TO THE EDITOR
Cathelicidins comprise an important family of antimicrobial peptides that are essential for protection of skin against bacterial infection in mice (Nizet et al., 2001), and are relevant to infection in human skin diseases such as atopic dermatitis (Ong et al., 2002). The human cathelicidin gene (Camp) is expressed as an inactive precursor protein (hCAP18) and is present in neutrophils, mast cells, keratinocytes, and eccrine glands (Braff et al., 2005). hCAP18 consists of an N-terminal signal peptide, a cathelin domain, and a C-terminal region encoding the antimicrobial domain (Sorensen et al., 2001). After proteolytic processing of hCAP18 to peptides such as LL-37, the peptide exerts antimicrobial activity that protects the host from infection. In this study we determined if cathelicidin is produced by sebocytes, thereby establishing its potential to add to defense of the skin by this cell.
The sebaceous gland has been suspected to provide protection to the external skin surface, but its functions were only recently understood (Zouboulis, 2003). Lipids produced by the sebocyte exhibit antibacterial activity (Georgel et al., 2005) and peptides of the β-defensin family have been found in human pilosebaceous units (Chronnell et al., 2001). Since cathelicidin has synergistic activity with β-defensins, it was of interest to determine the expression of cathelicidin in the sebaceous gland.
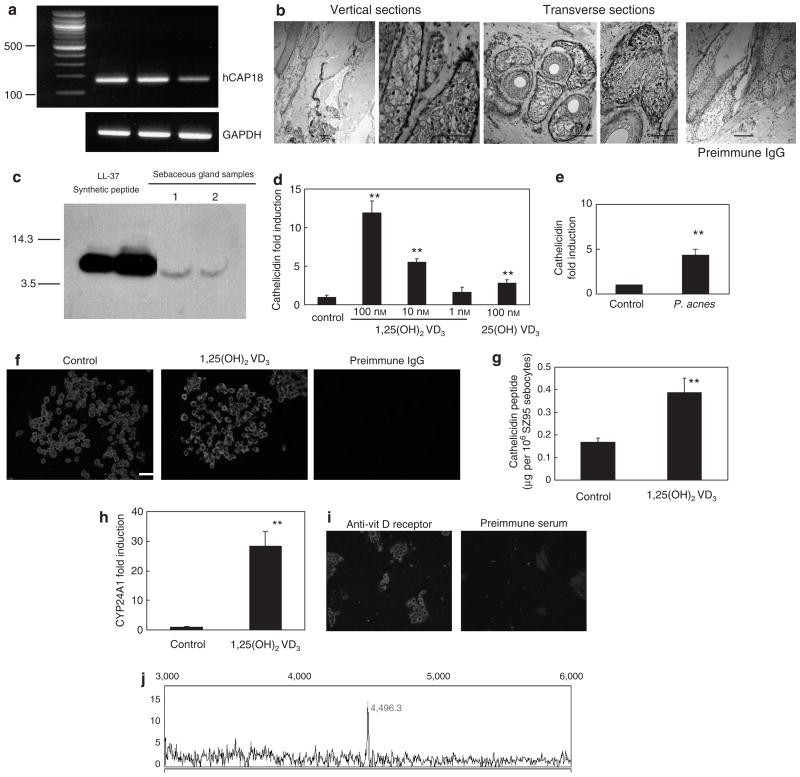
hCAP18 mRNA was detected by reverse transcriptase-PCR (RT-PCR) in isolated human sebaceous glands microdissected from normal scalp skin (Figure 1a). Cathelicidin protein was detected in sebaceous glands in normal human scalp tissue examined with anti-LL37 antibody (Murakami et al., 2002; Figure 1b). Western blot analysis of extracts from isolated human sebaceous glands demonstrated a single band corresponding to LL-37 (Figure 1c). This finding is in contrast to cathelicidin isolated from neutrophils, where the predominant form seen was the hCAP18 precursor (Sorensen et al., 2001).
Figure 1. Cathelicidin is present in sebaceous glands isolated from normal scalp, in isolated sebaceous gland extracts, and in SZ95 sebocytes, where it is inducible by 1,25(OH)2 vitamin D3.
(a) PCR amplification of hCAP18 mRNA from three independent isolates of normal scalp human sebaceous glands. (b) Histological sections of normal human scalp tissue examined for cathelicidin expression by immunohistochemistry with the anti-LL37 antibody. Human cathelicidin peptide was detected in both vertical and transverse scalp sections, and in eccrine structures, including glands and ducts. No staining was detected in the tissue sections stained with preimmune IgG. (c) Human sebaceous gland extracts evaluated by western blot with antibody to LL-37. Right two lanes show a single band at a size consistent with the expected 5-kDa mature form of LL-37 and similar to the LL-37 synthetic peptide in the left two lanes. (d) Real-time RT-PCR for hCAP18 SZ95 sebocytes treated with 1,25(OH)2 vitamin D3 or the vehicle for 24 hours. (e) Real-time RT-PCR for hCAP18 in SZ95 treated with P. acnes culture supernatant for 24 hours. (f) SZ95 sebocytes treated with the vehicle, or 100 nM 1,25(OH)2 vitamin D3 for 24 hours and stained with anti-LL-37 antibody or preimmune IgG. (g) Analysis of cathelicidin in SZ95 sebocyte extracts evaluated by ELISA. (h) Real-time RT-PCR for CYP24A1 in SZ95 treated with 1,25(OH)2 vitamin D3 (100 nM) or with the vehicle for 24 hours. (i) SZ95 sebocytes stained with anti-vitamin D receptor antibody. (j) Surface-enhanced laser desorption/ionization–time of flight–mass spectrometry analyses of cathelicidin isolated from SZ95 sebocyte extracts. A peak at 4,496-Da corresponding to the mature LL-37 peptide was detected in SZ95 sebocytes (**P < 0.01; Student’s t-test, Bars = 20 μm). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
hCAP18 mRNA was also detectable in SZ95 sebocytes (Zouboulis et al., 1999) by quantitative real-time RT-PCR, and was induced by 1,25(OH)2 vitamin D3 (Figure 1d). The expression of hCAP18 mRNA was also induced by Propionibacterium acnes (P. acnes) culture supernatant (Figure 1e). Cathelicidin protein was also detected by immunofluorescence and this was enhanced with 1,25(OH)2 vitamin D3 (100 nM) (Figure 1f). The concentrations of cathelicidin detected by ELISA were 0.17 μg per 106 SZ95 sebocytes (vehicle control) and 0.39 μg per 106 SZ95 sebocytes (100 nM 1,25(OH)2 vitamin D3) (Figure 1g).
To further define the response of SZ95 sebocytes to vitamin D, the expression of the vitamin D-dependent gene CYP24A1 (24-hydroxylase), and vitamin D receptor, was examined. CYP24A1 mRNA was induced 28-fold by 100 nM of 1,25(OH)2 vitamin D3 (Figure 1h). The vitamin D receptor was abundantly detected by immunofluorescence (Figure 1i). These results confirmed that SZ95 cells were vitamin D responsive.
To more precisely determine the mass and sequence of the cathelicidin peptide, SZ95 sebocytes were evaluated by surface-enhanced laser desorption/ionization–time of flight–mass spectrometry with anti-LL37 antibody capture. Surface-enhanced laser desorption/ionization–time of flight–mass spectrometry detected a single peak with a molecular mass of 4,496-Da (Figure 1j), identical to the expected mass of the LL-37 when processed in neutrophils.
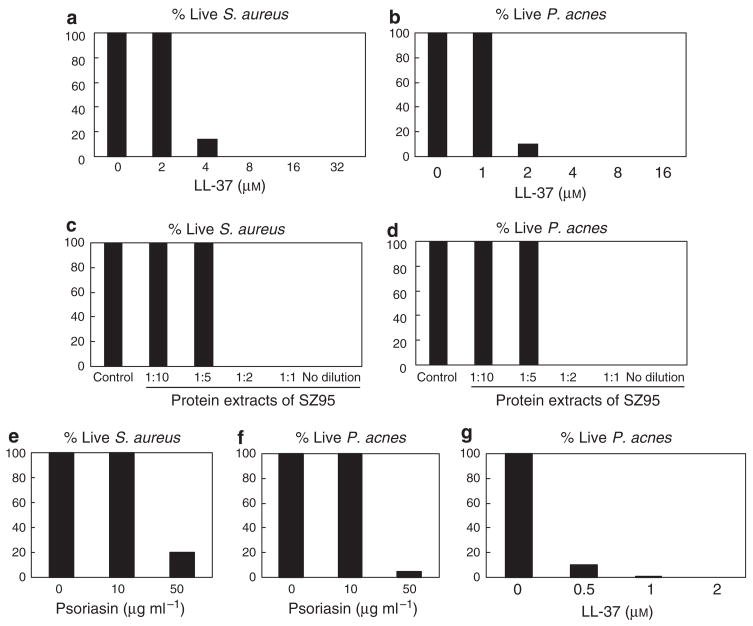
To investigate the relevance of this finding to the antimicrobial function of sebocytes, we next determined if LL-37 could kill bacteria such as Staphylococcus aureus (S. aureus) and P. acnes, both potential pathogens of the human pilosebaceous unit. Under low-nutrient conditions, synthetic LL-37 (8 μM) killed 100% of S. aureus wild-type strain SA113 (ATCC 35556) (Figure 2a) and synthetic LL-37 (4 μM) killed 100% of P. acnes (Figure 2b). Acid-soluble protein extracts of SZ95 sebocytes also showed activity against S. aureus (Figure 2c) and P. acnes (Figure 2d). However, the amount of LL-37 in these extracts was insufficient to explain the antimicrobial activity: the concentration of LL-37 in SZ95 acid-soluble protein extracts as measured by ELISA was 0.17 μg ml−1 (0.038 μM).
Figure 2. Synthetic LL-37 peptide, acid-soluble protein extracts of SZ95 sebocytes, and psoriasin kill S. aureus and P. acnes. LL-37 and psoriasin show a synergistic effect against P. acnes.
The antimicrobial activity of synthetic LL-37 (a, b), acid-soluble protein extracts of SZ95 sebocytes (c, d), and psoriasin (e, f) was evaluated against S. aureus (a, c, e) and P. acnes (b, d, f, g) by solution killing assay. Bacteria were incubated for 3 hours at 37 °C in 10 mM sodium phosphate buffer (pH 6.5) supplemented with 100 mM NaCl. Recombinant psoriasin was a gift from A. Büchau and S. Kellermann, Dermatology, Heinrich-Heine University, Düsseldorf, Germany. Values are percentage of living bacteria compared with the control. Data shown are representative of triplicate determinations from three independent experiments.
Since other antimicrobials such as β-defensins (Chronnell et al., 2001) and psoriasin (Glaser et al., 2005) are expressed in sebocytes, and antimicrobial peptides have additive or synergistic functions when combinded (Nagaoka et al., 2000), we next examined the potency of LL-37 against P. acnes when combinded with another antimicrobial peptide found in the sebocyte. Psoriasin alone (50 μg ml−1) killed 80% of S. aureus (Figure 2e) and 95% of P. acnes (Figure 2f). When combined with psoriasin (10 μg ml−1), LL-37 was much more effective against P. acnes (Figure 2g).
Taken together, these data show that cathelicidin is expressed in sebocytes and processed to the active peptide LL-37, where it may potentially aid in skin defense. Cathelicidin expression in sebocytes was both constitutive and inducible. This is reminiscent of the expression of cathelicidin in keratinocytes, where it is induced in vivo by infection, inflammation, or 1,25(OH)2 vitamin D3 (Frohm et al., 1997; Weber et al., 2005). We show that SZ95 sebocytes respond similarly to keratinocytes and increased cathelicidin when exposed to vitamin D3 or extracts of P. acnes. This observation suggests a potential novel role for vitamin D in defense of the follicle. However, it remains to be determined what is the relative role of cathelicidin in sebocyte antimicrobial action. The form of cathelicidin produced by sebocytes (LL-37) can kill skin microflora in vitro such as S. aureus or P. acnes, but this is only likely to be relevant when it works together with other antimicrobial agents. The total antimicrobial activity in sebocytes is likely due to all antimicrobial peptides acting together, and in vivo is probably also aided by the antimicrobial action of fatty acids, lipids, and the hostile pH of the skin surface. Human β-defensin-2 mRNA can also be induced in SZ95 sebocytes by P. acnes (Nagy et al., 2006), and psoriasin adds to the mixture. We show here that combining only two antimicrobials (LL-37 and psoriasin) produced a synergistic effect against P. acnes and greatly increased the potency of cathelicidin. The actual concentration of β-defensins, psoriasin, or other potential antimicrobial peptides in the sebaceous gland remains unknown, but the current findings support the conclusion that the sebaceous gland contributes to epithelial defense by the release of multiple antimicrobial molecules to the skin surface. LL-37 may contribute more to inflammation surrounding the sebaceous gland than in antimicrobial defense (Yamasaki et al., 2007).
Abbreviations
- P. acnes
Propionibacterium acnes
- RT-PCR
reverse transcriptase-PCR
- S. aureus
Staphylococcus aureus
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- Chronnell CM, Ghali LR, Ali RS, Quinn AG, Holland DB, Bull JJ, et al. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol. 2001;117:1120–5. doi: 10.1046/j.0022-202x.2001.01569.x. [DOI] [PubMed] [Google Scholar]
- Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, et al. A TLR2-responsive lipid effector pathway protects mammals against Gram-positive bacterial skin infections. Infect Immun. 2005;73:4512–21. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroeder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin antimicrobial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–5. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–9. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–9. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–2. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Elevated serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC. Sebaceous gland in human skin—the fantastic future of a skin appendage. J Invest Dermatol. 2003;120:xiv–xv. doi: 10.1046/j.1523-1747.2003.12263.x. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–20. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]