Abstract
A number of studies have demonstrated the influence of nicotine on fetal development. This study determined the expression of choline acetyltransferase (ChAT), vesicular acetylcholine transporter (VAChT), and high-affinity choline transporter (CHT1) in the forebrain and hindbrain following chronic prenatal nicotine exposure in the rat fetus (maternal rats were subcutaneously injected with nicotine at different gestation periods). We also measured the effect of chronic nicotine exposure on fetal blood pO2, pCO2, pH, Na+ and K+ concentrations, as well as lactic acid levels. Maternal nicotine exposure during pregnancy was associated with a decrease in fetal pO2 coupled with a significant increase in pCO2 and lactic acid as well as restricted fetal growth. Additionally, maternal nicotine administration also reduced ChAT, VAChT, and CHT1 mRNA levels in the fetal brain. Nicotine-induced fetal hypoxic responses and reduced cholinergic marker expression in the brain were more severe when nicotine was started in early gestation. Our results provide new information about the effects of repeated exposure to nicotine in utero on the expression of central ChAT, VAChT, and CHT1 in the rat fetus. These results indicate that repeated hypoxic episodes or/and a direct effect of nicotine on the central cholinergic system during pregnancy may contribute to brain developmental problems in fetal origin.
Keywords: Nicotine, Rat fetus, Hypoxia, ChAT, VAChT, CHT1
1. Introduction
Smoking during pregnancy induces a wide range of pathological abnormalities in both experimental animals and humans, including an increased mortality, low birth weight, sudden infant death syndrome, as well as dysfunction of the central nervous system (CNS) (Lichtensteiger et al., 1988; Slotkin et al., 1987; Birnbaum et al., 1994; Bassi et al., 1984; Leichter, 1989; Mochizuki et al., 1985; Theodore, 1998). Nicotine exposure is a well known consequence of cigarette smoking. Nicotine can easily penetrate the placental barrier and affect the fetus in utero (Lichtensteiger et al., 1988). A number of studies have demonstrated that cigarette smoking during pregnancy causes fetal growth retardation (Birnbaum et al., 1994; Bassi et al., 1984; Leichter, 1989.). This study focused on the effect of episodes of hypoxia induced by a high dose of nicotine administrated subcutaneously to pregnant rats on the fetal brain and determined the effect of nicotine on oxygenic status and central cholinergic markers in the fetus.
The traditional method for collecting fetal blood samples is decapitation of the rat fetuses. The resulting fetal blood samples are mixture of arterial and venous blood, as well as other fluids. This makes analysis of blood values complicated and difficult. In the present study, we developed a method to collect fetal blood samples directly from the fetal heart in fetal rats for measurement of blood pO2, pCO2, O2 saturation, pH, and lactic acid (Mao et al., 2007).
Heavy smoking during pregnancy may cause brain problems in the offspring (Winzer-Serhan, 2008), suggesting that the development of the fetus is significantly affected in utero. In central cholinergic systems, the neurotransmitter acetylcholine (Ach) is synthesized from choline and acetyl-CoA by choline acetyltransferase (ChAT) (Ohno et al., 2001), and then translocated into synaptic vesicles by the vesicular acetylcholine transporter (VAChT) in cholinergic nerve terminals (Eiden, 1998). Transporting of choline into the nerve terminal by Na+-dependent high-affinity choline transporter (CHT1) is the rate-limiting step in Ach synthesis (Okuda et al., 2000; Birks, 1957). Thus, if these cholinergic enzyme and transporters are abnormal, Ach synthesis in the CNS will be affected. Acute or chronic injection of nicotine in rats can cause changes in the release of Ach (Rada et al., 2001). ChAT activity in the brain could be significantly reduced by nicotine (Trauth et al., 2000). In the present study, the mRNA expression of ChAT, VAChT, and CHT1 were measured in the forebrain and hindbrain of rat fetuses under the condition of maternal exposure to a high dose of nicotine. Notably, these three cholinergic elements exist at nerve terminals and synapse structures in the CNS. They may play an important role in the development of synapses and network circuits in the fetal brain during critical developmental stages. Deficit of these cholinergic enzyme and transporters may be linked to poor development of brain functions. Therefore, determining the effect of exposure to nicotine during pregnancy on the expression of ChAT, VAChT, and CHT1 mRNA in the fetal brain in association with repeated hypoxic episodes may yield new information regarding nicotine-induced fetal brain damage. Slotkin et al. found that infusion of nicotine would cause periods of ischemia (and consequent hypoxia) by the peak of plasma levels (Slotkin, 1998). Although steady infusion is a good method for studying effects of nicotine in smoking, cigarette smoking also may cause episode changes of chemical concentrations in blood. Therefore, this study focused on repeated episodes by injections (twice per day during the gestational periods) instead of consistent infusion in determination of the effect of prenatal nicotine on mRNA of central cholinergic markers in the fetus.
2. Material and methods
2.1. Animals and experiments
Pregnant Sprague-Dawley rats (Shanghai Experimental Animal Center) were divided into experimental and control groups (n = 7/each group). Group 1 (from early gestation) was subcutaneously (s.c.) injected with 1.5 mg/kg nicotine hydrogen tartrate (Sigma, St. Louis, MO) in 0.3 ml 0.9% NaCl solution, twice daily, from gestational day 3 to 21 [The dose of nicotine was based on previous reports that used 2-3 mg/kg for subcutaneously injection (Mannucci et al., 2005; Kalpana et al., 2005)]. Group 2 (from middle gestation) were injected with the same dose of nicotine from gestational day 9 to 21. Animals in group 3 (from late gestation) were treated with the nicotine dosing from gestational day 15 to 21. Control groups received 0.3 ml saline (0.9% NaCl), twice daily, subcutaneously from gestational day 3, or 9, or 15. The injection solution was prepared freshly and subcutaneous treatment was performed at 9:00 a.m. and 6:00 p.m. On gestational day 21, rats in all groups were anesthetized 30 min after treatment. A small cut was made at the middle of abdomen of rats and fetuses were removed and weighed. Blood samples were collected for determination of blood values as described below. The fetal brain tissue was immediately frozen in liquid nitrogen and stored at -80 °C before collecting mRNA. Fetal forebrain and hindbrain were prepared for measuring CHAT, CHT1, and VAChT mRNA. All procedures used in this study have been approved by the Institute Animal Care Service.
2.2. Measuring blood values
Under condition of aesthesis, 1.0 ml maternal blood was collected from maternal abdominalis aorta. Fetal blood samples were collected from the fetal heart directly using intracardiac puncture as we reported recently (Mao et al., 2007). All blood samples were collected in chilled heparinzed syringes. Fetal blood samples were pooled from the same litter. Blood pH, pO2, pCO2, O2 saturation, sodium, potassium concentrations, and lactic acid levels were determined with a Nova eleven-electrolyte analyzer (Nova Biochemical, Model pHOx Plus L, Waltham, MA) at 39 °C base. The remaining blood samples were centrifuged at 4 °C for 15 min, and plasma osmolality was measured with an advanced digimatic osmometer (Model 3MO, Advanced Instruments, Needlham heights, MA).
2.3. Measuring fetal body weight
Each rat had about 10-12 fetuses. Three or four fetuses from each mother were used for measuring fetal weight and the remaining fetuses were used for other experiments. Immediately following opening the abdomen of maternal rats, fetuses were weighed. The fetal body was then dried in an oven at 70 °C for 8 h to remove water from tissue, and the dried fetal body was weighed.
2.4. Measuring mRNA of the cholinergic markers in the fetal brain by real-time PCR
Relative quantitative real-time PCR analysis was employed for ChAT, CHT1, and VAChT gene expression in the fetal forebrain and hindbrain. The rat primers were designed based on the published rat mRNA and genomic sequences in GenBank (Table 1).
Table 1.
Relative gene primers sequence
| Target gene | Primer sequence | Genebank No. | Production length (bp) | |
|---|---|---|---|---|
| 18S | Forward primer | 5′-GCGGTTCTATTTTGTTGG-3′ | M11188.1 | 120 |
| Reverse primer | 5′-AATGCTTTCGCTCTGGTC-3′ | |||
| ChAT | Forward primer | 5′GATGTTCTGCTGTTATGGACCCG-3′ | XM001061520.1 | 232 |
| Reverse primer | 5′-AGTCAAGATTGCTTGGCTTGGTT-3′ | |||
| VAChT | Forward primer | 5′GTGCGAGGACGACTACAACTATT-3′ | U09211.1 | 101 |
| Reverse primer | 5′-TCTTGCAGAATGACCATCTTGAC-3′ | |||
| CHT1 | Forward primer | 5′-ATGGAACTTGCTGATAGGATGGA-3′ | NM053521.1 | 121 |
| Reverse primer | 5′ATGATTCTCCCGATTGTTCTACAG-3′ |
Purification of total RNA from the fetal forebrain and hindbrain tissue was performed using a protocol adapted from published methods (Yen et al., 2002). The tissue was homogenized in TRIZOL (60-100 mg tissue in 1 ml TRIZOL) (Invitrogen Life Technologies, USA). RNA was then immediately isolated, precipitated, washed, and stored in 75% ethanol at -70 °C until analysis. RNA was only used if the ratio between spectrophotometer readings (260 nm:280 nm) were between 1.8 and 2.0, denoting minimum contamination from cellular proteins. Samples were digested with DNAse I to degrade any DNA present in the RNA isolation. A reverse transcription and first strand cDNA synthesis was performed using MMLV-RT reverse transcriptase (Invitrogen Life Technologies, USA). The first strand cDNA was checked using the housekeeping gene to assess the quality of the reverse transcription. First strand synthesis was achieved using 2 μg of total RNA. Each representative cDNA pool was aliquoted and stored at -20 °C.
ChAT, CHT1, and VAChT specific PCR assay was optimized using total RNA from fetal tissue in the absence of SYBY Green I (TaKaRa Biotechnology, Japan). The CT value assigned to a particular PCR plate well, and thus reflects the point during the reaction at which a sufficient number of amplicons have accumulated. CT values were collected at linearity and used to calculate the 18S rRNA corrected CT (or ΔCT) for ChAT, CHT1, and VAChT gene. Values from the control and experiment groups were then used to calculate the mean corrected difference in CT for ChAT, CHT1, and VAChT (ΔCT ± S.E.). The extent of the response is determined by two mean (ΔΔCT), while a negative value suggests repression of ChAT, CHT1, and VAChT gene expression, so the relative degree of response is calculated by two mean (ΔΔCT) (Kenneth and Schmittgen, 2001). The internal control 18S was used. For confirmation of amplification presence and purity, the PCR product was determined on a 1.5% Agrose gel.
Real-time PCR (BIO-Rad icycler iQ, USA) was performed in a 96 well plate. 12.5 μl 2 × SYBR Green master mix (Takara, Japan), 0.5 μl forward primer, 0.5 μl reverse primer, 2.0 μl cDNA, and 9.5 μl dd water were added for a total volume of 25 μl per well. The tubes were incubated in a thermocycler at 95 °C for 2 min. There were 45 cycles of PCR amplification performed consisting of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. Relative gene expression was calculated by the following equation: relative gene expression (RGE) = 2-ΔΔCt. The relative gene expression was normalized to 18S rRNA. The values were calculated as 2-(TCt-NCt), where T represents the level of experimental groups, N represents the control group whose expression level was calculated, and Ct is the delta threshold cycle between the gene of interest and 18S.
2.5. Statistics
Statistical analysis was performed with SPSS software. Comparison between the treatments was determined with one-way ANOVA followed by Tukey test or t-test. All data are expressed as mean ± S.E.
3. Results
3.1. Blood values
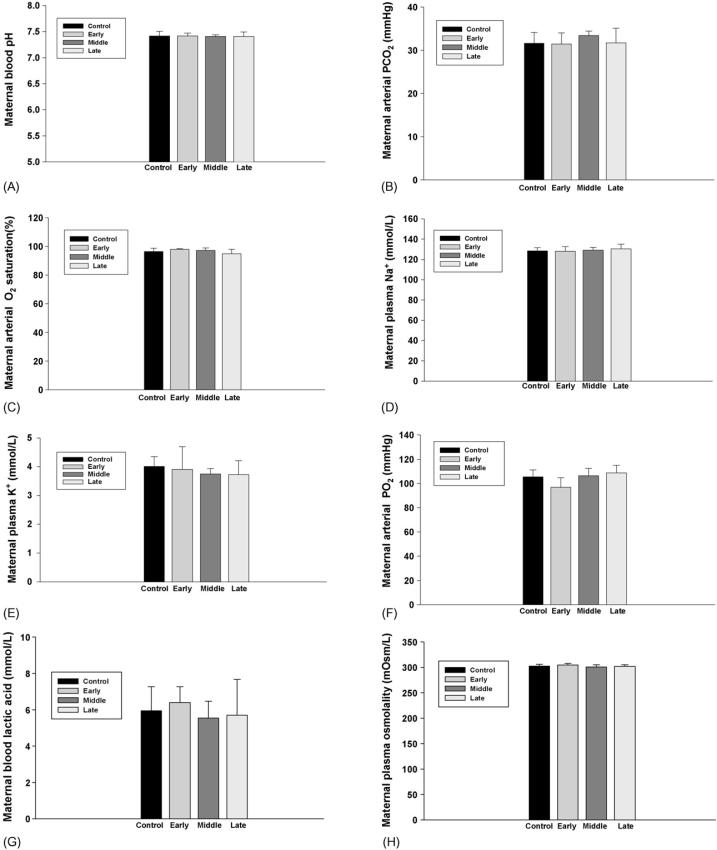
Maternal blood pH, pO2, pCO2, O2 saturation, Na+ and K+ concentrations, and plasma osmolality were not changed in all experimental and control groups following administration of nicotine or the saline. Maternal blood lactic acid levels also were not changed by subcutaneous nicotine in the present study (Fig. 1).
Fig. 1.
The effect of subcutaneous injection (s.c.) of nicotine in maternal rats on maternal blood pH, pCO2, pO2, O2 saturation, Na+ and K+ levels and plasma osmolality, lactic acid levels. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21.
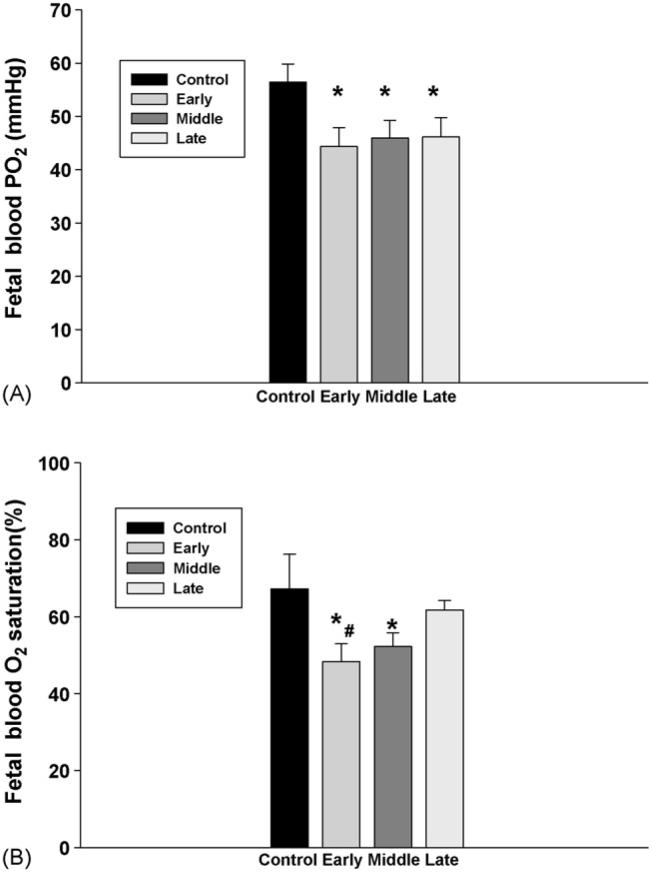
However, there were significant changes in fetal blood values following subcutaneously injection of nicotine in maternal rats. First, fetal blood pO2 and O2 saturation were significantly decreased in the rats treated with nicotine from the early and middle gestation (Fig. 2), while fetal pCO2 levels were significantly increased by subcutaneous nicotine in all treatment groups; furthermore, fetal blood pCO2 was higher in the rats starting nicotine administration from the early gestational age than that from the late gestation (Fig. 3).
Fig. 2.
The effect of subcutaneous injection (s.c.) of nicotine in maternal rats on fetal blood pO2, O2 saturation. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21. * and #p < 0.05; * vs. the Control; # vs. the late group.
Fig. 3.
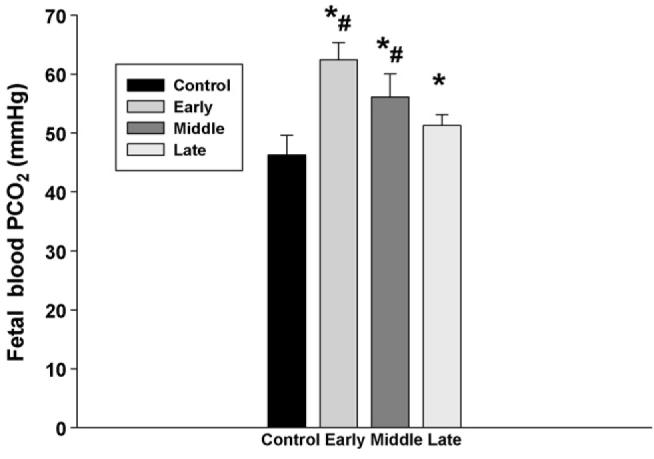
The effect of subcutaneous injection of nicotine in maternal rats on fetal blood PCO2. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21. * and #p < 0.05; * vs. the Control; # vs. the late group.
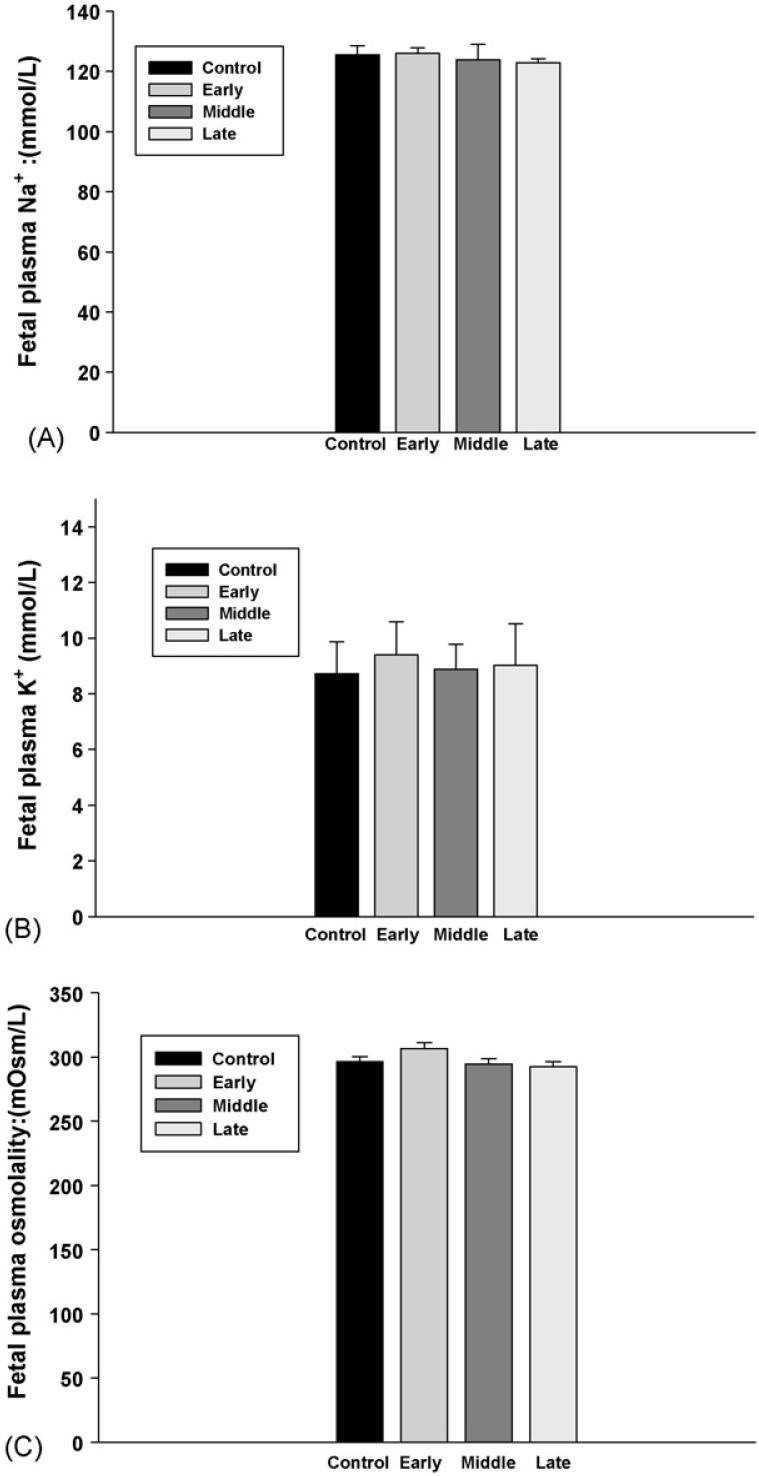
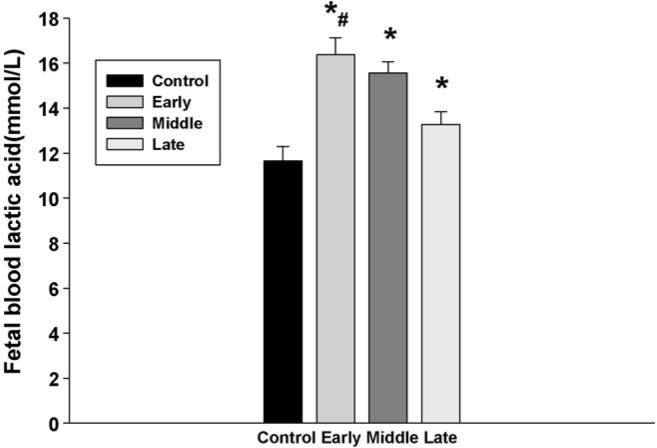
Fetal blood electrolytes, including Na+ and K+, were not changed in all groups. In addition, there was no significant change in fetal plasma osmolality (Fig. 4). However, fetal blood lactic acid levels were significantly increased following injection of nicotine in maternal rats, and the increase of fetal lactic acid was higher in the animals that were treated with nicotine from the early gestation than those from the later gestation (Fig. 5). In addition, although subcutaneous nicotine did not cause significant change of fetal blood pH in the rats that were injected with nicotine from the middle or the late gestation, nicotine decreased fetal blood pH if the maternal rats were administrated with nicotine from early gestation (Fig. 6).
Fig. 4.
The effect of subcutaneous injection of nicotine in maternal rats on fetal blood Na+ and K+ levels and plasma osmolality. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21.
Fig. 5.
The effect of subcutaneous injection of nicotine in maternal rats on fetal blood lactic acid levels. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21. * and #p < 0.05; * vs. the Control; # vs. the late group.
Fig. 6.
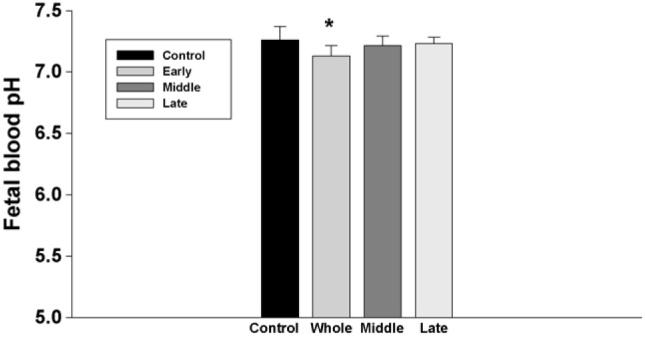
The effect of subcutaneous injection of nicotine in maternal rats on fetal blood pH. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21.
3.2. Fetal body weight
Following exposure to nicotine, both wet and dry body weight of fetuses were significantly decreased and the difference was greater in the animals that were treated with nicotine from the early gestation compared to those treated from late gestation (Fig. 7).
Fig. 7.
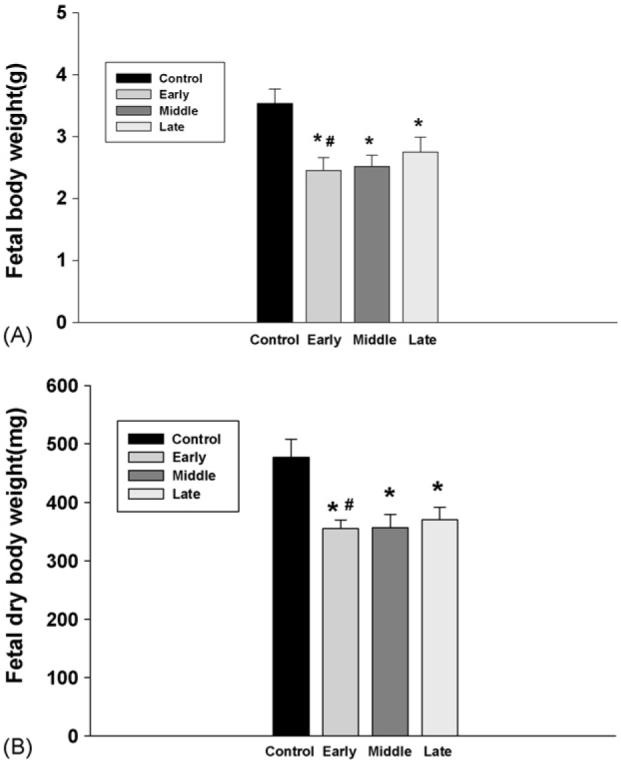
The effect of subcutaneous injection of nicotine in maternal rats on fetal body weight. Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21. * and #p<0.05; * vs. the Control; # vs. the late group.
3.3. Fetal brain cholinergic marker mRNA
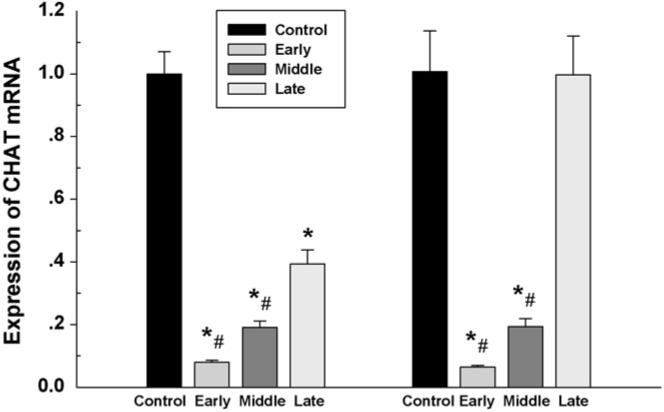
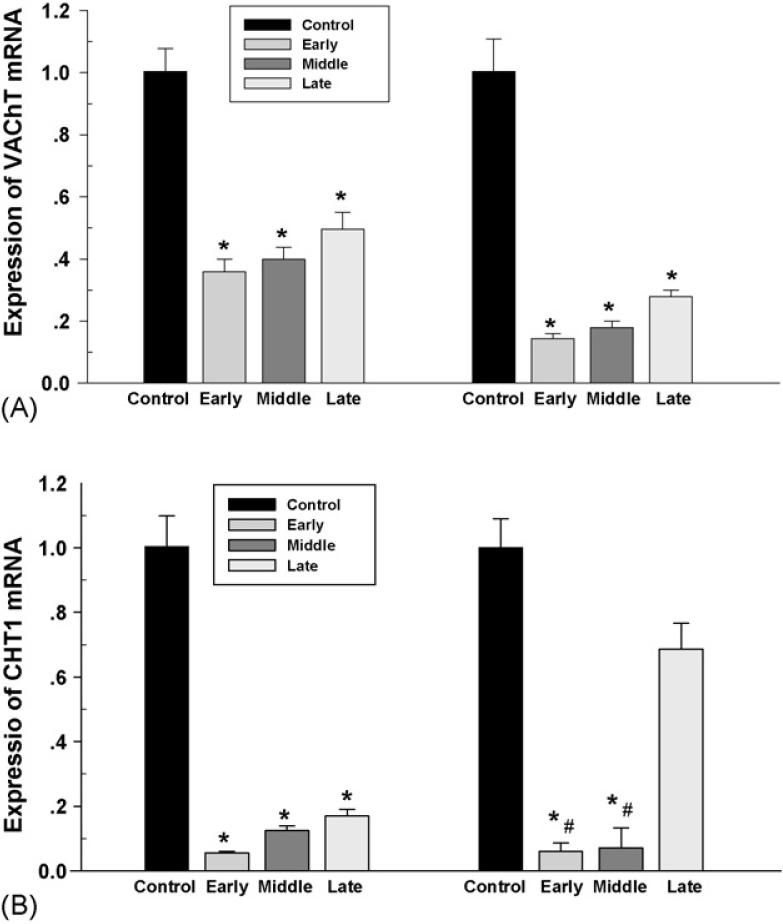
The fetal brain from all groups was collected on day 21 of gestation for analysis of the mRNA. Real-time PCR demonstrated expression of ChAT, CHT1 and VAChT mRNA in both fetal forebrain and hindbrain. Nicotine administrated in maternal rats from the early and middle gestation caused a significant reduction of fetal ChAT mRNA in both forebrain and hindbrain. However, using nicotine from the late gestation decreased ChAT mRNA in the fetal forebrain, not in the hindbrain. In addition, reduction of ChAT mRNA was greater in rats treated with nicotine from the early gestation compared to those that began treatment in late gestation (Fig. 8). CHT1 and VAChT were also decreased in a similar pattern in both fetal forebrain and hindbrain, and a greater reduction was found in the fetal hindbrain in the rats administrated with nicotine from the early and middle gestation compared to that from the late gestation (Fig. 9).
Fig. 8.
The effect of subcutaneous injection of nicotine in maternal rats on ChAT mRNA levels in the fetal forebrain (left) and hindbrain (right). Control: the control animals; early; maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21. * and #p < 0.05; * vs. the Control; # vs. the late group.
Fig. 9.
The effect of subcutaneous injection of nicotine in maternal rats on VAChT and CHT1 mRNA levels in the fetal forebrain (left) and hindbrain (right). Control: the control animals; early: maternal s.c. nicotine from GD 3 to 21; middle: maternal s.c. nicotine from GD 9 to 21; late: maternal s.c. nicotine from GD 15 to 21. * and #p < 0.05; * vs. Control; # vs. late group.
4. Discussion
In fetuses of large animals, various results such as a decrease or unchanged fetal blood pO2 by nicotine infusions in the maternal have been reported (Ayromlooi et al., 1981; Manning et al., 1978). Although previous studies have demonstrated effects of smoking or nicotine on arterial pO2, O2 saturation, and pCO2 in adults, there is limited information regarding influence of nicotine on the fetal blood pO2, O2 saturation, and pCO2 in rodents. This was due to difficulty of collecting arterial blood from rodent fetuses. In most physiological and functional experiments in fetal rats, blood collection depends on decapitation. In this way, the blood collected was actually a mixture from arteries and veins, as well as other fluids. This makes analysis complicated. Particularly for analysis of blood pO2, pCO2, and O2 saturation that could be strikingly different between arterial and vein blood. We recently demonstrated the method of collecting pure blood directly from the fetal heart for determing arterial values, including pH, pO2, pCO2, O2 saturation (Mao et al., 2007). In the present study, using the similar method, we found fetal blood pO2, O2 saturation were decreased following administration of nicotine into maternal rats, while pCO2 was increased. These results suggest that maternal exposure to a high dose of nicotine resulted in fetal hypoxia in utero in rats. In addition, nicotine administration from the early gestation showed greater impact on the fetal blood oxygen compared that from the late gestation, indicating that either prolonged exposure of nicotine or time points during pregnancy for nicotine treatment, or both, play a role in producing lower pO2 and O2 saturation in the fetal blood in utero.
Fetal blood pO2 was higher compared to the arterial pO2 levels in the ovine fetus in utero (Shi et al., 2004, 2005). Although fetal blood was collected within a few minutes, during this short period, fetal rats out of the womb may start to breath. This could be the cause for a relatively higher fetal blood pO2 for both control and experimental groups. Considering blood samples from the control fetuses were collected in the exactly same way, the comparison between the control and the experimental groups treated with nicotine is acceptable. However, the present study did not exclude possibility that chronic treatment with nicotine during pregnancy might damage fetal lung development that may comprise respiratory functions following birth, which could be another reason of low fetal pO2 found in our experiments.
Fetal blood lactic acid was increased in the present study. Lactic acid levels may rise when pathological conditions involving reduced blood flow or a reduction in the delivery of oxygen throughout the body. A high level of lactic acid indicates lactic acidosis. Lactic acidosis has been reported in patients with tissue hypoxia (Geetinder et al., 1993; Daniel et al., 2005). Previous studies have shown that nicotine can induce Ach release (Rada et al., 2001), and cholinergic stimulation is important for body fluid regulation (Xu et al., 2001). Thus, both maternal and fetal blood Na+ and K+ concentration and plasma osmolality were monitored in the present study. They were not changed, which does not support that possible changes in body fluids may contribute to a high lactic acid. It is well known that a high lactic acid could be from conditions that prevent adequate oxygen from reaching the body's cells. Our results showed that fetal pO2 and O2 saturation were reduced following use of nicotine. This may play a role in production of additional lactic acid in the fetus. Furthermore, fetal pH was lowered in the animals treated with nicotine from the early gestation, not from the middle or late gestation, suggesting that the longer of exposure to nicotine during pregnancy, the higher of risk of metabolic changes involving lactic acidosis in the fetus. The present study is the first to demonstrate that lactic acid in rat fetuses was increased by maternal exposure to nicotine, and suggests that lactic acidosis may be involved in nicotine-induced fetal problems during development in utero.
The present study showed a decrease of fetal body weight in response to nicotine exposure. It is known that body fluids are much higher in fetuses than that in adults. Conditions like injection of nicotine might influence fetal body fluids and subsequently cause reduction of body weight. However, our study demonstrated that dry body weight after removing water from the fetus also was lowered. In addition, fetal blood Na+ and plasma osmolality were not changed. Therefore, it is confirmed that maternal nicotine significantly affected fetal growth in utero. Smoking or nicotine during pregnancy may compromise uteroplacental blood flow and thus result in poor fetal development. Chronic stimulation of nicotinic receptors might result in unbalanced receptor activation (Lips et al., 2005). Subcutaneous injection of nicotine (0.5 or 5 mg/kg body wt) resulted in a marked and prolonged reduction in uterine blood flow and intrauterine oxygen tension (Hammer et al., 1981). Atrophic and hypovascular changes of placental villi were detected from smoking mothers. These results suggest that the retarded fetal growth in heavy smokers may be due to the impairment of utero-placental circulation by nicotine (Mochizuki et al., 1985; Philip et al., 1984; Van der Veen, 1982). There is evidence that nicotine can pass the placenta from the maternal side (Lichtensteiger et al., 1988). A number of studies have confirmed that hypoxia is one of the major causes for in utero growth restriction (Slotkin, 1998). In the present study, fetal pO2 and O2 saturation were decreased following administration of nicotine. Episodic hypoxic insult may contribute to fetal growth restriction. Therefore, a combined effect from placental malfunctions and in utero hypoxia may be pathological mechanisms for the in utero growth retardation.
Exposure to nicotine during pregnancy causes not only in utero growth retardation, but also poor development of fetal brain, and evidence indicated impaired central functions following prenatal exposure to nicotine. Nicotine acts as a cholinergic neurotransmitter in the fetal brain, eliciting abnormalities of cell proliferation and differentiation, leading to shortfalls in the number of cells, and eventually altering synaptic activity (Court et al., 1995). In the present study, we focused on the cholinergic molecular in the fetal brain in association with repeated episodes of hypoxia in utero.
Schütz et al. suggested that independent transcription of the vesicular acetylcholine transporter and choline acetyltransferase genes provides a mechanism for regulating expression of these two proteins in different types of cholinergic neurons centrally (Schütz et al., 2001). Choline acetyltransferase activity has been reported in fetal development and it plays a role in the shaping of visual cortical plasticity (Court et al., 1995; Walch et al., 1989). ChAT mRNA expression was detected in spinal cord, septal area, striatum, cortex, and hippocampus in neonatal and adult rat CNS (Cavicchioli et al., 1991). In the human fetus at 8-22 weeks of gestation, activities of ChAT and acetylcholinesterase (AChE) were comparable to those of adult brain tissue. Cortical ChAT activity decreased significantly with gestational age. These indicate that during the second trimester of human fetal development, cortical cholinergic functions may be preceded by relatively high ChAT activity (Perry et al., 1986). In our experiments on rat fetuses, we measured three cholinergic elements - VAChT, ChAT, and CHT1 - during different gestational periods after exposure to nicotine. The results extend published data on VAChT, ChAT, and CHT1 in the fetal brain. We found that all three cholinergic mRNA are present in both forebrain and hindbrain of the rat fetus. Furthermore, prenatal exposure to nicotine from the early, middle, or late gestation significantly decreased ChAT mRNA in the fetal forebrain and lowered ChAT mRNA in the fetal hindbrain only when exposed from early or middle gestation. In addition, nicotine exposure from the early gestation reduced more ChAT mRNA than that from late gestation. These results suggest that prolonged treatment with nicotine or time points for exposure to nicotine during the developmental stages may be linked to increased susceptibility to molecular deficits in the brain and different brain regions in the fetus may present different levels of responses in ChAT activity in the face of the challenge. In other words, our data indicate that the longer of exposure to a high dose of nicotine during pregnancy, the higher risk of poor development of the cholinergic molecules in the fetal brain. This may lead to psychological problems after birth. Thus, during a critical developmental period, nicotine exposure produces brain cholinergic imbalances that may contribute to brain damage evoked by repeated hypoxic episodes.
ChAT is the rate-limiting enzyme for the synthesis of Ach (Ohno et al., 2001). Ach is then transported into synaptic vesicles by VAChT in the cholinergic nerve terminal. VAChT has been shown to be a reliable and specific marker to identify cholinergic synapses (Gilmor et al., 1996), and to be present not only in cholinergic neurons, but also in cholinoceptive neurons (Butcher and Woolf, 1984). A high-affinity re-uptake of choline is essential for a new cycle of cellular ACh synthesis, and in cholinergic neurons this re-uptake is the rate-limiting via a high-affinity choline transporter (CHT1) (Haga and Noda, 1973; Kuhar and Murrin, 1978). Together, ChAT, VAChT, and CHT1 play an important role in nerve terminals and synapses. These cholinergic enzyme and transporters may be particularly important in development of synapses in the formation of neural network in the CNS during the early developmental periods before birth.
Previous study suggested that hypoxia produces impairment of the cholinergic system perhaps through an alteration of acetylcholine release (Gibson et al., 1983). In the present study, VAChT and CHT1 mRNA were significantly decreased in both forebrain and hindbrain in the fetal CNS by prenatal nicotine. This adds new insights of molecular mechanisms on impact of nicotine on the fetal brain. Notably, cholinergic VAChT, ChAT, and CHT1 mRNA in the fetal brain were markedly depressed following repeated exposure to nicotine during pregnancy. Cholinergic deficits were the neurochemical changes recognized in neurodegenerative brain as shown by decreases in ChAT and other cholinergic components (Perry et al., 1977). Prenatal exposure to smoking or nicotine has been demonstrated to be linked to a poor development of brain functions such as learning and memory (Falk et al., 2005a,b). Smoking during early pregnancy affects the expression of both nicotinic and muscarinic acetylcholine receptors in human brainstem and cerebellum (Falk et al., 2005b). Our study showed a significant decrease of VAChT, ChAT, and CHT1 mRNA associated with repeated fetal hypoxia. This opened up intriguing possibilities for future studies of cholinergic mechanisms for nicotine-induced brain damage or dysfunction during the fetal development in pregnancy. For example, whether other neurotransmitter systems (GABA, glutamate, catecholamine) could be affected under the condition used in the present study is an interesting question that should be addressed.
In utero hypoxia due to nicotine may directly or indirectly contribute to the reduction of ChAT, VAChT, and CHT1 mRNA in the fetal brain. The effect of hypoxia on the neurotransmitter phenotype of rat cholinergic neurons was analyzed using a dissociated fetal rat culture system. At exposure to 0.5-1.5% O2, there was a time-dependent decrease in ChAT activity (Flavin et al., 1992). Therefore, it is possible that the repeated exposure to a high dose of nicotine may lead to hypoxia-induced brain damage, such as the neurochemical changes and cholinergic deficits in the fetal brain.
In conclusion, this study provides evidence that repeated exposure of nicotine during pregnancy could induce in utero hypoxia in rodents, including changes of fetal blood pO2, pCO2, lactic acid, and pH, as well as growth. In addition, nicotine administration in mothers during pregnancy could cause deficits of ChAT, VAChT, and CHT1 in the fetal forebrain and hindbrain associated with repeated hypoxic episodes. Considering these cholinergic molecules reflect the density of cholinergic innervations during development, our findings extend the understanding of nicotine-induced brain problems during prenatal periods. The effect of prenatal nicotine on the rat fetus is closely related to both changes in fetal oxidation in utero and to prolonged espousing periods or “window” time for administration of nicotine during pregnancy.
Acknowledgements
We thank P. Hui, F. Xu, and Y. Liu for technical assistance for this work. We also thank Kurt Meyer for his help on the manuscript. Supported by National Natural Science Foundation (No.30570915), Natural Science Foundation of Jiangsu Grant (05KJB31019), Jiangsu Natural Science Key Grant (BK2006703), Suzhou Key Lab Grant (SZS0602), Suzhou Social Development Research Grant (ssy0632), Soochow University Program Project Grant (No.90134602), Suzhou International Cooperation Grant (N2134703), Suda Medical Key Grant (EE134704), and Grant from J. Lv.
References
- Ayromlooi J, Desiderio D, Tobias M. Effect of nicotine sulfate on the hemodynamics and acid base balance of chronically instrumented pregnant sheep. Dev. Pharmacol. Ther. 1981;3:205–213. doi: 10.1159/000457444. [DOI] [PubMed] [Google Scholar]
- Bassi JA, Rosso P, Moessinger AC. Fetal growth retardation due to maternal tobacco smoke exposure in the rat. Pediatr. Res. 1984;18:127–130. doi: 10.1203/00006450-198402000-00002. [DOI] [PubMed] [Google Scholar]
- Birnbaum SC, Kien N, Martucci RW. Nicotine- or epinephrine-induced uteroplacental vasoconstriction and fetal growth in the rat. Toxicology. 1994;94:69–80. doi: 10.1016/0300-483x(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Birks M. Acetylcholine metabolism at nerve-endings. Br. Med. Bull. 1957;13:157–161. doi: 10.1093/oxfordjournals.bmb.a069605. [DOI] [PubMed] [Google Scholar]
- Butcher LL, Woolf NJ. Histochemical distribution of acetylcholinesterase in the central nervous system: clues to the localization of cholinergic neurons. J. Pharmacol. Exp. Ther. 1984;277:728–738. [Google Scholar]
- Cavicchioli L, Flanigan TP, Dickson JG, Vantini G, Dal Toso R, Fusco M, Walsh FS, Leon A. Choline acetyltransferase messenger RNA expression in developing and adult rat brain: regulation by nerve growth factor. Brain Res. Mol. Brain Res. 1991;9:319–325. doi: 10.1016/0169-328x(91)90079-d. [DOI] [PubMed] [Google Scholar]
- Court JA, Perry EK, Spurden D, Griffiths M, Kerwin JM, Morris CM, Johnson M, Oakley AE, Birdsall NJ, Clementi F. The role of the cholinergic system in the development of the human cerebellum. Brain Res. Dev. Brain Res. 1995;90:159–167. doi: 10.1016/0165-3806(96)83496-1. [DOI] [PubMed] [Google Scholar]
- Daniel E, Warren, Donald CJ. The role of mineralized tissue in the buffering of lactic acid during anoxia and exercise in the leopard frog Rana pipiens. J. Exp. Biol. 2005;208:1117–1124. doi: 10.1242/jeb.01490. [DOI] [PubMed] [Google Scholar]
- Eiden LE. The cholinergic gene locus. J. Neurochem. 1998;70:2227–2240. doi: 10.1046/j.1471-4159.1998.70062227.x. [DOI] [PubMed] [Google Scholar]
- Falk L, Nordberg A, Seiger A, Kjaeldgaard A, Hellstrom-Lindahl E, Gerstein M, Huleihel M, Mane R, Stilman M, Kashtuzki I, Hallak M, Golan H. Remodeling of hippocampal GABAergic system in adult offspring after maternal hypoxia and magnesium sulfate load: immunohistochemical study. Exp. Neurol. 2005a;196:18–29. doi: 10.1016/j.expneurol.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Falk L, Nordberg A, Seiger A. Smoking during early pregnancy affects the expression pattern of both nicotinic and muscarinic acetylcholine receptors in human first trimester brainstem and cerebellum. Neuroscience. 2005b;132:389–397. doi: 10.1016/j.neuroscience.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Flavin MP, Yang Y, Riopelle RJ. The effect of hypoxia on neurotransmitter phenotype of forebrain cholinergic neurons. Brain Res. 1992;583:201–206. doi: 10.1016/s0006-8993(10)80025-3. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Pelmas CJ, Peterson C. Cholinergic drugs and 4-aminopyridine alter hypoxic-induced behavioral deficits. Pharmacol. Biochem. Behav. 1983;18(6):909–916. doi: 10.1016/s0091-3057(83)80014-8. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. Expression of the putative vesicular acetylcholine transporter in rat brain and localization in cholinergic synaptic vesicles. J. Neurosci. 1996;16:2179–2190. doi: 10.1523/JNEUROSCI.16-07-02179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetinder C, Allen IA, Cummings C, Tierney LM., Jr. Lactic acidosis complicating the acquired immunodeficiency syndrome. Ann. Intern. Med. 1993;118:37–39. doi: 10.7326/0003-4819-118-1-199301010-00007. [DOI] [PubMed] [Google Scholar]
- Haga T, Noda H. Choline uptake systems of rat brain synaptosomes. Biochim. Biophys. Acta. 1973;291:564–575. doi: 10.1016/0005-2736(73)90508-7. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Goldman H, Mitchell JA. Effects of nicotine on uterine blood flow and intrauterine oxygen tension in the rat. J. Reprod. Fertil. 1981;63:163–168. doi: 10.1530/jrf.0.0630163. [DOI] [PubMed] [Google Scholar]
- Kalpana C, Rajasekharan KN, Menon VP. Modulatory effects of curcumin and curcumin analog on circulatory lipid profiles during nicotine-induced toxicity in Wistar rats. J. Med. Food. 2005;8(2):246–250. doi: 10.1089/jmf.2005.8.246. [DOI] [PubMed] [Google Scholar]
- Kenneth JL, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Murrin LC. Sodium-dependent, high affinity choline uptake. J. Neurochem. 1978;30:15–21. doi: 10.1111/j.1471-4159.1978.tb07029.x. [DOI] [PubMed] [Google Scholar]
- Leichter J. Growth of fetuses of rats exposed to ethanol and cigarette smoke during gestation. Growth Dev. Aging. 1989;53:129–134. [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog. Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, Kummer W. Nicotinic acetylcholine receptors in rat and human placenta. Placenta. 2005;26:735–746. doi: 10.1016/j.placenta.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Manning F, Walker D, Feyerabend C. The effect of nicotine on fetal breathing movements in conscious pregnant ewes. Obstet. Gynecol. 1978;52:563–568. [PubMed] [Google Scholar]
- Mannucci C, Catania MA, Adamo EB, Bellomo M, Caputi AP, Calapai G. Long-term effects of high doses of nicotine on feeding behavior and brain nitric oxide synthase activity in female mice. J. Pharmacol. Sci. 2005;98(3):232–238. doi: 10.1254/jphs.fpe05001x. [DOI] [PubMed] [Google Scholar]
- Mao C, Guan J, Yuan X, Lv J, Zhu H, Miao Y, Chen R, Zhou Y, Xu Z. Got pure blood in fetal rats? Pediatr. Hematol. Oncol. 2007;24:457–460. doi: 10.1080/08880010701451467. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Maruo T, Masuko K. Mechanism of foetal growth retardation caused by smoking during pregnancy. Hung. Acta Physiol. 1985;65:295–304. [PubMed] [Google Scholar]
- Ohno K, Tsujino A, Brengman JM, Harper CM, Bajzer Z, Udd B, Beyring R, Robb S, Kirkham FJ, Engel AG. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2017–2022. doi: 10.1073/pnas.98.4.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- Perry EK, Smith CJ, Atack JR, Candy JM, Johnson M, Perry RH. Neocortical cholinergic enzyme and receptor activities in the human fetal brain. J. Neurochem. 1986;47:1262–1269. doi: 10.1111/j.1471-4159.1986.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- Philip K, Pateisky N, Endler M. Effects of smoking on uteroplacental blood flow. Gynecol. Obstet. Invest. 1984;17:179–182. doi: 10.1159/000299145. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl.) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Schütz B, Weihe E, Eiden LE. Independent patterns of transcription for the products of the rat cholinergic gene locus. Neuroscience. 2001;104:633–642. doi: 10.1016/s0306-4522(01)00100-2. [DOI] [PubMed] [Google Scholar]
- Shi L, Guerra C, Yao J, Xu Z. Vasopressin mechanism-mediated pressor responses caused by central angiotensin II in the ovine fetus. Pediatr. Res. 2004;56:756–762. doi: 10.1203/01.PDR.0000141519.85908.68. [DOI] [PubMed] [Google Scholar]
- Shi L, Hui J, Zhang Y, Morrissey P, Yao Y, Xu Z. The association of cardiovascular responses with brain c-fos expression after central carbachol in the near-term ovine fetus. Neuropsychopharmacology. 2005;30:2162–2168. doi: 10.1038/sj.npp.1300738. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ. Effects of prenatal nicotine on biochemical development of rat brain regions: maternal infusion via osmotic mini-pumps. J. Pharmacol. Exp. Ther. 1987;240:602–611. [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? Pharmacol. Exp. Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Van der Veen F, Fox H. The effects of cigarette smoking on the human placenta: a light and electron microscopic study. Placenta. 1982;3:243–256. doi: 10.1016/s0143-4004(82)80002-7. [DOI] [PubMed] [Google Scholar]
- Walch C, Schliebs R, Bigl V. Effect of early visual pattern deprivation on development and laminar distribution of cholinergic markers in rat visual cortex. EXS. 1989;57:295–304. doi: 10.1007/978-3-0348-9138-7_29. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front. Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Xu Z, Pekarek E, Ge J, Yao J. Functional relationship between subfornical organ cholinergic stimulation and cellular activation in the hypothalamus and AV3V region. Brain Res. 2001;922:191–200. doi: 10.1016/s0006-8993(01)03166-3. [DOI] [PubMed] [Google Scholar]
- Yen K, Linda L, Jennifer M, Dominick D. Differential expression of nicotine acetylcholine receptor subunits in fetal and neonatal mouse thymus. J. Neuroimmunol. 2002;130:140–154. doi: 10.1016/s0165-5728(02)00220-5. [DOI] [PubMed] [Google Scholar]