 | 1,018 | 82.4 | 21.8 | 1.14 | 0.97 | 1.34 | 1.07 | 0.90 | 1.27 |
| Breastfeeding duration | |||||||||||
| Never | 888 | 19.6 | 217 | 17.6 | 19.6 | 1.00 | 1.00 | ||||
| < 3 months | 1,411 | 31.1 | 358 | 29.1 | 20.2 | 1.04 | 0.86 | 1.25 | 1.00 | 0.83 | 1.22 |
| 3 -< 6 months | 639 | 14.1 | 189 | 15.3 | 22.8 | 1.21 | 0.97 | 1.51 | 1.14 | 0.91 | 1.43 |
| ≥ 6 months | 1,596 | 35.2 | 468 | 38.0 | 22.7 | 1.20 | 1.00 | 1.44 | 1.11 | 0.92 | 1.35 |
| Exclusive breastfeeding | |||||||||||
| Never | 888 | 19.6 | 217 | 17.6 | 19.6 | 1.00 | 1.00 | ||||
| < 4 months | 3,122 | 68.9 | 868 | 70.5 | 21.8 | 1.14 | 0.96 | 1.34 | 1.07 | 0.90 | 1.27 |
| ≥ 4 months | 524 | 11.6 | 146 | 11.9 | 21.8 | 1.14 | 0.90 | 1.44 | 1.07 | 0.84 | 1.38 |
| Bronchial hyper-responsiveness at 8 years (7,171 attended clinic visit, of which 6,551 had FEV1 > 70% and 3,920 had Methacholine Challenge) | |||||||||||
| Ever breastfed | |||||||||||
| No | 614 | 18.6 | 105 | 17.1 | 14.6 | 1.00 | 1.00 | ||||
| Yes | 2,691 | 81.4 | 510 | 82.9 | 15.9 | 1.11 | 0.88 | 1.39 | 1.14 | 0.90 | 1.44 |
| Breastfeeding duration | |||||||||||
| Never | 614 | 18.6 | 105 | 17.2 | 14.6 | 1.00 | 1.00 | ||||
| < 3 months | 992 | 30.1 | 197 | 32.1 | 16.6 | 1.16 | 0.90 | 1.50 | 1.16 | 0.89 | 1.50 |
| 3 - < 6 months | 493 | 15.0 | 100 | 16.3 | 16.9 | 1.19 | 0.88 | 1.60 | 1.21 | 0.90 | 1.65 |
| ≥ 6 months | 1,199 | 36.4 | 211 | 34.4 | 15.0 | 1.03 | 0.80 | 1.33 | 1.07 | 0.82 | 1.40 |
| Exclusive breastfeeding | |||||||||||
| Never | 614 | 18.6 | 105 | 17.1 | 14.6 | 1.00 | 1.00 | ||||
| < 4 months | 2,293 | 69.6 | 454 | 73.9 | 16.5 | 1.16 | 0.92 | 1.46 | 1.17 | 0.93 | 1.49 |
| ≥ 4 months | 388 | 11.8 | 55 | 9.0 | 12.4 | 0.83 | 0.58 | 1.18 | 0.87 | 0.61 | 1.26 |
Prevalence
Odds ratio
Models adjusted for sex, older siblings, maternal age, maternal allergy, maternal asthma, maternal smoking last 2 months of pregnancy, exposure to environmental tobacco smoke in the first 4 years of life
In crude analyses, breastfeeding had a modest inverse association with wheeze in the first three years of life (for example, OR for 6+ months relative to never breastfed = 0.64, 95% CI 0.57-0.71) and atopy was weakly positively related to breastfeeding (OR for 6+ months relative to never = 1.20, 95% CI 1.00-1.44) (Table 1). After adjustment for confounders, breastfeeding remained protective for early wheeze (OR for 6+ months relative to never breastfed = 0.80, 95% CI 0.70-0.90) but the weak relation with atopy was further attenuated and not statistically significant (OR = 1.11, 95% CI 0.92-1.35) (Table 1). Maternal age and maternal history of allergy were the most important confounders of the slight relationship between breastfeeding and atopy (data not shown). When the 70 children reporting antihistamine use were excluded from analyses, the results were not materially different. There was no appreciable association with BHR or current wheezing in either crude or adjusted analyses.
We found no clear evidence of effect modification of the association between breastfeeding and any of the outcomes by any of the factors that were included as confounders: sex, maternal age (dichotomized at the median), maternal smoking in last 2 months of pregnancy (yes, no), environmental tobacco smoke in the first 4 years of life (none, some), maternal asthma, maternal allergy, and older siblings (yes/no) (data not shown). Based on the findings of Wright et al. (2001)6 that exclusive breastfeeding for at least 4 months led to a very high increased risk of later asthma in the subgroup of children who were both atopic and had an asthmatic mother, we also calculated odds ratios for each of our breastfeeding metrics (ever/never, duration, and exclusivity) in relation to the comparable outcome of current asthma at 7½ years (defined as doctor’s diagnosis plus either wheeze or medication use in the past 12 months) stratified by the four combined categories of child’s atopy (based on skin prick testing) and mother’s history of asthma. The analysis comparing exclusive breastfeeding for 4+ months with exclusive breastfeeding for < 4 months does not suggest increased risk of asthma among atopic children with asthmatic mothers (crude OR for asthma = 0.55, 95% CI 0.20-1.55, OR for BHR = 0.67, 95% CI 0.12, 3.65). Although our study is much larger than that of Wright et al., subset analyses remain limited by small numbers; e.g., there were only 6 asthmatic children among the 198 who were exclusively breastfed more than 4 months and were both atopic and had asthmatic mothers and only 2 children with BHR among the 98 children with BHR data who had asthmatic mothers and were atopic. Based on a recent suggestion that the association between breastfeeding and atopy might depend jointly on parental atopy and gender of the child27, we also examined possible effect modification by the combination of child gender and parental atopy. We found no evidence for this three-way interaction -- ORs for atopy in relation to ever-never breastfeeding were close to 1.0 for all combinations of child gender and parental atopy.
Children with wheeze reported by three months of age appeared to be breastfed for longer periods than children without early wheeze (Figure 1). The mean duration of breastfeeding for children who did not wheeze in the first three months of life was 6.6 months (standard error (SE) 0.10), compared with 8.9 months (SE 0.24) for children with wheeze (log-rank test for homogeneity p < 0.0001).
Figure 1.
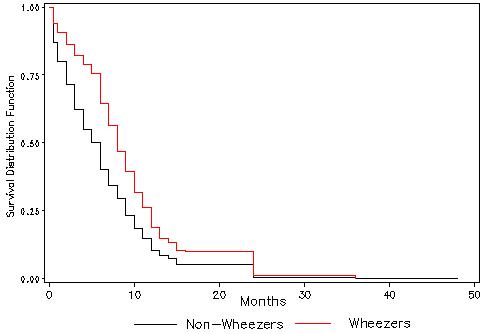
Kaplan Meyer curves for breastfeeding duration in children with and without early wheeze.
The Bayesian joint model included a random effect variable that suggested that children with susceptibility to wheeze were more likely to be breastfed longer and to develop allergic outcomes. However, results from this Bayesian analysis were virtually identical to those from logistic regression models. The Bayesian joint model also adjusted for possible effects of selection bias due to the loss of subjects for the visits at ages seven and eight when atopy and BHR were determined. In the Bayesian joint model, breastfeeding remained protective against early wheeze, slightly positively, but not statistically significantly associated with atopy [relative to never breastfed, OR = 0.95 for less than 3 months, 1.10 for 3-6 months and 1.18 (95% CI 0.93-1.48) for 6+ months of breastfeeding], and was not associated with bronchial hyperresponsiveness [relative to never breastfed, OR = 1.10 for less than 3 months, 1.08 for 3-6 months and 1.03 (95% CI 0.78-1.39) for 6+ months of breastfeeding] for later wheeze.
Discussion
In this large prospective study with objective outcome measures at ages seven and eight years and extensive data on potential confounders and effect modifiers, we found no evidence that breastfeeding increased the risk of later wheeze, asthma diagnosis, atopy, or BHR. We found no evidence of deleterious effects of breastfeeding either in the dataset as a whole or in subsets defined by parental atopy, child atopy, gender, family size, various combinations of these factors or by other risk factors in these data.
As in most previous prospective studies, we found evidence for a modest protective effect of breastfeeding on wheezing in the first few years of life 5, 28, 29. At this age, wheezing is predominantly associated with viral illnesses, and breastfeeding protect by reducing respiratory infections. Although we did not ask about asthma in the first few years of life, due to uncertainty of this diagnosis in young children, the finding of a protective effect of early wheezing is consistent with results from studies that examined early asthma30-32. In our data, the protective effect of breastfeeding on wheeze in early life did not extend into later childhood (7½ years), consistent with most other studies4,5. This is not surprising, however, because asthma phenotypes in later childhood are more likely to be atopic and would only be associated if breastfeeding modulates atopy. In contrast to our findings, a few prospective studies have reported adverse effects of breastfeeding on later wheeze or asthma risk7,8. In another study, breastfeeding was related to increased risk of asthma in later childhood only among the subgroup of 99 children of asthmatic mothers6, while breastfeeding was not related to atopy to aeroallergens. For atopy, in unadjusted analyses, we found a very slight increased risk of borderline statistical significance for six or more months of breast feeding. However, after adjustment for confounders, especially maternal history of allergy and maternal age, breastfeeding was no longer associated with atopy. In three much smaller studies with measures of atopy to aeroallergens at age 5 years or after, breastfeeding was a modest risk factor 7,8,10 but there was no increased risk in another6.
The divergent findings on effects of breastfeeding across studies may result by chance due to small numbers, especially in subset analysis. Differences in breastfeeding practices across time and place, variability in ages at which children are studied, or methodological issues including recall bias of feeding history, divergent criteria for allergic outcomes, and confounding may also contribute to discrepancies in the literature. By using data on breastfeeding and a wide array of potential confounders collected prospectively from the large ALSPAC birth cohort, in whom objective measures of atopy and bronchial hyperresponsiveness were obtained in later childhood, we were able to address many of the major methodological issues.
Divergent findings across studies may also be related to differences in environmental exposures for different populations. For example, increased microbial loads have been associated with increased risk of wheeze from lower respiratory infections in children, but in decreased risk of asthma and allergy in later childhood and adolescence33. In the ALSPAC cohort, 53% had older siblings, 11% attended daycare in the first two years of life, 70% had exposure to pets in the first two years, and at least 54% had used antibiotics in the first 15 months of life.
Although not well explored in the previous literature, reverse causation could contribute to positive associations between breastfeeding and allergic outcomes, if the duration of breastfeeding is influenced by early manifestations of atopic or asthmatic phenotypes16-18. To our knowledge, only one study has evaluated reverse causation in a quantitative manner. In the Melbourne Atopy Cohort study, Lowe et al.17 found that early signs of atopic disease led to prolongation of exclusive breastfeeding. Mirshahi et al10 attempted to examine reverse causation with respect to itchy rash in the first four weeks or three months of life. Although limited by small numbers, they reported that “there were trends indicating a tendency to prolong breastfeeding and delay the introduction of solid foods among children with early features of eczema”. Although we did not collect data on itchy rash with sufficient time course detail to allow assessment of reverse causation by this phenotype, we found that mothers breastfed babies who wheezed in the first 3 months of life for a slightly longer time than nonwheezy babies, suggesting that early manifestations of asthma phenotypes may play a part in a mother’s decision to continue breastfeeding. However, results from a joint model using Bayesian methods indicated that this modest difference in duration of breastfeeding based on early infant wheezing was not sufficient to bias the relationships between breastfeeding and the health outcomes examined.
As in virtually all previous birth cohorts we experienced substantive loss to follow-up for outcome assessment in later childhood, although the attrition occurred steadily over the time course of the study. Children who were breastfed were more likely to participate in the later clinic visits when atopy and BHR were measured (Table 1). However, in order for selection bias to occur, loss to follow-up must be jointly dependent on the exposure of interest and the outcomes under study with sufficient strength to influence the association34. Therefore, we evaluated the possibility of informative missingness of later childhood outcomes, contingent on breastfeeding and risk factors for developing the conditions under study, using Bayesian methods. We did not find evidence that the pattern of missing data in this study appreciably influenced our findings.
The major benefits of this study for examination of the possible effects of breastfeeding on later development of atopy and asthma phenotypes include its large size and the use of objective outcome measures. For example, almost 7,000 children underwent skin-prick testing and over 4,000 children had measures of bronchial hyperresponsiveness. This compares with approximately 700 children with skin prick testing and approximately 800 with bronchial hyperresponsiveness testing in the largest study with objective outcomes to report a deleterious effect of breastfeeding on these outcomes 7. In addition, our study is one of the largest prospective studies with questionnaire-based assessment of symptoms and diagnoses in later childhood. Thus we had improved power to investigate any potential effect modification with a reduced possibility of random variations in associations between breastfeeding and respiratory or allergic outcomes within small strata. For example, unusually large positive odds ratios of 8.7 have been reported for exclusive breastfeeding in relation to asthma among children of asthmatic mothers 6. Among the 99 children with an asthmatic mother in that study, there was only one asthmatic in the never breastfed group limiting the ability to reliably estimate associations. In contrast, our comparable analysis included 728 asthmatic children with an asthmatic mother. Likewise, Mandhane et al.27, in follow-up of the finding of Wright et al. 6 regarding interaction with maternal asthma, found no such interaction but on additional stratification by child gender found some strong increased risks (OR = 7.4) for breastfeeding in relation to atopy among the subgroup of 55 boys with atopic fathers. Although multi-way interaction analyses are often interesting to contemplate, they require sample sizes that are far larger than any published study of breastfeeding and respiratory outcomes 35. Very large birth cohorts (approximately 100,000 children each) currently being assembled in the US, Norway, Denmark and other countries may have sufficient power to examine properly these higher-order interactions that could be of relevance to the study of breastfeeding and allergy or asthma.
A trial in which women are randomized to breastfeeding or not, and children are examined in later childhood without loss of follow-up could answer definitively the question of whether breastfeeding leads to later risk of atopy or asthma. However, such a trial is not feasible. A recent study randomized communities in Belarus to receive or not receive a hospital and clinic-based breastfeeding promotion intervention11. The intervention successfully increased the duration of breastfeeding; for example at three months the prevalence of breastfeeding was 72.7% in intervention communities compared with 60.0% for control with much greater differences for exclusive breastfeeding (43.3% versus 6.4%). Being in an intervention community had no significant impact on skin test positivity (OR for atopy to any of 5 common antigens was 1.2, 95% CI 0.5-2.6). An increased risk of atopy (OR = 2.0, 95% CI 1.1-3.4) appeared only in a sensitivity analysis removing the six clinics out of 31 with suspiciously high rates of atopy, resulting in exclusion of 26.1% of the intervention subjects versus 12.3% of the control group. Notably, no increased risks of wheezing, asthma, or eczema were found.
Our study supports the body of evidence that breastfeeding does not adversely affect children with regard to asthma and other allergic outcomes. Even if breastfeeding does not protect against atopy or asthma in later childhood, the protection against wheezing illness in the first few years of life is a benefit for respiratory health. Given some recent findings from smaller studies or subset analyses suggesting deleterious effects, these results should provide reassurance to expectant mothers, including those with atopy, regarding current public health guidelines that encourage breastfeeding.
Supplementary Material
Acknowledgements
We are extremely grateful to all the mothers who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC study team which comprises interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
The UK Medical Research Council, the Wellcome Trust and the University of Bristol provide core support for ALSPAC. The analysis was supported by the Division of Intramural Research, National Institute of Environmental Health Sciences, USA.
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Children
- BHR
Bronchial hyper-responsiveness
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- OR
Odds ratio
Footnotes
Conflict of interest statement
None declared.
References
- 1.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2002;(1) doi: 10.1002/14651858.CD003517. CD003517. [DOI] [PubMed] [Google Scholar]
- 3.Oddy WH. Breastfeeding protects against illness and infection in infants and children: a review of the evidence. Breastfeed Rev. 2001;9(2):11–8. [PubMed] [Google Scholar]
- 4.Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139(2):261–6. doi: 10.1067/mpd.2001.117006. [DOI] [PubMed] [Google Scholar]
- 5.van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. 2003;58(9):833–43. doi: 10.1034/j.1398-9995.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 6.Wright AL, Holberg CJ, Taussig LM, Martinez FD. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax. 2001;56(3):192–7. doi: 10.1136/thorax.56.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360(9337):901–7. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 8.da Costa Lima R, Victora CG, Menezes AM, Barros FC. Do risk factors for childhood infections and malnutrition protect against asthma? A study of Brazilian male adolescents. Am J Public Health. 2003;93(11):1858–64. doi: 10.2105/ajph.93.11.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegienka G, Ownby DR, Havstad S, Williams LK, Johnson CC. Breastfeeding history and childhood allergic status in a prospective birth cohort. Ann Allergy Asthma Immunol. 2006;97(1):78–83. doi: 10.1016/S1081-1206(10)61374-9. [DOI] [PubMed] [Google Scholar]
- 10.Mihrshahi S, Ampon R, Webb K, et al. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clin ExpAllergy. 2007;37(5):671–679. doi: 10.1111/j.1365-2222.2007.02696.x. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Mazer B, the Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomized trial. BMJ. 2007;335:815. doi: 10.1136/bmj.39304.464016.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becquet R, Leroy V, Salmi LR. Breastfeeding, atopy, and asthma. Lancet. 2003;361(9352):174. doi: 10.1016/S0140-6736(03)12203-9. author reply 175-6. [DOI] [PubMed] [Google Scholar]
- 13.Friedman NJ, Zeiger RS. The role of breast-feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115(6):1238–1248. doi: 10.1016/j.jaci.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 14.Golding J, Pembrey M, Jones R. ALSPAC--the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MS. Does breast feeding help protect against atopic disease? Biology, methodology, and a golden jubilee of controversy. J Pediatr. 1988;112(2):181–90. doi: 10.1016/s0022-3476(88)80054-4. [DOI] [PubMed] [Google Scholar]
- 16.Laubereau B, Brockow I, Zirngibl A, et al. Effect of breast-feeding on the development of atopic dermatitis during the first 3 years of life--results from the GINI-birth cohort study. J Pediatr. 2004;144(5):602–7. doi: 10.1016/j.jpeds.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Lowe AJ, Carlin JB, Bennett CM, et al. Atopic disease and breast-feeding - Cause or consequence? J Allergy Clin Immunol. 2006;117(3):682–687. doi: 10.1016/j.jaci.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Muraro A, Dreborg S, Halken S, et al. Dietary prevention of allergic diseases in infants and small children. Part III: Critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol. 2004;15(4):291–307. doi: 10.1111/j.1399-3038.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 19.De Stavola BL, Nitsch D, dos Santos Silva I, et al. Statistical issues in life course epidemiology. Am J Epidemiol. 2006;163(1):84–96. doi: 10.1093/aje/kwj003. [DOI] [PubMed] [Google Scholar]
- 20.Roberts G, Peckitt C, Northstone K, Strachan D, Lack G, Henderson J, Golding J, the ALSPAC Study Team Relationship between aeroallergen and food allergen sensitization in childhood. Clin Exp Allergy. 2005;35(7):933–940. doi: 10.1111/j.1365-2222.2005.02280.x. [DOI] [PubMed] [Google Scholar]
- 21.ATS Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 22.Yan K, Salome C, Woolcock AJ. Rapid method for measurement of bronchial responsiveness. Thorax. 1983;38(10):760–5. doi: 10.1136/thx.38.10.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison P. Survival Analysis Using the SAS System: A Practical Guide. SAS Institute; North Carolina: 1995. [Google Scholar]
- 25.Dunson DB, Perreault SD. Factor analytic models of clustered multivariate data with informative censoring. Biometrics. 2001;57:302–308. doi: 10.1111/j.0006-341x.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 26.Touloumi G, Pocock SJ, Babiker AG, Darbyshire JH. Estimation and comparison of rates of change in longitudinal studies with informative drop-outs. Stat Med. 1999;18:1215–1233. doi: 10.1002/(sici)1097-0258(19990530)18:10<1215::aid-sim118>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Mandhane PJ, Greene JM, Sears MR. Interactions between breast-feeding, specific parental atopy, and sex on development of asthma and atopy. J Allergy Clin Immunol. 2007;119(6):1359–1366. doi: 10.1016/j.jaci.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Linneberg A, Simonsen JB, Petersen J, Stensballe LG, Benn CS. Differential effects of risk factors on infant wheeze and atopic dermatitis emphasize a different etiology. J Allergy Clin Immunol. 2006;117(1):184–9. doi: 10.1016/j.jaci.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 29.Wickman M, Melen E, Berglind N, et al. Strategies for preventing wheezing and asthma in small children. Allergy. 2003;58(8):742–7. doi: 10.1034/j.1398-9995.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 30.Kull I, Almqvist C, Lilja G, Pershagen G, Wickman M. Breast-feeding reduces the risk of asthma during the first 4 years of life. J Allergy Clin Immunol. 2004;114(4):755–60. doi: 10.1016/j.jaci.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Rothenbacher D, Weyermann M, Beermann C, Brenner H. Breastfeeding, soluble CD14 concentration in breast milk and risk of atopic dermatitis and asthma in early childhood: birth cohort study. Clin Exp Allergy. 2005;35(8):1014–21. doi: 10.1111/j.1365-2222.2005.02298.x. [DOI] [PubMed] [Google Scholar]
- 32.Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101(5):587–93. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- 33.Von Mutius E. Influences in allergy: epidemiology and the environment. J Allergy Clin Immunol. 2004;113(3):373–9. doi: 10.1016/j.jaci.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Hennekens CH, Buring JE. Epidemiology in Medicine. Little Brown and Co.; Boston: 1987. p. 170. [Google Scholar]
- 35.Smith PG, Day NE. The design of case-control studies: the influence of confounding and interaction effects. Int J Epidemiol. 1984;13(3):356–65. doi: 10.1093/ije/13.3.356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


