Abstract
Cigarette smoke and hemodynamic stress both contribute to vascular inflammation and associated atherosclerosis. We recently demonstrated direct activation of complement components C4 and C3 on human endothelial cells (EC). The present study was designed to explore complement activation on bone marrow microvascular endothelial cells (BMEC) and human umbilical vein endothelial cells (HUVEC) in response to endothelial cell injury by tobacco smoke extract, shear stress, or other known inflammatory and atherogenic mediators, lipopolysaccharide (LPS) and INF-γ. Following treatment, confluent EC monolayers were exposed to plasma (60 min, 37 °C), and cell surface deposition of stable complement derivatives C4d, iC3b and SC5b-9 was measured in situ using an ELISA approach. Consistent with previous results, moderate levels of C4d, iC3b and SC5b-9 deposition were observed on native EC monolayers exposed to human plasma. Tobacco smoke and shear stress enhanced EC C4d deposition. In contrast, LPS and INF-γ failed to affect EC mediated complement activation, despite evidence of EC activation illustrated by ICAM-1 expression. The combination of tobacco smoke and shear stress nearly doubled EC C4d expression. No increases in iC3b or SC5b-9 were noted, suggesting inhibition of classical and alternative pathway C3 convertase assembly or activity. Indeed, concomitantly increased surface expression of complement regulatory proteins CD35 (CR1) and CD55 was observed following EC exposure to tobacco smoke and shear stress. These results suggest that a balance between complement activation and regulation exists at the EC surface, and may impact vascular injury leading to thrombosis, arteriosclerosis, and atherogenesis.
Keywords: Cigarette smoke, Shear stress, Complement activation, Endothelial cells
Introduction
Vascular pathology leading to thrombosis, arteriosclerosis and atherogenesis is influenced by several recognized risk factors including dyslipidemia, arterial injury including cigarette smoke and hypertension, inflammation, and activation of the adaptive immune response. Among these, cigarette smoke and altered hemodynamic stress are major risk factors related to vascular inflammation and disease [1-6]. In vitro effects of cigarette smoke on vascular endothelial cells include impaired endothelial cell survival [7], induction of apoptosis [8], increased expression of vascular cell adhesion molecule-1 (VCAM-1), enhanced leukocyte adhesion [9], and increased endothelial cell inflammation [10]. Hemodynamic shear stress can inhibit inflammatory gene expression by vascular endothelial cells [11] under physiologic blood flow conditions, and induce endothelial cell activation and apoptosis [12], as well as platelet activation [13] under altered blood flow conditions.
Complement activation is an important component of the local and systemic inflammatory response and participates in instructing adaptive immunity. Complement activation is affected directly by cigarette smoke. Cigarette smoke has been described to induce plasma chemotaxins and anaphylatoxins, C3a and C5a [14]. Moreover, Kew et al. [15] reported that tobacco smoke extract modifies C3 in vitro, leading to activation of the alternative complement pathway. We postulate that complement activation on altered vascular endothelial cells serves both protective and pathologic functions. Complement activation under physiologic conditions may contribute to the clearance of apoptotic cells and cellular debris to prevent local necrosis and associated vascular damage. In a pathologic setting, complement activation may enhance inflammation and influence the adaptive immune response in the arterial wall, leading to atherosclerosis and arteriosclerosis associated with organ transplant rejection.
Complement components have been identified within atherosclerotic lesions, and complement-derived inflammatory mediators likely play a role in vascular injury [16-18]. Endothelial cells express several complement receptors, including gC1qR/p33 (gC1qR) [19], which recognizes the globular domain of the first component of the classical complement pathway, C1q. gC1qR has the capacity to directly activate the classical complement pathway [20]. We recently demonstrated classical pathway complement activation on human umbilical vein endothelial cells and on immortalized endothelial cell lines including human bone marrow microvascular endothelial cells. We also observed that elevated shear stress enhanced classical pathway complement activation on vascular endothelial cells in vitro [21].
The present study was designed to explore the effects of several known inflammatory and atherosclerotic risk factors, tobacco smoke and shear stress, as well as lipopolysaccharide (LPS) and INF-γ, on complement activation on endothelial cells. In addition, the expression of endothelial cell surface complement regulatory proteins, complement receptor 1 (CR1, CD35), decay-accelerating factor (DAF, CD55), and protectin (CD59) [22-24] was measured. CR1 regulates both classical and alternative pathway complement activation, and functions in the clearance of opsonized particles or cellular debris. DAF inhibits activation of complement components C3 and C5 by accelerating the decay of C3 and C5 convertases. CD59 prevents the assembly of cytolytic membrane attack complexes (MAC, C5b-9).
Materials and methods
Cell culture
Studies were performed using the human bone marrow microvascular endothelial cell line (BMEC) described previously [25]. BMEC were maintained in Dulbecco's Modified Eagle Media (DMEM) containing 5% fetal bovine serum (FBS) at 37°C in a normal atmospheric environment with 5% CO2 (Invitrogen Corp, Carlsbad, CA). Cells were used between passage 14 and 30. Results were confirmed using primary human umbilical vein endothelial cells (HUVEC) (ScienCell Research Laboratory, San Diego, CA). These were maintained in endothelial cell medium (ECM) (ScienCell Research Laboratory) supplemented with 5% FBS and used between passages 2 and 3. All cells were grown to confluence on type I collagen (0.1895 mg/ml) (Rat tail collagen type I, Becton Dickinson, Lincoln Park, NJ).
Antibodies
74.5.2 [26], a murine monoclonal antibody against gC1qR, was used at a concentration of 50 μg/ml. A murine monoclonal anti-human ICAM-1 (CD54) antibody (Sigma-Aldrich Corp., St. Louis, MO) was used at a dilution of 1:100 (~20 μg/ml). Biotinylated monoclonal anti-human C4d, iC3b and SC5b-9 antibodies were obtained from Quidel Corporation (San Diego, CA), and diluted 1:200 (~1-5 μg/ml). Murine monoclonal anti-human CD35, CD55, and CD59 antibodies were obtained from Ancell Corporation (Bayport, MN), and used at concentrations of 1 μg/ml, 10 μg/ml, and 5 μg/ml, respectively. MOPC21 (mouse IgGκ) (Sigma-Aldrich) was used as a nonimmune primary control antibody. All antibodies were diluted in 0.01 M HEPES (hydroxyethyl piperazine ethanesulfonic acid) buffered modified Tyrode's solution (HBMT) [21].
Platelet poor plasma
Anticoagulated (0.32% sodium citrate) normal human platelet poor plasma (PPP) was prepared as previously described [26].
Tobacco smoke extract
Tobacco smoke extract (TS) was made from 2R4F research cigarettes [27]. Mainstream smoke was generated in a Borgwaldt smoking machine and bubbled through sterile phosphate buffered saline (PBS) at 1.6 puffs/ml for each cigarette (8 puffs in 5 ml PBS) according to a standard Federal Trade Commission (FTC) protocol. This protocol mimics a standardized human smoking pattern (puff duration, 2 s; frequency, 1 puff/min; volume 25 ml/puff). The final concentration of tobacco smoke in cell culture medium was expressed as puffs/ml medium.
Tobacco smoke treatment of EC
Confluent EC monolayers were washed twice with PBS, and incubated overnight (37 °C) with medium containing 0.5% FBS. This medium was replaced with fresh medium containing 0.5% FBS and TS at a final concentration of 0.03 puffs/ml. Although there is no standardized way to express cigarette smoke extract, this level of TS is similar to concentrations used in other studies examining TS effects on endothelial cells [28-30]. EC were incubated for 24 h before characterization of TS effects. Control monolayers were incubated in the absence of TS.
Shear stress treatment of EC
Following treatment with TS extract, control or TS-treated EC monolayers were exposed to 1/10 volume of normal human platelet poor plasma (PPP) and 40 μg/ml PPACK (D-phenylalany-L-prolyl-L-arginine chloromethyl ketone, Calbiochem, San Diego, CA). EC were submitted to constant shear stress for one hour at 18 dynes/cm2 in a cone and plate shearing device (courtesy of Dr. David Varon, BDR Technologies Ltd., Israel) at room temperature. Arteries can usually adjust to maintain a wall shear stress at this level [31].
Stimulation of EC with LPS and INF-γ
Confluent endothelial cell monolayers were treated with 1 μg/ml LPS or 100 U/ml INF-γ for 24 h in culture with 0.5% FBS.
Complement activation and deposition on EC
C4d, C3b and C5b-9 deposition were measured on control and EC monolayers treated with LPS, INF-γ, TS, and/or shear stress. Complement activation was measured after incubation with 1/10 PPP for 60 min at 37 °C. After washing with PBS, EC were fixed with 0.5% glutaraldehyde (15 min, 37 °C) (Sigma-Aldrich Corp., St. Louis, MO). Glutaraldehyde was neutralized subsequently with 100 mM glycine-0.1% BSA. Deposition of complement components was detected using biotinylated anti C4d, anti iC3b, and anti SC5b-9 antibodies. Primary antibody binding was detected using either alkaline phosphatase conjugated streptavidin (Immunopure Streptavidin, Pierce Biotechnology Inc.) or an alkaline phosphatase conjugated goat anti mouse antibody, followed by addition of 1 mg/ml p-nitrophenyl phosphate substrate (pNPP) (Pierce Biotechnology, Inc.). Color development was quantified at 405 nm (reference at 490 nm) using a Thermomax microplate reader (Molecular Devices Corp, Palo, Alto, CA). Baseline complement deposition was assessed on control EC monolayers.
Expression of ICAM-1, gC1qR and complement inhibitors on activated EC monolayers
Expression of the EC activation marker, ICAM-1 (intracellular adhesion molecule 1), was measured using a monoclonal anti-human ICAM-1 antibody. Monoclonal antibody 74.5.2 was used to measure changes in gC1qR expression. The expression of EC surface complement regulatory proteins was quantified using monoclonal murine anti-human CD35, CD55 and CD59 antibodies.
Statistics
Statistical analysis was performed by paired t-test or ANOVA analysis, as specified below. Data from experiments repeated on 4 to 8 separate occasions were evaluated.
Results
Complement activation and deposition
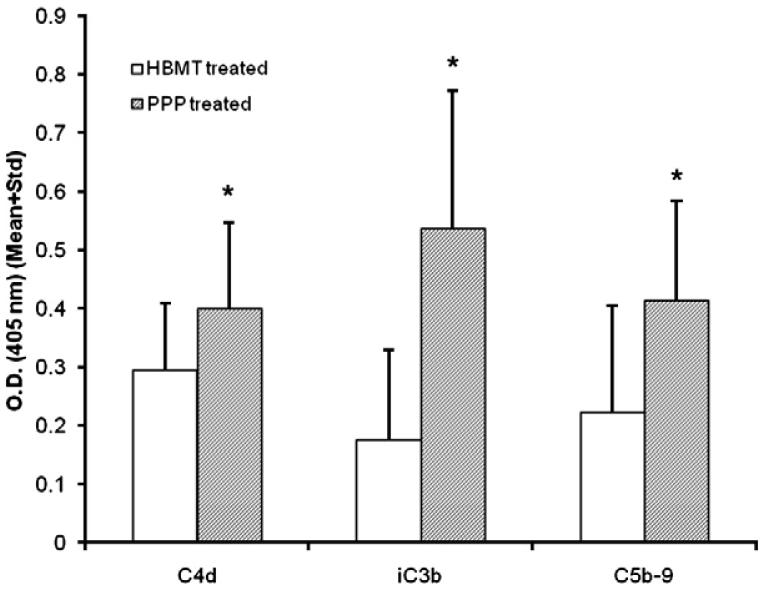
Complement activation was observed on unstimulated, confluent BMEC monolayers following exposure to diluted PPP (1/10). The data are summarized in Fig. 1. Compared to nonspecific, background antibody binding in the absence of plasma, statistically significant (paired t-test) deposition of C4d (n = 9, P<0.05), C3b (n = 9, P<0.05), and C5b-9 (n = 9, P<0.05) was noted by ELISA using monoclonal antibodies directed against stable complement fragments C4d, iC3b, and SC5b-9. Although increases in C4d and SC5b-9 antibody binding over background were modest, anti C3b antibody binding increased by greater than 2-fold.
Figure 1.
Complement activation on resting BMEC monolayers. Confluent EC monolayers were incubated in growth medium supplemented with 0.5% FBS in presence of 1/10 PPP (60 min, 37°C). Complement activation/deposition was assessed by ELISA using monoclonal antibodies against stable complement activation fragments, C4d, iC3b, and SC5b-9. Primary antibody binding was detected with an alkaline phosphatase conjugated reporter and p-NPP substrate, as described in Materials and methods. Color development was quantified at 405 nm (reference at 490 nm). Compared to cells treated with buffer (HBMT), statistically significant (paired t-test) deposition of C4d (n = 9, P<0.05), iC3b (n = 9, P<0.05) and SC5b-9 (n = 9, P<0.05) is shown following exposure of cells to plasma (PPP). Values represent mean+Standard deviation (Std).
Modest increases in anti C4d antibody binding of approximately 25% were observed (Table 1) following BMEC treatment with TS (0.03 puffs/ml) (n = 4, P<0.05). Lower doses of TS (0.015 puffs/ml) were ineffective. Higher doses (0.06 puffs/ml) resulted in cell death (data not shown).
Table 1.
Changes in C4d, C3b and C5b-9 deposition with chemical or mechanical stimulation of BMEC monolayers
| Baseline | TS03 | Shear | TS03+Shear | LPS | INF-γ | |
|---|---|---|---|---|---|---|
| C4d | 1 | 1.25±0.17* (n=4) | 1.33±0.28* (n=6) | 1.92±0.41* (n=4) | 1.09±0.25 (n=5) | 1.09±0.24 (n=5) |
| iC3b | 1 | 1.03±0.31 (n=8) | 0.83±0.57 (n=8) | 1.09±0.51 (n=8) | 1.01±0.12 (n=5) | 0.97±0.14 (n=5) |
| SC5b-9 | 1 | 1.09±0.30 (n=8) | 1.10±0.74 (n=8) | 1.17±0.57 (n=8) | 0.96±0.12 (n=5) | 1.00±0.20 (n=5) |
Results were normalized to baseline C4d, iC3b and SC5b-9 deposition observed on unstimulated BMEC monolayers and presented as mean±STD.
(indicates statistical significance P<0.05 by ANOVA).
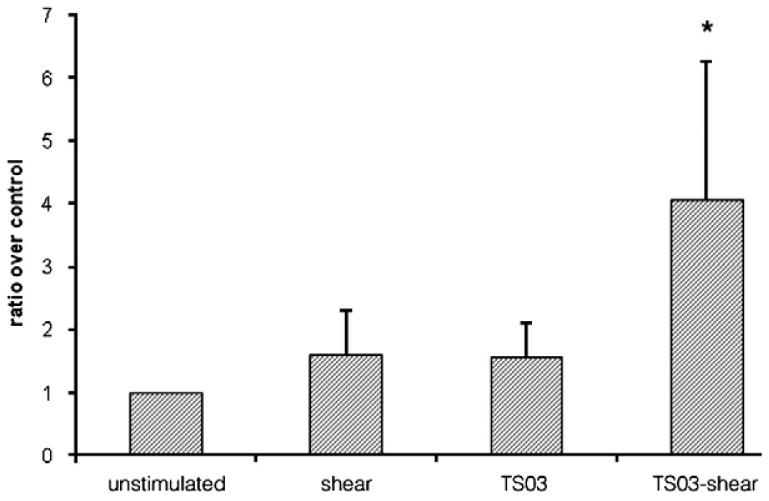
Shear stress (18 dynes/cm2 for 1 h) also enhanced C4d expression (Table 1) (n = 6, P<0.05) on BMEC monolayers. Notably, exposure of BMEC to combined stimulation with TS and shear stress produced a more marked effect (Table 1) (n = 4, P<0.05), approximately doubling C4 deposition. These results were confirmed using primary HUVEC (Fig. 2). Again, the combined exposure to TS (0.03 puffs/ml) and shear stress (18 dynes/cm2 for 1 hour) produced the greatest enhancement in C4d expression (4.1 ± 2.2 fold) compared to unstimulated EC monolayers (n = 4, P<0.05).
Figure 2.
Effect of tobacco smoke or/and shear stress on C4 deposition on HUVEC. Shear stress (18 dynes/cm2 for 1 h) enhanced C4d expression on the surface of HUVEC monolayers (n = 4, P = 0.08). Tobacco smoke (0.03 puffs/ml for 24 h) also enhanced C4d expression (n = 4, P = 0.06). Notably, C4d expression was significantly enhanced following exposure of HUVEC to combined stimulation with TS and shear stress (n = 4, P<0.05).
To determine if the observed deposition of C4 on EC monolayers reflected enhanced complement activation on the EC surface as compared to complement activation in fluid phase with subsequent cell surface C4b deposition, similar studies were performed with immobilized and glutaraldehyde fixed [21] RBC exposed to autologous plasma. C4d expression on RBC increased minimally in the presence of plasma: approximately 1.2 fold over background. In contrast, as reported previously [21], C4d expression on immobilized, glutaraldehyde fixed EC increased 3-5-fold over background.
Interestingly, no detectable downstream activation of the complement cascade, as measured by iC3b and SC5b-9 expression, was discerned on EC in response to BMEC treatment with TS and/or shear stress (Table 1). Moreover, no amplification in EC surface complement activation occurred following BMEC stimulation with LPS (1 μg/ml) or INF-γ (100 U/ml) (Table 1). These inflammatory mediators did, however, produce expected increases in ICAM-1 expression (Table 2).
Table 2.
ICAM-1 and gC1qRexpression on tobacco smoke (0.03 puffs/ml) (TS03) and/or shear stress (18 dyne/cm2), LPS (1 μg/ml) and INF-γ (100 U/ml) treated BMEC monolayers
| Baseline | TS03 | Shear | TS03+Shear | LPS | INF-γ | |
|---|---|---|---|---|---|---|
| ICAM-1 | 1 | 1.05±0.17 (n=6) | 1.31±0.2* (n=6) | 1.33±0.21* (n=4) | 1.35±0.15* (n=4) | 1.99±0.39* (n=4) |
| gC1qR (74.5.2) | 1 | 1.11±0.14 (n=6) | 1.63±0.38* (n=6) | 1.5±0.37* (n=4) | N.A. | N.A. |
Results were normalized to baseline ICAM-1 and gC1qR expression observed on unstimulated BMEC monolayers and presented as mean±STD.
(indicates statistical significance P<0.05 by ANOVA).
N.A. experiments not done.
Increased ICAM-1 expression of approximately 30% (n = 6, P<0.05) over baseline was observed following BMEC exposure to shear stress. TS (0.03 puffs/ml) did not alter ICAM-1 expression. Moreover, no additional increase in ICAM-1 expression occurred when BMEC were exposed to the combination of TS and shear stress. The data are summarized in Table 2.
A similar pattern of EC gC1qR expression was observed in response to TS and/or shear stress (Table 2). gC1qR is present on the EC surface and was recently shown to participate in classical complement pathway activation [20,21]. Compared to baseline, gC1qR expression increased by approximately 60% (n = 6, P<0.05) in response to shear stress, but was unaffected by TS (0.03 puffs/ml).
Expression of complement regulatory proteins
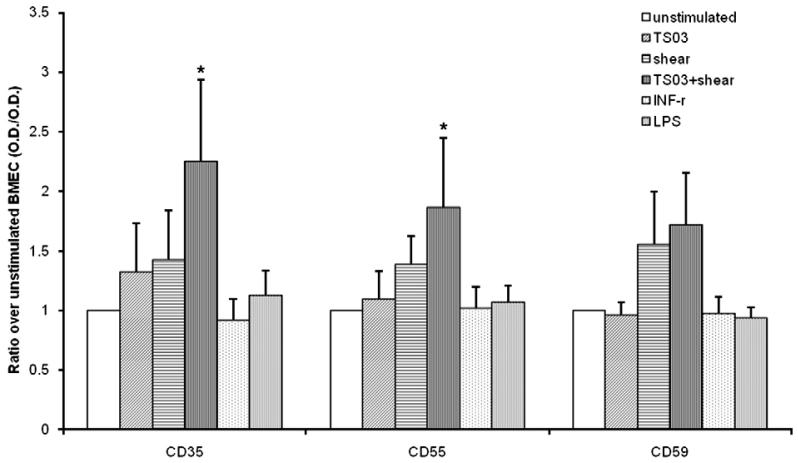
Fig. 3 compares the expression of cell surface complement regulatory proteins, CD35, CD55, and CD59 on unstimulated and activated BMEC. TS alone had no significant effect on BMEC surface complement regulatory protein expression. Shear stress modestly enhanced CD35, CD55, and CD59 expression, although these increases did not reach statistical significance. The combination of TS and shear stress markedly enhanced CD35 and CD55 expression. CD 59 expression also increased, but this change did not reach statistical significance (0.05<P<0.1).
Figure 3.
Changes in cell surface expression of complement regulators (Mean ± S.D.), CR1 (CD35) (n = 3), DAF (CD55) (n = 4), and Protectin (CD59) (n = 4) in response to BMEC exposure to TS (0.03 puffs/ml), chemical (LPS at 1 μg/ml or INF-γ at 100 U/ml), or mechanical shear stress (18 dyne/cm2). Results were normalized to baseline CD35, CD59 and Cd59 expression observed on untreated BMEC monolayers. The data were analyzed by single factor ANOVA. (*) Denotes a statistically significant increase (P<0.05).
Discussion
We recently demonstrated activation of the classical complement pathway on EC exposed to plasma or purified complement components, C1 and C4 [21]. This activation occurred independently of cell surface IgG or IgM, and may reflect an intrinsic capacity of EC to contribute to the inflammatory response. These observations were made using either glutaraldehyde fixed EC monolayers or unfixed EC in suspension. Complement activation was assessed using specific antibodies to stable activated complement fragments in either ELISA or flow cytometry assays, respectively [21]. The present study examined evidence for complement activation under more physiologic in vitro conditions using unfixed EC monolayers. Consistent with previous findings, the data demonstrate in situ activation of C4 and C3, as well as assembly of the cytolytic membrane attack complex, C5b-9 on EC. Comparatively greater C3b deposition was seen on BMEC monolayers than C4d deposition, suggesting the potential activation of both classical and alternative pathway C3 convertases. Moreover, C5b-9 levels on BMEC were low in comparison to C3b, consistent with active cell surface regulation of C5b-9 formation to prevent cell lysis. In general, complement activation on undisturbed EC monolayers in the present study was less than that previously observed on immobilized, glutaraldehyde fixed EC [21], suggesting that immobilization on poly-L lysine and fixation may have activated EC or otherwise altered the expression of complement activators and regulators on the EC surface.
We postulate that under physiologic conditions, in situ complement activation contributes to the clearance of apoptotic cells from vascular lesions to prevent necrosis and associated vascular damage associated with thrombosis and atherosclerosis. Under pathologic conditions, complement activation may contribute to tissue damage via generation of lytic C5b-9 complexes, fuel the inflammatory response via generation of C3a and C5a peptides [32], and impact the local adaptive immune response via C3d generation [33].
To further investigate conditions associated with EC mediated complement activation, additional studies evaluated effects of chemical and physical stimuli associated with vascular damage and predisposition to atherosclerosis. We compared the effects of tobacco smoke, elevated shear stress, LPS, and INFγ on in situ complement activation and expression of complement regulatory proteins on EC. BMEC monolayers were studied primarily. Results were confirmed using primary HUVEC.
As an atherogenic risk factor, tobacco smoke can activate the vascular endothelium, and induce thrombosis [34]. In the lung, tobacco smoke also damages epithelial cells and triggers local inflammation [35]. The present data provide evidence that tobacco smoke extract additionally enhances vascular endothelial cell surface C4 deposition. Thus, complement activation may be involved in vascular responses to cigarette smoking. Interestingly, combined exposure of EC to tobacco smoke extract and elevated shear stress resulted in a markedly increased C4 deposition, suggesting that the combination of shear stress and tobacco smoke may magnify complement activation in the vasculature. Under physiologic conditions, this may contribute to clearance of apoptotic cellular debris in responses to vascular injury, via recognition of C1q by macrophages [36] and C3b by macrophages and CR1 bearing cells [37].
Several investigators have reported that oxidant reactions induced by cigarette smoke are related to the development of cardiovascular disease [38-40]. The present study did not investigate if the observed complement activation was due to tobacco smoke-induced oxidant damage to EC. In addition, given the short half life of the reactive thioester bond in C4b [41], it has not been possible to determine whether the observed increase in EC surface C4d reflects TS induced fluid phase complement activation [14,15] with subsequent binding of C4b to EC surfaces, or enhanced classical pathway complement activation by EC affected by TS. In either case, complement activation on or near EC will contribute to vascular inflammation.
In contrast to TS, neither LPS nor INF-γ increased C4 activation on EC over baseline. These agents also had no effect on EC surface expression of complement regulatory proteins. Thus, EC injury by shear stress and/or tobacco smoke appears to trigger specific signaling pathways that lead to changes in the expression of surface membrane constituents involved in complement activation and regulation. Interestingly, EC mediated complement activation did not correlate with ICAM-1 or gC1qR expression in the present study.
Previous studies demonstrated upregulation of both ICAM-1 and gC1qR in response to inflammatory mediators, including LPS and INF-γ [42]. Similar observations were made in the present study with BMEC responding to shear stress but not tobacco smoke. gC1qR is thought to be involved in inflammation via its ability to activate the kinin system on EC [43,44]. More recently, in vitro evidence has been obtained to suggest that gC1qR can directly activate the classical complement cascade by recognizing C1q [20]. Despite the ability of purified gC1qR to activate the classical complement pathway, enhanced C4 activation on EC in the present study did not correlate with changes in gC1qR expression. These data are consistent with previous findings that blockade of EC gC1qR by monoclonal antibodies had only minimal impact on EC mediated complement activation [21]. Additional studies are required to understand the ligand binding selectivity of gC1qR under different conditions and in different cellular microenvironments.
Interestingly, in the present study, C4 deposition on EC monolayers stimulated by tobacco smoke and/or shear stress did not lead to increased activation or deposition of downstream complement components. This is expected to significantly limit the inflammatory effects of complement activation, since C3a and C5a are important in local inflammation and immune responses [45]. Compared to C3a and C5a, C4a, is a much weaker anaphylatoxin, and has comparatively little chemotactic activity [46]. Moreover, limited C5b-9 formation on EC may prevent cell lysis and tissue damage [47].
To investigate the limited deposition of complement components on EC injured by tobacco smoke and/or shear stress, we examined changes in the expression of EC surface complement regulatory proteins. Modest increases in the surface expression of CR1 (CD35), DAF (CD55), and CD59 were appreciated following EC exposure to shear stress, but these did not reach statistical significance. In contrast, statistically significant increases in CR1 (CD35) and DAF (CD55) were noted when EC were exposed to the combination of tobacco smoke and shear stress, a condition that was associated with the greatest increased in situ C4 activation. Tobacco smoke alone, however, did not enhance the expression of complement regulatory proteins.
CR1 (CD35) and DAF (CD55) are involved in regulating classical and alternative pathway C3 convertases [48]. Regulation of enhanced EC surface complement activation may be an important protective mechanism to prevent complement mediated cell injury and death, as well as to modulate the inflammatory response by regulating in situ C3a and C5a generation. In addition, C3d, a stable degradation product of C3b, plays a major role in instructing the adaptive immune response [33], associated particularly with graft rejection and thrombosis.
In conclusion, the present study provides novel insight into potential atherogenic effects of tobacco smoke and elevated shear stress. The data suggest that both stimuli lead to enhanced C4 deposition on endothelial cell surfaces. Regulation of complement activation at the cell surface by complement regulatory proteins likely modulates the inflammatory response and attendant pathologic sequelae. These data set the stage for further investigations into the impact of dysregulated in situ complement activation on EC in vascular pathogenesis.
Acknowledgments
This work was supported in part by grants HL67211 (EIP) and AI060866 (BG) from the National Institutes of Health, and an American Heart Association Heritage Affiliate post doctoral award # 0625900T (WY).
References
- [1].Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004 May 19;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- [2].Reynolds PR, Cosio MG, Hoidal JR. Cigarette smoke-induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2006 Sep;35(3):314–9. doi: 10.1165/rcmb.2005-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005 May 24;111(20):2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- [4].van d V, Postma DS, Timens W, Hylkema MN, Willemse BW, Boezen HM, et al. Acute effects of cigarette smoking on inflammation in healthy intermittent smokers. Respir Res. 2005;6:22. doi: 10.1186/1465-9921-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, et al. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998 Jan 14;279(2):119–24. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- [6].van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004 Aug;59(8):713–21. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang YM, Liu GT. Damaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanism. Biomed Environ Sci. 2004 Jun;17(2):121–34. [PubMed] [Google Scholar]
- [8].Vayssier-Taussat M, Camilli T, Aron Y, Meplan C, Hainaut P, Polla BS, et al. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am J Physiol Heart Circ Physiol. 2001 Mar;280(3):H1293–300. doi: 10.1152/ajpheart.2001.280.3.H1293. [DOI] [PubMed] [Google Scholar]
- [9].Nordskog BK, Fields WR, Hellmann GM. Kinetic analysis of cytokine response to cigarette smoke condensate by human endothelial and monocytic cells. Toxicology. 2005 Sep 1;212(23):87–97. doi: 10.1016/j.tox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [10].Witkowska AM. Soluble ICAM-1: a marker of vascular inflammation and lifestyle. Cytokine. 2005 Jul 21;31(2):127–34. doi: 10.1016/j.cyto.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [11].World CJ, Garin G, Berk B. Vascular shear stress and activation of inflammatory genes. Curr Atheroscler Rep. 2006 May;8(3):240–4. doi: 10.1007/s11883-006-0079-8. [DOI] [PubMed] [Google Scholar]
- [12].Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003 Apr;81(3):177–99. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- [13].Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996 Sep 1;88(5):1525–41. [PubMed] [Google Scholar]
- [14].Robbins RA, Nelson KJ, Gossman GL, Koyama S, Rennard SI. Complement activation by cigarette smoke. Am J Physiol. 1991 Apr;260(4 Pt 1):L254–9. doi: 10.1152/ajplung.1991.260.4.L254. [DOI] [PubMed] [Google Scholar]
- [15].Kew RR, Ghebrehiwet B, Janoff A. Cigarette smoke can activate the alternative pathway of complement in vitro by modifying the third component of complement. J Clin Invest. 1985 Mar;75(3):1000–7. doi: 10.1172/JCI111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peerschke EI, Minta JO, Zhou SZ, Bini A, Gotlieb A, Colman RW, et al. Expression of gC1q-R/p33 and its major ligands in human atherosclerotic lesions. Mol Immunol. 2004 Jul;41(8):759–66. doi: 10.1016/j.molimm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- [17].Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- [18].Tiong AY, Brieger D. Inflammation and coronary artery disease. Am Heart J. 2005 Jul;150(1):11–8. doi: 10.1016/j.ahj.2004.12.019. [DOI] [PubMed] [Google Scholar]
- [19].Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004 Jun;41(23):173–83. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- [20].Ghebrehiwet B, CebadaMora C, Tantral L, Jesty J, Peerschke EI. gC1qR/p33 serves as a molecular bridge between the complement and contact activation systems and is an important catalyst in inflammation. Adv Exp Med Biol. 2006;586:95–105. doi: 10.1007/0-387-34134-X_7. [DOI] [PubMed] [Google Scholar]
- [21].Yin W, Ghebrehiwet B, Weksler B, Peerschke EI. Classical pathway complement activation on human endothelial cells. Mol Immunol. 2007 Mar;44(9):2228–34. doi: 10.1016/j.molimm.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Collard CD, Bukusoglu C, Agah A, Colgan SP, Reenstra WR, Morgan BP, et al. Hypoxia-induced expression of complement receptor type 1 (CR1, CD35) in human vascular endothelial cells. Am J Physiol. 1999 Feb;276(2 Pt 1):C450–8. doi: 10.1152/ajpcell.1999.276.2.C450. [DOI] [PubMed] [Google Scholar]
- [23].Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006 Feb;118(23):127–36. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- [24].Langeggen H, Berge KE, Johnson E, Hetland G. Human umbilical vein endothelial cells express complement receptor 1 (CD35) and complement receptor 4 (CD11c/CD18) in vitro. Inflammation. 2002 Jun;26(3):103–10. doi: 10.1023/a:1015585530204. [DOI] [PubMed] [Google Scholar]
- [25].Schweitzer KM, Vicart P, Delouis C, Paulin D, Drager AM, Langenhuijsen MM, et al. Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest. 1997 Jan;76(1):25–36. [PubMed] [Google Scholar]
- [26].Peerschke EI, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006 Sep;4(9):2035–42. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- [27].Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, et al. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005 Jan 15;65(2):664–70. [PubMed] [Google Scholar]
- [28].Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1b to alter b-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007;21:1831–43. doi: 10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- [29].Raveendran M, Wang F, Senthil D, Utama J, Shen Y, Dudley D, et al. Endogenous nitric oxide activation protects against cigarette smoking induced apoptosis in endothelial cells. FEBS Lett. 2005;579:733–40. doi: 10.1016/j.febslet.2004.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang Q, Adiseshaiah P, Reddy SP. Matrix metalloproteinase/epidernal growth factor receptor/mitogen-activated protein kinase signaling regulate fra-1 induction by cigarette smoke in lung epithelial cells. Am J Respir Cell Mol Biol. 2005;32:72–81. doi: 10.1165/rcmb.2004-0198OC. [DOI] [PubMed] [Google Scholar]
- [31].Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988 Oct;112(10):1018–31. [PubMed] [Google Scholar]
- [32].Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000 Mar;46(3):209–22. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- [33].Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol Res. 2006;36(13):197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- [34].Tapson VF. The role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):71–7. doi: 10.1513/pats.200407-038MS. [DOI] [PubMed] [Google Scholar]
- [35].Friedrichs B, Miert E, Vanscheeuwijck P. Lung inflammation in rats following subchronic exposure to cigarette mainstream smoke. Exp Lung Res. 2006 May;32(5):151–79. doi: 10.1080/01902140600817457. [DOI] [PubMed] [Google Scholar]
- [36].Kohl J. The role of complement in danger sensing and transmission. Immunol Res. 2006;34(2):157–76. doi: 10.1385/IR:34:2:157. [DOI] [PubMed] [Google Scholar]
- [37].Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004 Nov;41(11):1089–98. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- [38].Bernhard D, Wang XL. Smoking, oxidant stress and cardiovascular disease. Curr Med Chem. 2007;14:1703–12. doi: 10.2174/092986707781058959. [DOI] [PubMed] [Google Scholar]
- [39].Isik B, Ceyland A, Isik R. Oxidant stress in smokers and non smokers. Inhal Toxicol. 2007;19:767–9. doi: 10.1080/08958370701401418. [DOI] [PubMed] [Google Scholar]
- [40].Chavez J, Cano C, Souki A, Bermudez V, Medina M, Ciszek A, et al. Effect of cigarette smoking on the oxidant/antioxidant balance in healthy subjects. Am J Ther. 2007;14:189–93. doi: 10.1097/01.psp.0000249918.19016.f6. [DOI] [PubMed] [Google Scholar]
- [41].Janeway CA, Travers P. The immune system in health and disease. Third edition Garland Publishing Inc; New York: 1997. Immunobiology. [Google Scholar]
- [42].Guo WX, Ghebrehiwet B, Weksler B, Schweitzer K, Peerschke EI. Up-regulation of endothelial cell binding proteins/receptors for complement component C1q by inflammatory cytokines. J Lab Clin Med. 1999 Jun;133(6):541–50. doi: 10.1016/s0022-2143(99)90183-x. [DOI] [PubMed] [Google Scholar]
- [43].Joseph K, Shibayama Y, Nakazawa Y, Peerschke EI, Ghebrehiwet B, Kaplan AP. Interaction of factor XII and high molecular weight kininogen with cytokeratin 1 and gC1qR of vascular endothelial cells and with aggregated Abeta protein of Alzheimer's disease. Immunopharmacology. 1999 Sep;43(23):203–10. doi: 10.1016/s0162-3109(99)00136-8. [DOI] [PubMed] [Google Scholar]
- [44].Joseph K, Tholanikunnel BG, Ghebrehiwet B, Kaplan AP. Interaction of high molecular weight kininogen binding proteins on endothelial cells. Thromb Haemost. 2004 Jan;91(1):61–70. doi: 10.1160/TH03-07-0471. [DOI] [PubMed] [Google Scholar]
- [45].Fregonese L, Swan FJ, van Schadewijk A, Dolhnikoff M, Santos MA, Daha MR, et al. Expression of the anaphylatoxin receptors C3aR and C5aR is increased in fatal asthma. J Allergy Clin Immunol. 2005 Jun;115(6):1148–54. doi: 10.1016/j.jaci.2005.01.068. [DOI] [PubMed] [Google Scholar]
- [46].Gorski JP, Hugli TE, Muller-Eberhard HJ. C4a: the third anaphylatoxin of the human complement system. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5299–302. doi: 10.1073/pnas.76.10.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oliveira GH, Brann CN, Becker K, Thohan V, Koerner MM, Loebe M, et al. Dynamic expression of the membrane attack complex (MAC) of the complement system in failing human myocardium. Am J Cardiol. 2006 Jun 1;97(11):1626–9. doi: 10.1016/j.amjcard.2005.12.056. [DOI] [PubMed] [Google Scholar]
- [48].Hourcade DE, Mitchell L, Kuttner-Kondo LA, Atkinson JP, Medof ME. Decay-accelerating factor (DAF), complement receptor 1 (CR1), and factor H dissociate the complement AP C3 convertase (C3bBb) via sites on the type A domain of Bb. J Biol Chem. 2002 Jan 11;277(2):1107–12. doi: 10.1074/jbc.M109322200. [DOI] [PubMed] [Google Scholar]