Abstract
The authors analyzed smokers’ facial expressions using the Facial Action Coding System (P. Ekman & W. V. Friesen, 1978) under varying smoking opportunity conditions. In Experiment 1, smokers first were told that they either could (told-yes) or could not (told-no) smoke during the study. Told-yes smokers reported higher urges than did told-no smokers. Unexpectedly, told-yes smokers became increasingly likely to manifest expressions related to negative affect and less likely to evince expressions related to positive affect, compared with told-no smokers. In Experiment 2, smokers were more likely to show positive affect-related expressions if the delay was 15 s than if it was 60 s. Craving may be related to both a desire to use and an impatient desire to use immediately.
Recent years have seen a resurgence of interest in the topic of drug craving (e.g., Lowman, Hunt, Litten, & Drummond, 2000). Laboratory studies have proliferated in which craving is provoked using a variety of manipulations, including drug deprivation, drug use imagery, and drug cue exposure (Niaura et al., 1988). Carter and Tiffany’s (1999) review confirmed that exposure to drug-related cues significantly increased self-reported urges, or cravings. Research also suggests that cue reactivity can be moderated by both person-level (e.g., heavy vs. occasional users) and situational factors.
One situational factor that appears to affect self-reported urge is drug use opportunity. We recently observed across a number of studies that addicted individuals who were informed that they had an opportunity to consume their drug reported higher urges than did those who did not anticipate use, in some cases double the levels (Wertz & Sayette, 2001b). Left unresolved was whether drug use opportunity affected self-reported urge ratings in the absence of in vivo exposure to drug cues. Some have argued that there is an important distinction between cue-specific urges and general urges brought about by abstinence in the absence of in vivo drug cues (Carter & Tiffany, 1999). These investigators recently found that smoking opportunity affected cue-specific urge ratings (Carter & Tiffany, 2001). One aim of the present study was to contrast the effects of instructed opportunity to smoke on self-reported urge prior to in vivo smoking cue exposure.
Carter and Tiffany (1999) also concluded that drug cue exposure affected nonverbal responding. Specifically, cue exposure increased heart rate, sweat gland activity, and skin temperature. Until recently, however, characterizing the emotional valence of cue reactivity has proven challenging (Sayette et al., 2000; Zinser, Fiore, Davidson, & Baker, 1999). Ideally, these nonverbal measures should be conceptually linked to a particular theory of craving rather than simply measures of convenience (see Kassel & Shiffman, 1992; Tiffany, 1991). For the present study, we adopted the conceptualization of Baker, Morse, and Sherman (1987), in which cravings are considered to be emotional states reflecting the activation of motivational (drug-acquisitive) systems that have particular response patterns involving self-report, behavioral, and physiological correlates.
One cue reactivity domain that has received relatively little attention is facial reactivity. Facial muscle movement has been assessed using facial electromyography (EMG) and observational approaches. With respect to EMG, Drobes and Tiffany (1997) found increased activation in the zygomatic muscle region during smoking cue exposure. Research examining the reflexive eyeblink following startling stimuli (startle probe) also has proven useful for examining craving-related processes (e.g., Elash, Tiffany, & Vrana, 1995). In contrast to EMG, there has been less examination of facial expressions related to craving using observational methods.
For more than 25 years, emotion researchers have used observational coding systems to identify facial expressions thought to be associated with emotion (see Ekman & Rosenberg, 1997; Sayette, Cohn, Wertz, Perrott, & Parrott, 2001). The Facial Action Coding System (FACS; Ekman & Friesen, 1978) is the most comprehensive of these approaches (Ekman & Rosenberg, 1997). Using FACS and viewing videotaped facial behavior in slow motion, coderscan manually code all possible facial displays, referred to as action units (AUs; Ekman & Friesen, 1978). Because FACS can detect subtle, barely visible signs of affect (Ekman & Friesen, 1975), its use during cue exposure can provide key information that otherwise could go unnoticed and, unlike EMG, does not require application of electrodes. Moreover, because it can reliably code rapid changes in expressions, it may be particularly sensitive for examining drug use opportunity during cue exposure.
In an initial study, we used FACS to code facial behavior while smokers held a lit cigarette (Sayette & Hufford, 1995). We found that smokers’ facial expressions changed dramatically in just a matter of seconds from the time they first saw their cigarette, when AUs associated with positive affect were most prevalent, to the time that they were informed that they could not smoke, when negative affect-related AUs were most common. These data suggested that under certain conditions cue-elicited urge could be associated with expressions linked to either positive or negative affect. Depending on one’s model of craving, these facial signals may index craving-related affect or may be a measure of craving itself, if one views craving to be fundamentally affective in nature (see Baker et al., 1987).
This preliminary study (Sayette & Hufford, 1995) suggested that FACS could provide important information regarding emotional experiences following cue exposure. Nevertheless, it was not designed to provide a strong test of smoking opportunity. Although most participants assumed they would be able to smoke when initially presented with their cigarettes, this information was not explicitly manipulated during cue presentation. In addition, in the midst of smoking cue exposure, all participants were informed that they would not be permitted to smoke. Finally, all participants knew they were only required to spend less than 1 hr in the laboratory. Thus, even after being told they could not smoke midway through the experiment, they may have realized that they could smoke fairly soon. An aim of the current research was to use FACS to examine the impact of smoking opportunity in a study that addressed some of the limitations of the Sayette and Hufford (1995) study.
A secondary aim of the present study was to investigate the extent to which two predetermined clusters of AUs would appear during smoking cue exposure. These clusters were selected following a review of videotapes from a prior study in which smokers held and looked at their lit cigarette without smoking (Sayette & Parrott, 1999). Certain AUs involving lip movement appeared repeatedly. These AUs included tightening of the corner of the lip with cheeks compressed against teeth, as well as lips pursed, sucked, tightened, or pressed together. These movements may reflect suppressed emotion (Malatesta, Culver, Tesman, & Shepard, 1989). Malatesta et al. concluded that such expressions “function in the service of self-regulation (i.e., to dampen negative affect)” (p. 49). In addition to AUs putatively linked to suppressed affect, we observed a second pattern of AUs, consisting of a tongue show and lip wipe, which might be associated with appetitive motivation (see Stewart, de Wit, & Eikelboom, 1984, for a discussion of appetitive motivation). These two clusters of expressions might reflect different components or types of craving (i.e., anticipatory excitement as well as impulse restraint; Breiner, Stritzke, & Lang, 1999; Sayette, 1999). To our knowledge, however, particular facial movements associated with craving have not yet been identified. Accordingly, we investigated the degree to which smokers would manifest these two types of facial expressions. Because FACS is labor intensive, taking about 1 hr to code a single minute of tape (Ekman, 1982), and because we wanted to sample a fairly long (31-s) interval, we focused on these two specific patterns of expression for analysis.
Experiment 1
Experiment 1 provided an opportunity to examine three types of data: (a) self-reported urge ratings, (b) facial expressions associated with positive and negative affect, and (c) facial expressions posited to be associated with appetitive motivation or suppressed affect. FACS and urge rating data reported in Experiment 1 were collected as part of a multidimensional investigation of the effects of cue exposure described elsewhere (see Sayette, Martin, Wertz, Shiffman, & Perrott, 2001, for methodological details). This study used a mixed factorial design, with Gender, Smoking Level (heavy smokers [HS] vs. light-smoking tobacco chippers [TC]), Abstinence (7 hr smoking abstinent vs. nonabstinent), and Smoking Opportunity as between-subject factors and Cue Exposure (control vs. smoking) as a within-subject factor, with control cue exposure preceding smoking cue exposure. (Because the present study focuses on smoking opportunity, we refer readers to Sayette, Martin, et al., 2001, for further discussion of the other factors.) Counter-balancing of cues was not used because urge ratings following smoking cue exposure tend to remain high, making it difficult to interpret effects of any subsequent control exposure (e.g., Hutchison, Niaura, & Swift, 1999). Participants’ own cigarettes served as the smoking cue to increase reactivity. A roll of electrical tape served as the control cue, as it was of similar size and weight to a cigarette yet unlikely to be associated with smoking (Sayette & Hufford, 1994).
We hypothesized that participants told they would be able to smoke (told-yes) would report higher urges than those informed that they could not smoke (told-no). Second, we predicted that told-yes smokers would be more likely to evince positive affect-related AUs and less likely to evince negative affect-related AUs than would told-no participants. We also examined whether smoking level or gender would moderate facial responding. A secondary aim was to provide preliminary documentation of the occurrences of two AU clusters appearing during smoking cue exposure that might be linked to appetitive motivation and suppressed emotion.
Method
Participants
Smokers (N = 253; 124 male, 129 female) aged 21-35 were recruited through advertisements in local newspapers and radio programs. Exclusion criteria included medical conditions that ethically contraindicated nicotine, and illiteracy. Informed consentwas obtained from all participants. Seventy-seven percent of the sample was Caucasian, 18% was African American, and 5% was Hispanic or Asian American. The TC group (n = 123) reported smoking five or fewer cigarettes per day on at least 2 days per week. The HS group (n = 130) smoked an average of at least 21 cigarettes per day. Both groups had to report smoking at these rates for at least 24 continuous months (Shiffman, Paty, Kassel, Gnys, & Zettler-Segal, 1994). Abstinent HS and abstinent TC individuals had to have carbon monoxide (CO) levels that did not exceed 16 ppm and 10 ppm, respectively.
Participants’ mean age was 24.6 years (SD = 4.2). They averaged 14.5 years of formal education (SD = 1.8), 7.9 years of smoking (SD = 5.1), 14.5 cigarettes per day (SD = 11.0), and 6.2 prior quit attempts (SD = 2.7). These values were similar across abstinence, gender, and smoking opportunity conditions (ps > .05). The only differences on these variables emerged for smoking level. Compared with the HS group, the TC group was slightly younger, 24.0 years (SD = 3.9) versus 25.2 years (SD = 5.3); had more years of formal education, 14.9 years (SD = 1.7) versus 14.1 years (SD = 1.8); and had smoked for fewer years, 6.4 years (SD = 4.4) versus 9.3 years (SD = 5.3), Fs(1, 237) > 5.0, ps < .02. Analyses of CO at study outset (CO 1) revealed main effects for smoking level and abstinence and a Group × Abstinence interaction, Fs(1, 237) > 33.0, ps < .0001. The abstinent TCs had the lowest CO levels, followed by nonabstinent TCs, abstinent HSs, and nonabstinent HSs.
Baseline Assessment Measures
Demographic information, smoking history and patterns, and current interest in quitting were assessed with standard forms (Sayette, Martin, et al., 2001).
Craving Response Measures
Urge rating scale
Participants’ urge to smoke was reported using a scale ranging from 0 (absolutely no urge to smoke at all) to 100 (strongest urge to smoke I’ve ever experienced; Juliano & Brandon, 1998; Sayette & Hufford, 1994).
Facial coding
Facial expressions were coded by a FACS-certified coder during several periods throughout the experiment (see below) using the exhaustive FACS. Exhaustive coding requires coding all visible movements of the face using a frame-by-frame analysis of videotape. First, AUs were coded during two intervals associated with control cue exposure: (a) initial 5 s that participants viewed control cue and (b) initial 5 s that participants touched control cue. These two periods provided an assessment of participants’ responses to a novel cue that was not smoking-related. FACS was also coded during three intervals associated with smoking cue exposure: (a) the initial 5 s that participants viewed smoking cues (Look), (b) initial 5 s that smokers touched the cigarette (Touch), and (c) first 10 s that participants held the lit cigarette (Hold).
Specific AUs and AU combinations were classified as positive AUs or negative AUs on the basis of a review of FACS literature (see footnotes 3 and 6 in Sayette & Parrott, 1999, for list of AUs and AU combinations related to positive and negative affect). Reliability was tested using comparison coding by a second FACS-certified coder of a random sample of 114 coding periods. These periods included samples for the different time intervals noted above. Kappa coefficients showed that positive AUs (.90) and negative AUs (.69) were coded reliably.
An extended smoking time period was examined using selective coding for the presence of what we referred to above as suppression (AUs 14, 18, 23, 24, and 28) and appetitive (AUs 19, 26, and 37) clusters. This was the entire 31-s interval during which participants held the lit cigarette without smoking it.
Procedure
Telephone screening and instructions
Participants who responded to advertisements underwent a phone interview designed to exclude those not meeting selection criteria. Eligible smokers were asked to attend a 2-hr laboratory session. Those assigned to the abstinent conditions were instructed to refrain from smoking for at least 7 hr and were told that breath samples would test whether they had abstained. All participants were told to bring a pack of their preferred brand of cigarettes to the laboratory session.
Laboratory set-up
Participants underwent cue exposure manipulations while seated in a comfortable chair behind a desk. Facing the desk was a mounted video camera. Participants were told that the camera and intercom facilitated communication and helped the investigator determine whether instructions were understood throughout the study.
Baseline assessment
Experimental sessions began between 3:00 p.m. and 5:00 p.m. On participants’ arrival, their written informed consent was obtained. To confirm abstinence, participants reported the last time they smoked and a CO 1 reading was recorded. Participants presented their pack of cigarettes and lighter to the experimenter. They completed baseline assessment, including the urge rating scale (Urge 1). At this time, all nonabstinent participants smoked a cigarette. During this 5-min interval, abstinent participants sat quietly. Participants then provided CO 2 and rated Urge 2.
Cue exposure
Prior to cue exposure, participants were instructed how to perform a simple response time task, which involved clicking a mouse button whenever a tone sounded (Sayette, Martin, et al., 2001). Next, a tray holding an inverted plastic bowl was placed on the desk. Participants then lifted the bowl, which revealed a roll of tape. After picking up the tape in their dominant hand, participants rated Urge 3. Two minutes later, the experimenter replaced the tray and bowl with a second tray and bowl. Participants rated Urge 4 and then lifted the bowl, which revealed their pack of cigarettes, an ashtray, and a lighter. They were told to remove one cigarette from the pack and light it without putting it in their mouths. They then held the lit cigarette and looked at it. After 31 s, they rated Urge 5, following which they extinguished the cigarette and completed several additional measures reported elsewhere (see Sayette, Martin, et al., 2001, for further details about cue exposure procedure). Finally, participants completed a form asking them about the study’s purpose, were debriefed, and were paid $45.
Results
Urge Rating Scale
Table 1 presents urge ratings throughout the study. A 2 (smoking level) × 2 (abstinence) × 2 (smoking opportunity) × 2 (gender) × 5 (time) repeated measures analysis of variance (ANOVA), with the five urge ratings as a repeated variable, revealed smoking level, smoking opportunity, and abstinence main effects, as well as Smoking Level × Abstinence, and Smoking Level × Abstinence × Smoking Opportunity interactions, Fs(1, 235) > 5.0, ps < .02. A trend appeared for a Smoking Level × Smoking Opportunity interaction, F(1, 235) = 3.6, p < .06, such that smoking opportunity effects tended to be particularly potent among the HS groups (see Table 1). A main effect for time wasevident, as well as Time × Abstinence, Time × Abstinence × Smoking Level, and Time × Abstinence × Smoking Opportunity interactions, Fs(4, 940) > 3.0, ps < .02. To examine these interactions, urge data were examined separately at each time period.
Table 1.
Means and Standard Deviations of Self-Reported Urge Ratings
| Told-no tobacco chippers |
Told-no heavy smokers |
Told-yes tobacco chippers |
Told-yes heavy smokers |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonabstinent |
Abstinent |
Nonabstinent |
Abstinent |
Nonabstinent |
Abstinent |
Nonabstinent |
Abstinent |
|||||||||
| Urge | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| 1 | 18.9 | 21.5 | 22.2 | 22.3 | 20.8 | 17.9 | 48.1 | 22.8 | 28.6 | 25.3 | 22.1 | 18.6 | 27.4 | 21.3 | 63.2 | 20.6 |
| 2 | 9.5 | 14.1 | 21.3 | 18.6 | 2.6 | 5.5 | 49.1 | 20.2 | 12.7 | 19.5 | 21.6 | 18.2 | 3.5 | 5.8 | 66.7 | 20.9 |
| 3 | 11.7 | 15.3 | 23.0 | 21.4 | 10.1 | 13.5 | 48.6 | 21.4 | 14.8 | 19.8 | 23.4 | 19.5 | 16.6 | 19.5 | 68.5 | 20.3 |
| 4 | 10.9 | 14.4 | 25.1 | 23.2 | 14.5 | 19.1 | 50.5 | 21.6 | 15.0 | 19.0 | 25.7 | 20.6 | 17.3 | 19.4 | 70.1 | 19.5 |
| 5 | 24.3 | 23.0 | 44.2 | 32.3 | 34.1 | 24.3 | 70.7 | 23.0 | 37.5 | 30.3 | 39.1 | 24.8 | 36.0 | 29.1 | 79.7 | 19.8 |
Note. Urge 1 was rated at postinitial instruction; Urge 2 was rated postsmoke for the nonabstinent smokers; Urge 3 was rated at control cue exposure; Urge 4 was rated at presmoking cue baseline; Urge 5 was rated at smoking cue exposure.
For Urge 1, at the outset of the study, a main effect for smoking level, F(1, 237) = 37.0, p < .0001, indicated that HSs reported higher initial urges (M = 40, SD = 27) than did TCs (M = 23, SD = 23). Abstinent smokers reported higher urges (M = 39, SD = 27) than did nonabstinent smokers (M = 24, SD = 22), F(1, 237) = 26.0, p < .0001. Of particular interest to the current study was a main effect for smoking opportunity, F(1, 237) = 7.5, p < .01, with told-yes smokers (M = 36, SD = 27) reporting higher urges than told-no smokers (M = 28, SD = 25). A Smoking Level × Abstinence interaction also appeared, F(1, 237) = 38.0, p < .0001. Post hoc tests revealed that abstinent HSs reported substantially higher urges (M = 55) than did participants in the other three conditions who had similar urges (Ms = 22-25, all ps < .0001; see Table 1).
Urge 2 tested the effects of smoking on urge ratings, as half the participants (those in nonabstinent conditions) were permitted to smoke. There were main effects for smoking level, F(1, 237) = 47.0, p < .0001; abstinence, F(1, 237) = 222.0, p < .0001; smoking opportunity, F(1, 237) = 5.9, p < .02; as well as Smoking Level × Abstinence, F(1, 237) = 107.0, p < .0001, and Smoking Level × Abstinence × Smoking Opportunity, F(1, 237) = 6.0, p < .02, interactions. Abstinence had a greater impact on HSs than on TCs (p < .0001). In addition, smoking opportunity was an especially important determinant of urge ratings among abstinent HSs (p < .001). Effects for smoking level, F(1, 236) = 54.0, p < .0001; abstinence, F(1, 236) = 121.0, p < .0001; smoking opportunity, F(1, 236) = 8.3, p < .01; and the Smoking Level × Abstinence interaction, F(1, 236) = 54.0, p < .0001, were maintained during the control exposure period (Urge 3). Similar results appeared during precigarette exposure assessment (Urge 4). Significant effects emerged for smoking level, F(1, 236) = 56.0, p < .0001; abstinence, F(1, 236) = 119.0, p < .0001; smoking opportunity, F(1, 236) = 6.1, p < .02; and the Smoking Level × Abstinence interaction, F(1, 236) = 41.0, p < .0001. During cigarette exposure (Urge 5), main effects appeared for both smoking level, F(1, 237) = 32.0, p < .0001, and abstinence, F(1, 237) = 54.0, p < .0001, and there was a significant Smoking Level × Abstinence interaction, F(1, 237) = 21.0, p < .0001. For both smoking opportunity conditions the abstinent HSs reported significantly higher urges than each of the remaining three groups (i.e., abstinent TCs, nonabstinent HSs, and nonabstinent TCs; ps < .001). Urge ratings among these latter three groups did not differ significantly (see Table 1).
Facial Expression
AUs
Because FACS data were dichotomous (presence or absence of particular AUs), unless otherwise noted, data were analyzed using logistic regression (PROC CAT MOD; SAS Institute, Inc., 2000). Separate analyses were conducted for positive and negative AUs during both control and smoking cue exposure.
Positive AUs during control exposure
Unlike self-report and physiological measures, there is no true baseline period for coding facial expression. Nevertheless, we were able to code facial reactivity during control cue exposure and found that participants did not tend to evince positive AUs during control exposure. A 2 (smoking level) × 2 (abstinence) × 2 (smoking opportunity) × 2 (gender) × 2 (time) repeated measures analysis with the two control exposure periods as the repeated variable examined positive AUs across the two control coding periods and revealed only a main effect for time, χ2(1, N = 253) = 12.8, p < .001, with more smokers (18%) expressing positive AUs during the first than the second (9%) control exposure period.
Negative AUs during control exposure
A similar analysis was conducted to examine negative AUs. Relatively few participants manifested negative AUs during either the first (14%) or second (13%) control exposure periods. No significant main effects or interactions emerged.
Positive AUs during smoking cue exposure
To test whether told-yes smokers were more likely to evince positive affect-related AUs than told-no participants, a 2 (smoking level) × 2 (abstinence) × 2 (smoking opportunity) × 2 (gender) × 3 (time) repeated measures analysis examined positive AUs across the three smoking cue coding periods. There was a main effect for abstinence, χ2(1, N = 253) = 9.68, p < .01, with abstinent smokers more likely to express positive AUs than nonabstinent smokers, and a marginal Smoking Level × Abstinence interaction, χ2(2,N = 253) = 3.64, p < .06, with abstinent HSs tending to be most likely to express positive AUs. A main effect for time, χ2(2, N = 253) = 63.3, p < .001, indicated that the likelihood of expressing positive AUs declined over time with 48% of participants displaying positive AUs during the Look period but only 25% doing so during Touch and Hold periods. There was no effect for gender. Of particular relevance to the present study, a significant Time × Smoking Opportunity interaction also appeared, χ2(2, N = 253) = 9.8, p < .01, such that, as indicated in Figure 1a, over the course of smoking cue exposure, told-yes participants became less likely to express positive AUs, relative to told-no participants.
Figure 1.
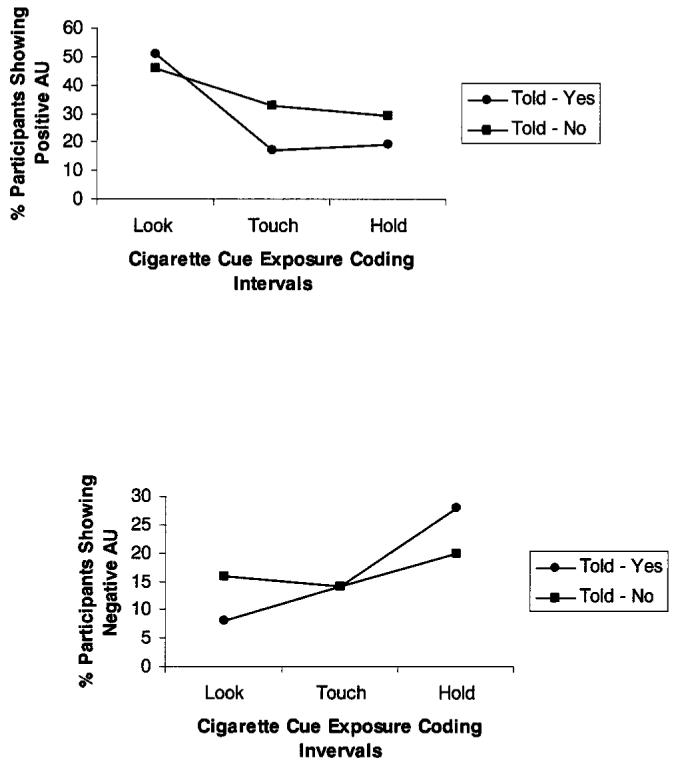
Experiment 1: Percentage of participants expressing positive affect-related action units (AUs) during smoking cue exposure (top panel) and percentage of participants expressing negative affect-related AUs during smoking cue exposure (bottom panel).
A post hoc analysis then examined smoking opportunity at each of the three smoking cue exposure time intervals. A significant effect of smoking opportunity appeared both during the Touch period, χ2(1, N = 253) = 7.19, p < .01, with told-no participants (33%) more likely than told-yes participants (17%) to express positive AUs, and during the Hold period, χ2(1, N = 253) = 4.78, p < .03, with told-no smokers (29%) more likely than told-yes smokers (19%) to express positive AUs.
Negative AUs during smoking cue exposure
We next investigated whether told-yes smokers would be less likely to evince negative-affect related AUs than would told-no participants. A 2 (smoking level) × 2 (abstinence) × 2 (smoking opportunity) × 2 (gender) × 3 (time) repeated measures analysis revealed a marginally significant effect for abstinence, χ2(1, N = 253) = 3.1, p < .08, with abstinent smokers tending to be less likely than nonabstinent smokers to express negative AUs. There was no effect for gender. A main effect of time, χ2(2, N = 253) = 7.2, p < .01, indicated that the likelihood of expressing negative AUs increased over time (from 12% during the Look period to 14% during the Touch period and 24% during the Hold period), and, pertinent to the present study, there was a Time × Smoking Opportunity interaction, χ2(2, N = 253) = 5.6, p < .02. As shown in Figure 1b, over the course of smoking cue exposure, told-yes smokers began to show more negative AUs, relative to told-no smokers. A post hoc analysis then examined smoking opportunity at each of the three smoking cue exposure time intervals. A main effect during the Look period revealed that told-yes participants (8%) were less likely than told-no participants (16%) to evince negative AUs. In addition, the effects of time on negative AUs were conducted separately for each smoking opportunity condition. There was a significant effect of time in the told-yes (p < .001) but not in the told-no condition. No other effects were significant.
Urge ratings during smoking cue exposure were significantly (though modestly) correlated with likelihood of expressing a positive AU during the Look period, r(253) = .18, p < .01; the Touch period, r(253) = .16, p < .01; and the Hold period, r(253) = .16, p < .01. Urge ratings were not correlated with likelihood of expressing a positive AU during the control exposure coding intervals. Urge ratings also were not correlated with occurrence of negative AUs during any of the control or smoking cue coding intervals (rs < .06).
Selected AUs during smoking cue exposure
During the 31 s that participants held the lit cigarette, we coded the occurrence of two predetermined clusters of AUs posited to appear during smoking cue exposure. Fifty-seven percent of participants showed AUs comprising the suppression cluster. A 2 (smoking level) × 2 (abstinence) × 2 (smoking opportunity) × 2 (gender) analysis revealed a main effect for smoker level, χ2(1, N = 253) = 4.53, p < .04, such that TCs were more likely to express these AUs (63%) than were HSs (50%). No other effects were significant. Thirteen percent of the sample showed AUs included in the appetitive cluster, and no significant effects appeared. Participants were more likely to display suppression AUs than they were to express appetitive AUs, F(1, 253) = 172.0, p < .0001. (During the Hold period, there was little overlap between appetitive responding and either positive or negative AUs, rs [n = 253] < .06, ns, or between suppression and either positive or negative AUs, rs [n = 253] < .07, ns.)
Discussion
Results of Experiment 1 revealed that smoking opportunity influenced self-reported urge prior to smoking cue exposure. Specifically, smokers told they could smoke during the study reported higher urges than did those told they could not smoke. Our literature review suggested that opportunity to use a drug influenced urge ratings during cue exposure (Wertz & Sayette, 2001b). The present data suggest that, at least for smokers, this smoking opportunity effect can appear even prior to in vivo smoking cue exposure.
Our finding that abstinent smokers were more likely to express positive AUs than were nonabstinent smokers during smoking cue exposure replicates the pattern found in our initial study (Sayette & Hufford, 1995). Contrary to our hypotheses, however, in the current study told-yes participants were neither more likely to evince positive AUs nor less likely to evince negative AUs than were told-no participants. Indeed, over the course of the three smoking cue coding periods, compared with told-no smokers, told-yes participants became less likely to express positive AUs and more likely to express negative AUs.
This unexpected finding may be attributed to the length of time that participants remained in their smoking opportunity condition. Perhaps, in the context of this laboratory setting, positive affect associated with anticipating imminent smoking can be maintained for only brief intervals. Initially, told-yes smokers may have believed that once they lit their cigarette they would be able to smoke immediately. Our data suggest that initially the smoking opportunity manipulation was successful, as told-yes participants were less likely than the told-no participants to evince negative AUs during the Look period. When informed that there would be a delay before smoking, however, told-yes participants may have begun to feel more keenly the frustration associated with the absence of smoking opportunity than did told-no participants. Indeed, told-no smokers may have had more time to prepare to cope with smoking cue exposure and thus may have experienced less distress. The realization that smoking would be postponed for a brief, but indefinite, period may have inadvertently altered the smoking opportunity condition for impatient told-yes smokers.
A recent study demonstrating smoking opportunity effects may have avoided this concern by restricting duration of the delay interval. Carter and Tiffany (2001) found that informing participants that they could smoke led to enhanced ratings of urge and positive affect, increased physiological responding, and greater drug-seeking behavior. It is important that in their cue-availability paradigm, the delay period was brief (their six-item measure was administered 8 s after cue presentation). Accordingly, Experiment 2 was designed to provide a more focused examination of the effects of smoking opportunity on facial expression during smoking cue exposure, by manipulating the duration of the interval.
Experiment 1 also provided initial descriptive data to suggest that certain clusters of AUs may appear during smoking cue exposure. The majority (57%) of participants manifested activation of the suppressed cluster. In contrast to the suppressed AUs, only 13% of smokers displayed the AUs that we posited to be associated with appetitive motivation. Further consideration of these data is provided in the General Discussion section.
Experiment 2
The primary aim of Experiment 2 was to test the effects of different delay periods on facial expression thought to be associated with affect. Abstinent smokers were randomly assigned to one of three latency conditions that varied with respect to how long they held a lit cigarette before they could smoke it. The latency intervals were 15 s (n = 18), 30 s (n = 19), and 60 s (n = 20). We predicted that the longer participants were required to wait, the more likely they would be to express negative AUs and the less likely they would be to express positive AUs. Specifically, we hypothesized that the shortest delay group would be more likely to manifest positive AUs and less likely to manifest negative AUs than would the longest latency group.
Method
Participants
Fifty-seven smokers (28 male and 29 female) aged 18-46 (M = 19.9, SD = 4.5) participated in return for course credit in an introductory psychology course. Informed consent was obtained from all participants. Eighty-nine percent of the sample was Caucasian, 10% was African American, and 2% was Asian American. Participants reported smoking an average of 13.6 (SD = 4.5) cigarettes per day for an average of 4.7 years (SD = 3.8) and reported smoking their initial cigarette within 2 hr of awakening. Groups did not differ on any of these variables. Participants reported to the lab between 10:00 a.m. and noon. They were told to stop smoking at 11:00 p.m. the previous night and to bring a pack of their cigarettes. A CO sample was collected to check abstinence compliance (M = 10.8 ppm, SD = 4.1). CO levels could not exceed 16 ppm.
Procedure
Participants sat in a comfortable chair behind a desk in an experimental room containing an intercom and a video camera. After completing the same baseline urge rating as in Experiment 1 and a variant of the Stroop color-naming task described elsewhere (Wertz & Sayette, 2001a), the tray used in Experiment 1 was brought into the room. Participants were asked if they wanted to smoke and, if so, they were asked to remove the cover and to light a cigarette without smoking it.1 They next were instructed to look at a timer, which had been placed on the desk to their right. The timer was set to count down from 15 s, 30 s, or 60 s, depending on condition. They were told that as soon as the timer reached zero they could smoke the lit cigarette. Participants immediately activated the timer and 5 s later rated (vocally) their urge to smoke. FACS was coded by a certified coder during the initial 15 s that participants were holding the lit cigarette. Positive and negative AUs were classified in the same manner as in Experiment 1. Reliability (κs = .98 for positive AUs and .79 for negative AUs) was checked using a second FACS-certified coder using the same methods as in Experiment 1.
Results and Discussion
Baseline urge ratings (M = 51.1, SD = 23.9) did not differ significantly across the three latency conditions. Across conditions, mean urge rating during cue exposure was 79.1 (SD = 20.0).
A 3 (latency) × 2 (time) repeated measures ANOVA revealed a significant Latency × Time interaction, F(2, 54) = 3.3, p < .05. Follow-up tests (Duncan’s) revealed that participants in the 15-s group had a significantly larger change from baseline (M = 38.6, SD = 20.7) than did the 30-s (M = 22.6, SD = 13.3) or 60-s (M = 23.4, SD = 30.7) groups (ps < .05).
Figure 2 shows the percentage of participants evincing negative AUs and positive AUs in the three latency conditions. A logistic regression analysis examined whether smoking latency condition and gender affected the likelihood of expressing positive AUs during the coding period. With respect to gender, a trend appeared indicating that women (59%) tended to be more likely to display positive AUs than were men (43%), χ2(1, N = 57) > 2.9, p < .09. There was a main effect for latency, χ2(2, N = 57) > 6.3, p < .05. Planned contrasts for latency revealed that participants in the 15-s group were significantly more likely to express a positive AU than were those in the 60-s group (p < .05; see Figure 2). The 30-s group did not differ significantly from either the 15-s or the 60-s group. A similar set of analyses was conducted for negative AUs. Although in the expected direction, latency did not significantly affect the probability that smokers would express a negative AU (see Figure 2).
Figure 2.
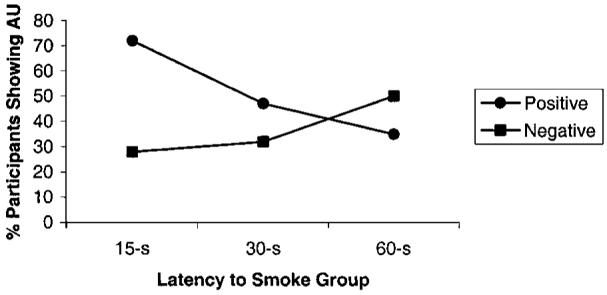
Experiment 2: Percentage of participants in each latency group expressing affect-related action units (AUs) during smoking cue exposure.
Across the entire sample, urge ratings during this cue exposure interval did not significantly correlate with the likelihood of evincing positive or negative AUs (rs < .16, p < .25). Although sample sizes were too small to reveal significant correlations, for exploratory purposes we also examined each delay condition separately. Urge ratings were in the direction of being positively correlated with the likelihood of evincing positive AUs in the 15-s group, r(18) = .20, p < .42, and inversely correlated in the 60-s condition, r(20) = -.37, p < .11. These two measures were uncorrelated in the 30-s condition, r(19) = .07, p < .79. Again, we emphasize that none of these correlations was significant in these small samples. Future research with larger samples is needed to determine whether positive AUs are positively correlated with urge ratings during brief latencies and negatively correlated with urge ratings during longer latencies. No pattern emerged across the three conditions for negative AUs (rs = -.02, .24, and -.00 for the 15-s, 30-s, and 60-s groups, respectively).
Similar to the findings in Experiment 1, 61% of participants expressed suppression AUs, and 8.8% expressed appetitive AUs. The occurrence of appetitive and suppression did not differ across latency conditions (Fs < 1.5). Appetitive responding and positive AUs were not correlated, r (n = 57) = -.19, ns. Appetitive responding and negative AUs were marginally correlated, r (n = 57) = .24, p < .08. Suppression and negative AUs were marginally correlated, r (n = 57) = .23, p < .09. Suppression and positive AUs were correlated, r (n = 57) = .30, p < .03.
General Discussion
One aim of the present study was to investigate the impact of smoking opportunity on self-reported urge to smoke. Findings from Experiment 1 indicated that participants told they could smoke reported higher urges than did those told they could not smoke. These data extend the findings from past studies (see Wertz & Sayette, 2001b) by suggesting that urge ratings can be influenced by smoking opportunity even prior to in vivo cue exposure. Data from Experiment 2 suggested further that urge ratings may be influenced not only by whether one expects to smoke but also by when one expects to smoke. That is, urge ratings increased more when participants were told they could smoke in 15 s than when they were told they could smoke in either 30 s or 60 s.
A primary goal of this research was to examine the effect of smoking opportunity on facial expressions thought to be associated with positive and negative affect. In Experiment 1, we found that the likelihood that smokers would evince a positive AU declined over the course of the smoking cue exposure and that this was more pronounced for smokers told they could smoke soon than for those told they could not smoke during the study. It is intriguing that the period during which smokers held their cigarette in Experiment 1 seemed to become increasingly negative for those told that soon they would be able to smoke. That is, as time passed, all participants became more likely to evince negative AUs, but this trend was especially pronounced among those instructed that they would be able to smoke soon.
Experiment 2 revealed that even brief differences in latency dramatically altered the affective tone of the experience. Nearly three fourths of the 15-s participants evinced positive AUs, compared with just one third of the 60-s participants. These differences do not seem to be due to a general increase in facial reactivity in the 15-s group, as there was no significant effect for negative AUs between the 15-s and 60-s latency conditions. Although just a matter of seconds, the additional delay may have turned what was generally a positive craving experience into a more negative experience. Zinser et al. (1999) found that a 60-s waiting period was associated with an electrophysiological index of approach motivation. The lack of a fine-grained temporal resolution in that study, however, makes it difficult to con-trast with our data. To the extent that positive AUs are linked to approach behavior, our 15-s condition would appear consistent with Zinser et al.’s conclusion that a brief latency cue exposure period can elicit approach motivation.
The present data suggest that under certain conditions (i.e., 15 s), craving may be linked to positive affect. This suggests that some of the perceived satisfaction generally associated with drug use may actually precede drug consumption. That is, craving itself may be satisfying to those who anticipate using the drug very soon. Given the lack of participant control and the generally artificial conditions found in the current study, we speculate that in the natural environment, the interval during which positive craving may occur could be extended. If so, the findings for the 15-s interval may be considered from an economic perspective. Loewenstein (1987) has described savoring as the “positive utility derived from anticipation of future consumption” (p. 667). Children who hoard their stash of Halloween candy rather than eating it, for example, may prefer savoring their candy to actual consumption. (Again the relevance of this example to the present data requires that positive affect be found when latency periods stretch well beyond 15 s.) Loewenstein (1987) also posited that, consistent with our findings, the pleasure derived from anticipating a reinforcer can increase as it approaches. Although speculative, it may prove useful to explain to smokers seeking abstinence that not all of the perceived satisfaction they are giving up is due to actual nicotine consumption and that part of what they miss is merely the anticipation of using (i.e., savoring). This approach of shifting drug effects from actual drug use to one’s beliefs and perceptions may share some features with successful expectancy challenge efforts described by Darkes and Goldman (1998).
Both Experiments 1 and 2 highlight the role that impatience can play in craving. That is, the findings suggest that cigarette craving is associated with not just a desire to smoke but an impatient desire to smoke immediately. This emphasis on temporal discounting is consistent with models of addiction in which craving is linked to impatience regarding drug use (e.g., Loewenstein, 1999).
Methodologically, these studies suggest difficulty examining craving during conditions in which smoking opportunity is present. Even a 60-s delay may be too long to maintain the positive allure of anticipating smoking in the laboratory setting. Most likely the time limit for maintaining a positive affective state is partly determined by what is happening during that delay. Perhaps if it seems that time is being spent productively in the service of eventual drug consumption (e.g., watching an experienced waiter remove the cork and pour a glass of wine), positive affect might be maintained for extended periods. Indeed, as suggested above, it is possible that in the natural environment, the savoring experience can extend well beyond the time intervals examined in the present study.
As noted above, Carter and Tiffany’s (2001) cue availability paradigm offers an attractively brief time interval for examining different smoking opportunity conditions. This approach requires smokers to take a number of cigarette puffs throughout the session, thereby eliminating total abstinence, which might subtly alter the craving experience (increasing or decreasing craving depending on the person and the assessment point). At this point, knowledge of the role of drug use opportunity is likely to be advanced through converging evidence from multiple methods.
Data from Experiments 1 and 2 similarly revealed that the majority of smokers displayed activation of the suppressed but not the appetitive AU cluster. It is interesting that, as discussed below, activation of the suppression cluster occurred more often for TCs than for HSs. The suppressed cluster may have been displayed for several reasons. Consistent with the conceptualization offered by Malatesta et al. (1989), this cluster may signal suppressed emotion reflecting both frustration and a desire for self-regulation that is required of smokers while they resist their impulse to smoke the cigarette (see also Newman & Bloom, 1981). (Note that all of the participants were smokers who were holding a lit cigarette in their hand and that even TCs reported a mean urge score during smoking cue exposure about one third of their maximum [see Table 1].) Accordingly, if this pattern were to appear in future studies, it would be useful to determine whether, like other cue reactivity measures (e.g., Rohsenow et al., 1994), facial reactivity provides important information regarding subsequent cessation efforts. There exists a wealth of data to suggest that particular AUs may provide important etiological information regarding schizo-phrenia, affective disorders, and other forms of psychopathology (see Ekman & Rosenberg, 1997). It remains to be seen whether the presence, or perhaps the intensity or duration, of particular AUs can prove useful for understanding or predicting drug relapse.
Alternative to the view that these AUs reflect an underlying emotional state related to craving is the possibility that these lip movements are instead manifestations of an interrupted smoking action plan (Tiffany, 1990). One consequence of this interruption is the activation of motoric or procedural responses associated with problem solving or anticipation of action. From this perspective, the observed lip movements may reflect preparatory movements by the participant to smoke, rather than an emotional state. A positron emission tomography study (Hsieh et al., 1994) offers data consistent with this position. These authors examined urge to scratch following histamine injections and found evidence of premotor cortical area activation, which is implicated in the preparation of an intended action. Whether these expressions reflect preparatory movements or underlying affective states likely could be disentangled empirically. In contrast to an affect explanation, a preparatory movement explanation suggests that this pattern of facial activation should occur only in cue exposure studies that involve drugs that are orally administered.
A third possible explanation for the frequent occurrence of these suppressed movements is that they may reflect a generalized frustration stemming from interrupting a wellpracticed routine. That is, these AUs might appear when any type of well-learned behavioral pattern is blocked and may not be specific to drug craving (see Sayette, 1999).
Most models of craving would predict that heavy smokers in withdrawal should experience the most intense cravings and, thus, should be especially likely to display any other signal of emotional arousal that indexes this motiva-tional state (Baker et al., 1987; Sayette, 1999). It is interesting that the occurrence of these suppressed AUs was not influenced by abstinence and that these AUs were more likely to be displayed by chippers than heavy smokers.
In contrast to the suppressed AUs, only about 10% of smokers across the two experiments displayed the AUs that we posited to be associated with appetitive motivation. It is possible that there is not a set of expressions linked to appetitive motivation, or that our selection of candidate AUs was misguided. Alternatively, our experimental procedure may have been suboptimal for eliciting this psychological state. Perhaps this pattern would have been more likely to occur if participants were told they could smoke immediately. When the opportunity to smoke was both imminent and salient, there is evidence that relative activation of the left frontal cortex, assessed using electroencephalograph, may index approach smoking motivation (Zinser et al., 1999).
Gender did not affect the likelihood of expressing either positive or negative AUs in Experiment 1. Consistent with our previous findings (Sayette & Hufford, 1995), however, in Experiment 2, women were more likely to display positive AUs than were men. It is not apparent to us why effects of gender should be inconsistent across both the present studies and our earlier work. The present study included smokers who were not currently interested in quitting. Consequently, we cannot be sure whether the patterns of facial expressions found here would generalize to those currently trying to quit. Previous research suggests that smokers interested in quitting, perhaps because they do not perceive an opportunity to smoke because of their attempted cessation, report lower urges than do those who are not interested (see Wertz & Sayette, 2001b).
The current findings indicate that instructions regarding smoking opportunity affect urge ratings and facial expressions. Future research that compares smoking opportunity with the opportunity to consume equally valued nondrug reinforcers would help determine whether urge and affect measures are specifically tied to the anticipation of a drug reinforcer.2
Our facial coding analyses focused on the occurrence of AUs. It also is possible to examine the duration and, in some cases, the intensity of AUs (Sayette, Cohn, et al., 2001). Our focus on occurrence was based on our past smoking cue exposure research (Sayette & Hufford, 1995), which suggested that findings with occurrence paralleled the other indices. In addition, because Experiment 2 only included abstinent smokers, it is unclear whether the different delay periods would also affect nonabstinent smokers. The absence of Smoking Opportunity × Abstinence interactions in Experiment 1 suggests, however, that as long as a sufficient urge is evident, smoking opportunity should influence responding (see also Sayette & Hufford, 1995).
This study cannot resolve the theoretical issue of whether these facial expressions are indexing craving per se, or simply emotional states associated with craving (cf. Baker et al., 1987; Sayette et al., 2000). Still, this research and our initial work (Sayette & Hufford, 1995) indicate that urges can occur with a wide range of affective tone. FACS may prove useful because it is not subject to many of the concerns raised against self-reported affect measures used in cue exposure studies (cf. Robinson & Berridge, 1993; Sayette et al., 2000; Zinser et al., 1999).
In summary, the present data add to an emerging body of work suggesting that opportunity for drug use affects urge responding and highlights more generally the central role of expectancy in addictions research (see Goldman, 2002). In addition to self-reported urge, the facial coding data presented here suggest that knowledge of both whether one perceives the opportunity to smoke and when this opportunity will occur, can influence affective tone during cue exposure. Facial expression, as measured by FACS, appears to be sensitive to drug use opportunity and is associated with craving self-report. Like many nonverbal cue reactivity measures, however, the specificity of particular AUs remains to be tested (see Sayette et al., 2000). Nevertheless, this is one of the largest studies using FACS ever conducted, and the data suggest that this method may prove as useful for understanding addictive disorders as it has for other areas of psychopathology (Ekman & Rosenberg, 1997).
Acknowledgments
This research was supported by Grant R01 DA10605 from the National Institute on Drug Abuse. We thank Saul Shiffman, George Loewenstein, and Carey Ryan for their helpful comments. We also thank Dominic Parrott, Dawn Giuffre, and the rest of the staff of the Alcohol and Smoking Research Laboratory for their assistance.
Footnotes
Several smokers did not wish to smoke and thus did not participate in this study. Demographic and sample size data noted above describe the sample of smokers who did participate and thus differs from data reported in Wertz and Sayette (2001a). The Wertz and Sayette (2001a) study focused on a color-naming task that was administered prior to the smoking opportunity manipulation, which is the focus here.
We thank an anonymous reviewer for this suggestion.
Contributor Information
Michael A. Sayette, Department of Psychology, University of Pittsburgh
Joan M. Wertz, Department of Psychology, University of Pittsburgh
Christopher S. Martin, Department of Psychiatry and Department of Psychology, University of Pittsburgh
Jeffrey F. Cohn, Department of Psychology, University of Pittsburgh
Michael A. Perrott, Department of Psychology, University of Pittsburgh
Jill Hobel, Department of Psychology, University of Pittsburgh.
References
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. In: Rivers C, editor. The Nebraska Symposium on Motivation: Vol. 34. Alcohol Use and Abuse. University of Nebraska Press; Lincoln: 1987. pp. 257–323. [PubMed] [Google Scholar]
- Breiner MJ, Stritzke WGK, Lang AR. Approaching avoidance: A step essential to the understanding of craving. Alcohol Research and Health. 1999;23:197–206. [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Darkes J, Goldman MS. Expectancy challenge and drinking reduction: Process and structure in the alcohol expectancy network. Experimental and Clinical Psychopharmacology. 1998;6:64–76. doi: 10.1037//1064-1297.6.1.64. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Ekman P. Methods for measuring facial action. In: Scherer KR, Ekman P, editors. Handbook of methods in nonverbal behavior research. Cambridge University Press; Cambridge, England: 1982. pp. 45–90. [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face: A guide to recognizing emotions from facial clues. Prentice Hall; Englewood Cliffs, NJ: 1975. [Google Scholar]
- Ekman P, Friesen WV. Facial Action Coding System. Consulting Psychologists Press; Palo Alto, CA: 1978. [Google Scholar]
- Ekman P, Rosenberg EL, editors. What the face reveals: Basic and applied studies of spontaneous expression using the Facial Action Coding System (FACS) Oxford University Press; New York: 1997. [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure:Self-report, psychophysiological, and startle probe responses. Experimental and Clinical Psychopharmacology. 1995;3:156–162. [Google Scholar]
- Goldman MA. Expectancy and risk for alcoholism: The unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcoholism: Clinical and Experimental Research. 2002;26:737–746. [PubMed] [Google Scholar]
- Hsieh JC, Hagermark O, Stahle-Backdahl M, Ericson K, Eriksson L, Stone-Elander S, et al. Urge to scratch represented in the human cerebral cortex during itch. Journal of Neurophysiology. 1994;72:3004–3008. doi: 10.1152/jn.1994.72.6.3004. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Experimental and Clinical Psychopharmacology. 1999;7:250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: Evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Advances in Behaviour Research and Therapy. 1992;14:141–167. [Google Scholar]
- Loewenstein G. Anticipation and the valuation of delayed consumption. Economic Journal. 1987;97:666–684. [Google Scholar]
- Loewenstein G. A visceral account of addiction. In: Elster J, Skog OJ, editors. Getting hooked: Rationality and addiction. Cambridge University Press; Cambridge, England: 1999. pp. 235–264. [Google Scholar]
- Lowman C, Hunt WA, Litten RZ, Drummond DC. Research perspectives on alcohol craving: An overview. Addiction. 2000;95:S45–S54. doi: 10.1080/09652140050111636. [DOI] [PubMed] [Google Scholar]
- Malatesta CZ, Culver C, Tesman JR, Shepard B. The development of emotion expression during the first two years of life. Monographs of the Society for Research in Child Development. 1989;54(12) Serial No. 219. [PubMed] [Google Scholar]
- Newman A, Bloom R. Self-control of smoking: I. Effects of experience with imposed, increasing, decreasing and random delays. Behaviour Research and Therapy. 1981;19:187–192. doi: 10.1016/0005-7967(81)90001-2. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, et al. Cue reactivity as a predictor of drinking among male alcoholics. Journal of Consulting and Clinical Psychology. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS system for Windows (Version 8) [Computer software] Author; Cary, NC: 2000. [Google Scholar]
- Sayette MA. Cognitive theory and research. In: Leonard K, Blane H, editors. Psychological theories of drinking and alcoholism. 2nd ed. Guilford Press; New York: 1999. pp. 247–291. [Google Scholar]
- Sayette MA, Cohn JF, Wertz JM, Perrott MA, Parrott DJ. A psychometric evaluation of the Facial Action Coding System for assessing spontaneous expression. Journal of Nonverbal Behavior. 2001;25:167–185. [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Urge and affect: A facial coding analysis of smokers. Experimental and Clinical Psychopharmacology. 1995;3:417–423. [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Parrott DJ. Effects of olfactory stimuli on urge reduction in smokers. Experimental and Clinical Psychopharmacology. 1999;7:151–159. doi: 10.1037//1064-1297.7.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. The role of conditioned and unconditioned drug effects in the self-administration of opiates. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. The application of 1980s psychology to 1990s smoking research. British Journal of Addiction. 1991;86:617–620. doi: 10.1111/j.1360-0443.1991.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: Impact on an electrophysiological index of approach motivation. Journal of Abnormal Psychology. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]


