Abstract
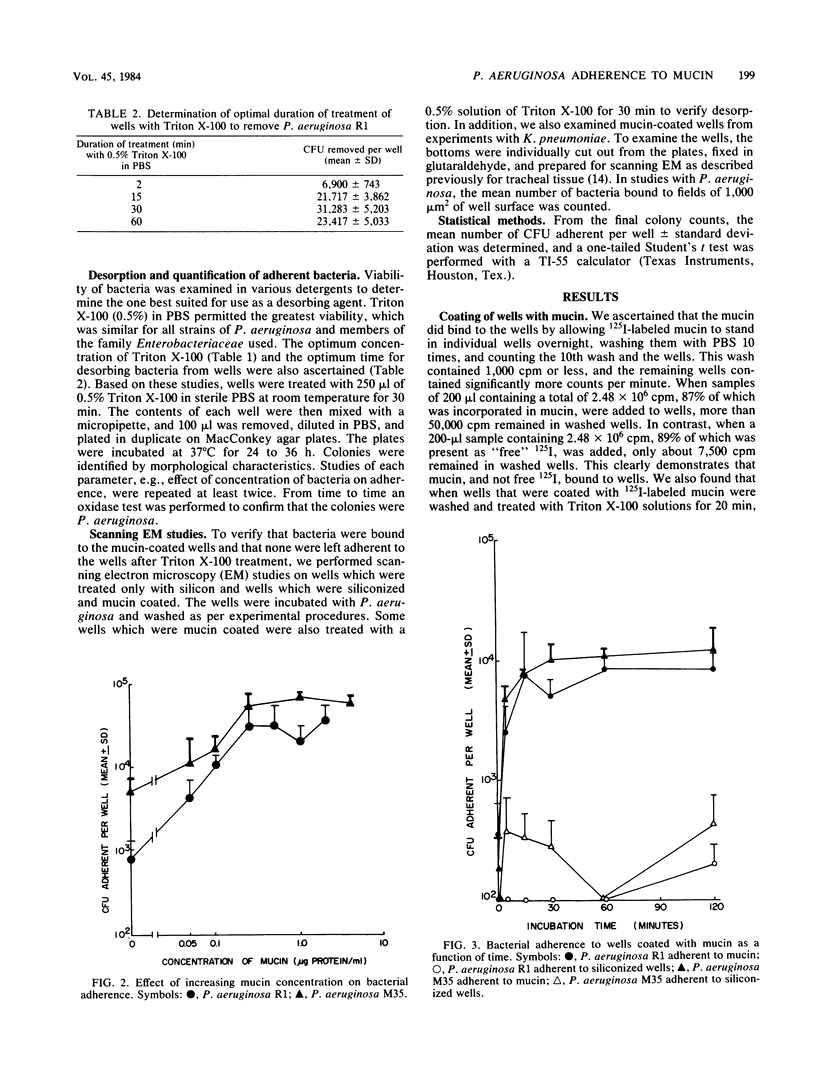
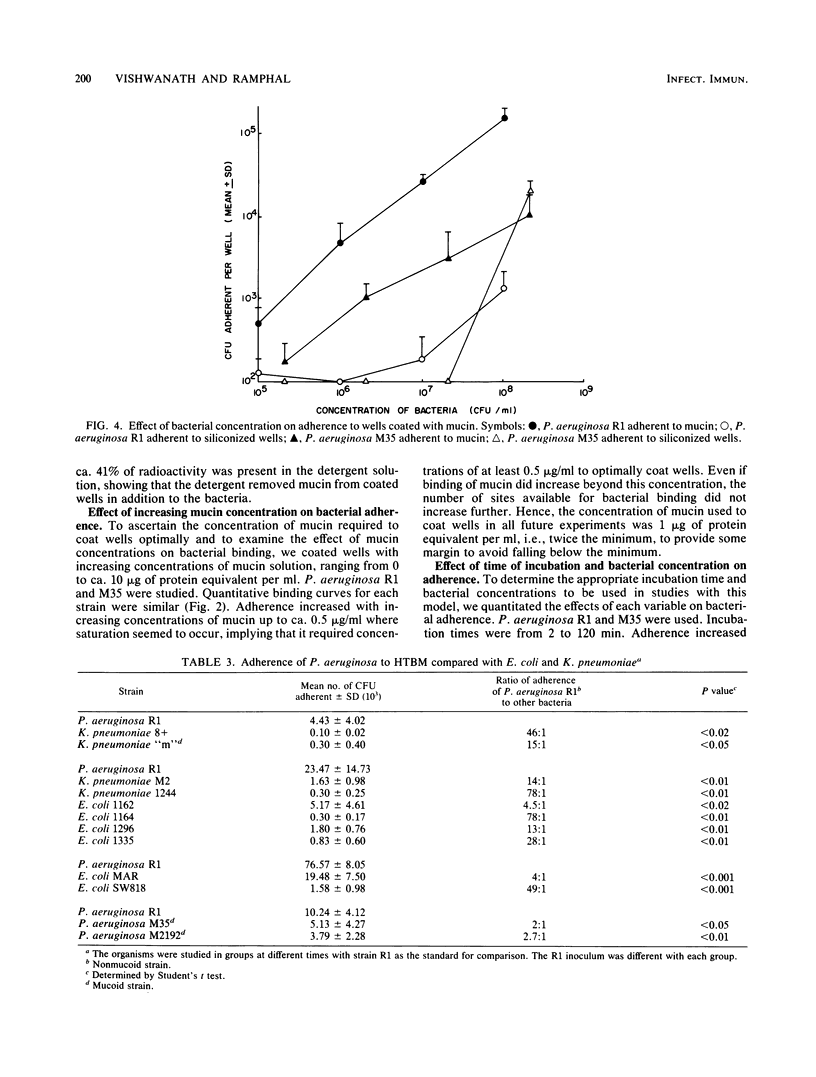
A microtiter plate assay was developed to study the adherence of Pseudomonas aeruginosa to purified human tracheobronchial mucin. The wells of the plates were treated with silicon to minimize nonspecific binding of bacteria and then coated with a solution of purified human tracheobronchial mucin. Bacteria were added to the wells, and the plates were incubated at 37 degrees C. The wells were washed 15 times in an automated microtiter plate washer, and the bacteria bound to wells were desorbed with Triton X-100 and plated for enumeration. Scanning electron microscopy verified bacterial adherence to the mucin-coated wells and desorption of bacteria by Triton X-100. Adherence of P. aeruginosa increased as the concentration of mucin used to coat the wells was increased, with saturation occurring at 0.5 microgram of mucin protein per ml. Other parameters that affected adherence included the time of incubation and concentration of bacteria. Similar studies with strains of Escherichia coli and Klebsiella pneumoniae indicated a relative lack of binding of these bacteria to mucin. In comparing different strains of P. aeruginosa, there were small differences in binding between strains. It is inferred that there may be specific sites on human tracheobronchial mucin which facilitate this preferential binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W., Iyer R. N., Carlson D. M., Polony I. Human respiratory tract secretion. Mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch Biochem Biophys. 1976 Nov;177(1):95–104. doi: 10.1016/0003-9861(76)90419-7. [DOI] [PubMed] [Google Scholar]
- Chick S., Harber M. J., Mackenzie R., Asscher A. W. Modified method for studying bacterial adhesion to isolated uroepithelial cells and uromucoid. Infect Immun. 1981 Oct;34(1):256–261. doi: 10.1128/iai.34.1.256-261.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson R., Mossberg B., Camner P., Afzelius B. A. The immotile-cilia syndrome. A congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N Engl J Med. 1977 Jul 7;297(1):1–6. doi: 10.1056/NEJM197707072970101. [DOI] [PubMed] [Google Scholar]
- FLOREY H. Mucin and the protection of the body. Proc R Soc Lond B Biol Sci. 1955 Jan 27;143(911):147–158. doi: 10.1098/rspb.1955.0001. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vidriero M. T. Airway mucus; production and composition. Chest. 1981 Dec;80(6 Suppl):799–804. [PubMed] [Google Scholar]
- Lowbury E. J., Thom B. T., Lilly H. A., Babb J. R., Whittall K. Sources of infection with Pseudomonas aeruginosa in patients with tracheostomy. J Med Microbiol. 1970 Feb;3(1):39–56. doi: 10.1099/00222615-3-1-39. [DOI] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Fischlschweiger W., Shands J. W., Jr, Small P. A., Jr Murine influenzal tracheitis: a model for the study of influenza and tracheal epithelial repair. Am Rev Respir Dis. 1979 Dec;120(6):1313–1324. doi: 10.1164/arrd.1979.120.6.1313. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983 Jul;41(1):345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Nicotra M. B. Pseudomonas aeruginosa mucoid strain. Its significance in adult chest diseases. Am Rev Respir Dis. 1982 Nov;126(5):833–836. doi: 10.1164/arrd.1982.126.5.833. [DOI] [PubMed] [Google Scholar]
- Roberts G. P. Isolation and characterisation of glycoproteins from sputum. Eur J Biochem. 1974 Dec 16;50(1):265–280. doi: 10.1111/j.1432-1033.1974.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Stevens R. M., Teres D., Skillman J. J., Feingold D. S. Pneumonia in an intensive care unit. A 30-month experience. Arch Intern Med. 1974 Jul;134(1):106–111. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]



