Abstract
This paper addresses common questions that clinicians face when treating pregnant women with opioid dependence. Guidance is provided to aid clinical decision-making, based on both research evidence and the collective clinical experience of the authors which include investigators in the Maternal Opioid Treatment: Human Experimental Research (MOTHER) project. MOTHER is a double-blind, double-dummy, flexible–dosing, parallel-group clinical trial examining the comparative safety and efficacy of methadone and buprenorphine for the opioid dependence treatment among pregnant women and their neonates. The paper begins with a discussion of appropriate assessment during pregnancy, and then addresses clinical management stages, including maintenance medication selection, induction and stabilization, opioid agonist medication management before, during and after delivery, pain management, breast-feeding, and transfer to aftercare. Lastly, other important clinical issues including managing co-occurring psychiatric disorders and medication interactions are discussed.
Keywords: pregnancy, substance abuse, pharmacologic treatment, opioid dependence, methadone, buprenorphine
1. Introduction
1.1 Rationale for Treating Pregnant Opioid-Dependent Patients with Agonist Medication
Opioid-dependent pregnant women face tremendous stigma from their family, social networks, and society. Health care providers can mitigate this source of stress by directly addressing their patient’s fears, guilt and treatment resistance. Historically, there has been considerable debate about the optimal management of opioid dependent pregnant women, given the potential risks of medications to the fetus. A particular concern has been the occurrence of neonatal withdrawal. Based on these concerns, some pregnant opioid-dependent women are treated inadequately, with either no medication or sub-therapeutic levels of medication in order to reduce the exposure and risk for physical dependence in the fetus. However, the benefits of methadone are well documented. While not formally approved by the United States Food and Drug Administration (FDA) for treatment during pregnancy, methadone has been the current recommended standard of care for opioid-dependent pregnant women since the early 1990s (National Institutes of Health Consensus Development Panel, 1998).
In pregnant patients, methadone substantially minimizes the peak and trough in maternal serum opioid levels that typically occur with repeated use of short-acting opioids (i.e., heroin), thereby reducing the harm the fetus encounters as a result of repeated intoxication and withdrawal (e.g., Kaltenbach et al., 1998). Compared to other approaches to treatment of opioid dependence available at the time, methadone maintenance was the most cost-effective, producing the greatest reductions in heroin use, criminal activity, and days of hospitalizations (Gerstein et al., 1992). Thus, the benefits of methadone are clear. Methadone maintenance relative to medication-assisted withdrawal provides superior relapse prevention, reduces fetal exposure to illicit drug use and other maternal risk behaviors, improves adherence with obstetrical care, and enhances neonatal outcomes (e.g., heavier birth weight; see Kaltenbach et al., 1998 for a review of this topic).
Following the approval of buprenorphine in non-pregnant populations as a treatment for opioid dependence, women have conceived while on this medication – and other women have entered treatment requesting buprenorphine due to its unique pharmacology and its availability in the private practitioner setting. Thus, there is new interest in better understanding the use of opioid maintenance medications during pregnancy and evaluating the suitability of buprenorphine to be approved by the FDA for use during pregnancy.
1.2 Risk-Benefit Assessment
In spite of the strong evidence supporting the use of methadone in pregnancy, methadone is not without risk or side effects. Neonatal withdrawal following methadone exposure is often of longer duration than with heroin exposure (Wilson et al., 1981). Methadone administration appears to alter fetal activity and heart rate (Jansson et al., 2005; Ramirez-Cacho et al., 2006). However, as described above, the benefits that methadone provides within a comprehensive care setting to this patient population far outweigh the potential risks of treatment.
Although only approved for the treatment of opioid-dependence in non-pregnant populations, buprenorphine is being prescribed frequently to opioid-dependent pregnant patients. Thus, patients should be informed that studies of the safety and efficacy of prenatal exposure to buprenorphine are currently in progress, the data regarding the prenatal effects of buprenorphine are incomplete compared to methadone, and recommendations regarding buprenorphine during pregnancy are necessarily in flux. Currently available data do not indicate that buprenorphine treatment during pregnancy is associated with greater risk to the mother or embryo/fetus than treatment with methadone (Jones et al., 2005b, LeJeune et al., 2006, Fischer et al., 2006). However, management of patients with buprenorphine presents unique challenges and potential benefits compared to those encountered with methadone, the accepted standard of care. Given the different pharmacology of the two medications, methadone and buprenorphine cannot be used interchangeably and methadone maintained patients are usually not always good candidates for buprenorphine.
1.3 Objectives of this Paper
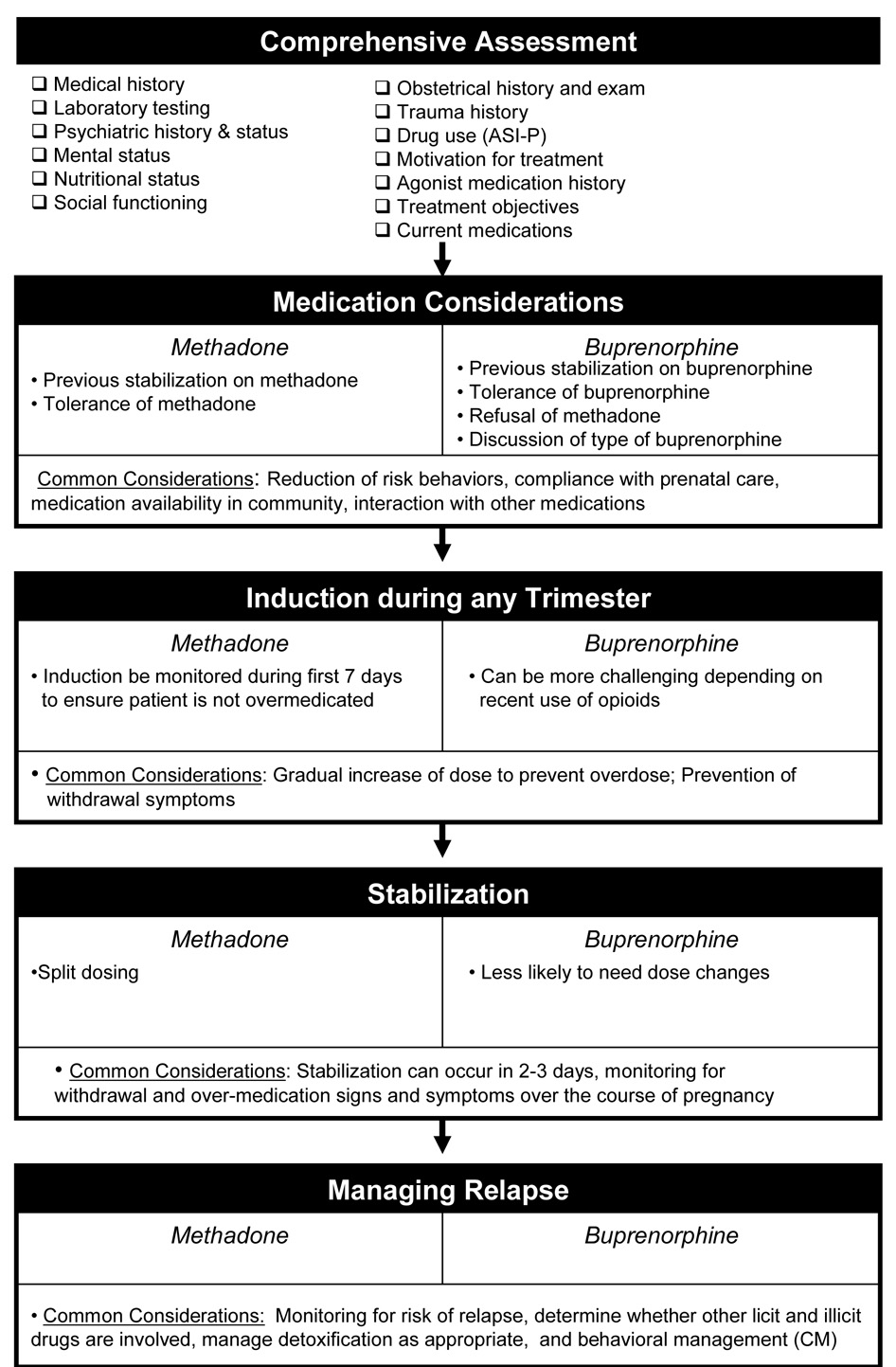
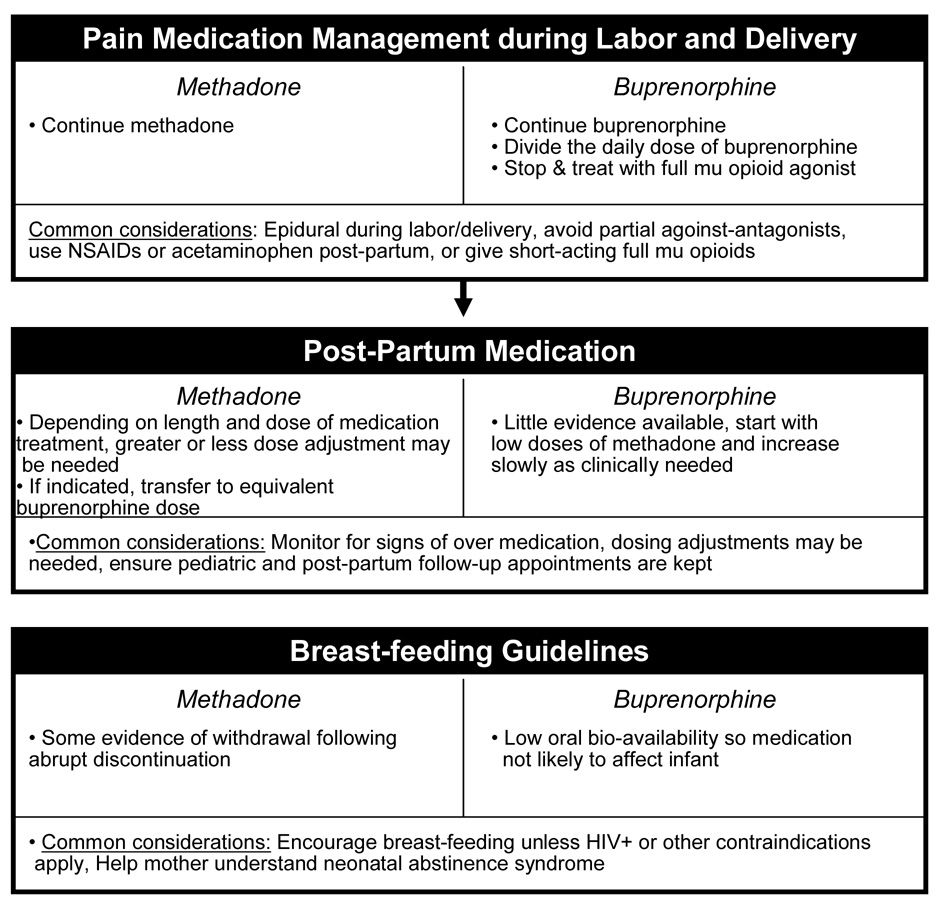
Evidence-based guidance is needed to optimize the care of the thousands of pregnant women each year who are prescribed either methadone or buprenorphine. This paper focuses on the appropriate use of methadone and buprenorphine in management of opioid-dependent pregnant women based both on the collective published literature and the clinical and research experiences of the authors. The Maternal Opioid Treatment: Human Experimental Research (MOTHER) project began in 2005 to examine the comparative safety and efficacy of methadone and buprenorphine in the treatment of opioid-dependence among pregnant women and their neonates. MOTHER is a double-blind, double-dummy, flexible–dosing, parallel-group clinical trial that involves eight clinical sites. It is the first large-scale study to formally examine the relative merits of each of the currently available opioid agonist agents in pregnant opioid dependent women. As Figure 1 summarizes, numerous issues need consideration when prescribing opioid agonist therapy and managing the pregnant opioid-dependent patient. This paper is focused on the clinical issues relevant to management of opioid-dependent pregnant women prior to and during delivery. Thus, neonatal management including neonatal withdrawal is beyond the scope of this paper.
Figure 1. Considerations in the Clinical Management of the Opioid-Dependent Pregnant Woman.
Considerations specific to methadone and buprenorphine are listed on the left and right, respectively, of each module. Considerations that apply to both medications are listed in the shared panel at the bottom of each module.
2. Assessing the Pregnant Opioid-Dependent Woman
Patient assessment at treatment entry serves two purposes, namely to establish rapport and trust with the individual and to gather information to optimize treatment.
2.1 Assessment to Build a Therapeutic Alliance
A pregnant woman expressing interest in receiving substance dependence treatment has taken a courageous step and should be treated accordingly. From first contact, every effort is needed to facilitate treatment entry. Timely assistance, scheduling flexibility and appropriate empathy and optimism for change is needed. The common treatment resistance and demanding behaviors are best viewed as treatment challenges and normal parts of substance dependence. Blaming the mother for her previous behavior will not facilitate positive behavior change. Intake appointments should be completed within the same day or no later than 2 working days after first contact to significantly reduce the attrition rate between initial contact and the intake appointment (Festinger et al., 1995, 1996, 2002; Stark et al., 1990; Stasiewicz & Stalker, 1999).
2.2 Opioid Agonist Medication History
Obtaining a medication and addiction treatment history will facilitate the selection of the optimal treatment strategy. Some patients, already maintained on methadone or buprenorphine prior to conception, may choose to remain on their current regimen, switch to the other medication, or seek medically-assisted withdrawal (also known as, detoxification or taper). Other patients will present naive to opioid agonist treatment. Factors to consider in choosing the best pharmacological approach to treat these patients are discussed below (see 3. initiation section). Declining medication assisted treatment often results from the patient misunderstanding the effects of methadone or buprenorphine during pregnancy. Equally, misinformation may result from a health care provider who is unfamiliar with opioid dependence or from family or significant others who encourage the pregnant woman to avoid medication in an attempt to prevent medication-related harm to the fetus or neonatal abstinence syndrome following delivery. Reviewing with the patient (and as appropriate, other concerned parties) the risks and benefits of opioid maintenance during pregnancy and the risks associated with medically-assisted withdrawal for the mother and neonate allows a fully-informed decision by the patient about treatment.
2.3 Motivation for Treatment
There are numerous reasons why patients seek or continue in opioid agonist treatment subsequent to pregnancy awareness. Clearly, the responsibility of raising a child and the changes in physiology associated with pregnancy can be powerful motivating factors for healthy behavior. Factors leading patients to treatment have been widely categorized into internal (e.g., mental anguish, sense of failure in professional and/or personal life) and external reasons (e.g., legal involvement). Motivating factors in pregnant opioid dependent patients (Jones et al., submitted a) paralleled the findings from a more general substance abusing population showing that severity of the substance problem itself and associated health problems were the strongest predictors of treatment readiness and entry (Handelsman et al., 2005).
2.4 Assessment Tools
Guidelines for comprehensive assessment of pregnant and non-pregnant opioid-dependent patients are available (CSAT 1993, 2005). These assessments focus on obstetric/gynecological status, nutrition, social functioning, medical and psychiatric history. A comprehensive evaluation may start with the Addiction Severity Index (ASI: McLellan et al., 1992), an assessment instrument that examines seven domains of functioning affected by substance addiction, namely medical, legal, employment, alcohol, drugs, psychological and family/social. Thus, the ASI is an assessment tool that can inform treatment planning. Ideally, clinicians should use a version of the ASI tailored to women and pregnancy (Comfort et al., 1999). In addition to assisting with initial treatment planning, results of regular ASI assessments can aid in assessing treatment progress and addressing relapse, if it occurs. The ASI has predictive validity in pregnant patients, with greater medical and drug ASI problem severity being associated with longer treatment retention in intensive comprehensive care (Kissin et al., 2004; Jones et al., submitted a).
2.5 Objectives for Treatment and Stabilization of Opioid Agonist Medication
Opioid-agonist treatment objectives for pregnant patients are similar to those of their non-pregnant counterparts: to prevent opioid withdrawal signs/symptoms, to provide a comfortable induction onto the medication, and then to block the euphoric effects of illicit opioids while also attenuating the motivation (i.e., craving, social interactions) to use illicit opioids and other drugs. In addition, there are pregnancy specific objectives of eliminating the fetal exposure to illicit opioids and other drugs and attempting to stabilize the intrauterine environment. Meeting these patient objectives may enhance treatment retention, particularly involvement in prenatal care, which is significantly associated with positive pregnancy and neonatal outcomes (Jones et al., 2006a). By eliminating the illicit opioid and other drug use, the opioid-dependent patients can begin to focus on repairing relationships, obtaining fulfilling employment, and engaging in rewarding recreational activities. Moreover, the process of prenatal bonding with her unborn child takes on added significance. No medication has been found to change all the behaviors and psychiatric disorders associated with illicit drug use. Thus, behavioral and psychosocial interventions specific to the problems facing opioid-dependent pregnant women are vital for initiating and sustaining substance abstinence (Finnegan, 1991).
3. Initiation of Opioid-Agonist Medication in Dependent Pregnant Patients
For opioid-dependent patients entering treatment upon pregnancy awareness, methadone is the ‘standard of care’ (CSAT, 2005). Patients stabilized on methadone before pregnancy should remain on it, especially since there have been case studies associating such morbidities as stillbirth, fetal distress and premature delivery with detoxification from methadone (Blinick et al., 1969; Rementeria & Nunag, 1973; Zuspan et al., 1975). Further, transition from methadone to buprenorphine introduces the possibility for destabilization and the potential maternal and fetal risks of transferring a stabilized methadone patient onto buprenorphine have not been fully evaluated, nor has a medication transition procedure that avoids withdrawal risk been developed.
Since methadone is the only medication recommended for pregnant opioid-dependent patients, buprenorphine should be prescribed only when the benefits outweigh the risks and the patient has refused methadone (CSAT, 2004). Thus, it is likely that many patients may be transferred from buprenorphine to methadone. Based on clinical recommendations for pregnant patients (Dunlop et al., 2003), the following approach is suggested. For patients maintained on buprenorphine 2–4 mg, transfer to 20 mg methadone; for those maintained on 6–8mg of buprenorphine, transfer to 30 mg methadone; for those maintained on 8 mg or more of buprenorphine, an initial dose of 40 mg Methadone is suggested. Higher initial methadone doses are not recommended on the first day due to concerns regarding over-sedation. This first dose should be followed with observations and clinical judgment should be used for initial and subsequent doses. Due to the residual blockade from buprenorphine it is possible that the subsequent doses could be less than the total day 1 dose.
Although buprenorphine has not been approved by the FDA for use in pregnancy, the reality is that the introduction of buprenorphine has provided an additional treatment option for non-pregnant patients and increased the opportunity for patients to potentially become pregnant while using this medication (CSAT, 2005). One aim of the MOTHER trial is to gain experience with buprenorphine during pregnancy and compare maternal and neonatal outcomes to a similar methadone group. These results may provide adequate data for the FDA to review the suitability of using these medications during pregnancy.
If the clinician determines that the risk/benefit favors buprenorphine, Subutex® (buprenorphine alone) is the preferred medication over Suboxone® (buprenorphine+naloxone). Subutex® is preferred for two reasons. The first is in order to avoid prenatal exposure to naloxone. Pre-clinical data suggest that fetal naloxone exposure produces maternal and subsequently fetal hormonal changes (Brunton et al., 2005; Douglas et al., 2005). The second is to reduce the likelihood of precipitating maternal and fetal withdrawal if buprenorphine was crushed and injected. For stabilized Suboxone® patients, guidelines recommend transfer to Subutex® following confirmation of pregnancy (CSAT, 2005). However, data to support the safety and efficacy of this recommendation are not currently available. Furthermore, the risk of diversion of take home Subutex® needs consideration.
When prescribing either methadone or buprenorphine, it is important to review with the patient the need to avoid both licit and illicit drugs for their health, treatment success and potential interaction of the medication with these substances (e.g., these drugs in combination with alcohol or benzodiazepines can be fatal; White et al., 1999). Table I shows, the potential for drug interactions exists. So, as with all pregnant patients, consideration needs to be given when additional medications are prescribed, especially in regards to their potential incompatibility with opioid agonist therapy.
Table I.
Selected medications known to alter the effect of methadone or buprenorphine
| Indication and Medication | Methadone Effect | Buprenorphine Effect | Reference |
|---|---|---|---|
| Alcohol abuse | |||
| Disulfiram | No change in effect | No change in effect | Tong et al, 1980; George et al, 2000 |
| Naltrexone | Risk of opioid withdrawal | Risk of opioid withdrawal | Kosten et al, 1990; Eissenberg et al, 1996; |
| Anti-convulsant | |||
| Carbamazepine | Decreased effect of Methadone | No change in effect | Paetzold et al, 2000; Eap et al, 2002; Schlatter et al, 1999 |
| Anti-depressants | |||
| Desipramine | Increased serum levels of desipramine | No change in effect | Oliveto, 1995; Kosten et al., 1992 |
| Fluoxetine | Increased effect due to inhibition of opioid metabolism. The effect appears less prominent than that between fluvoxamine and methadone and may be unlikely to have clinical consequences | No change in effect | Oliveto et al., 1995; Iribarne et al., 1998b Bertschy et al., 1996 |
| Fluvoxamine | Increased effect due to inhibition of opioid metabolism | Increased effect due decreasing opioid metabolism | Bertschy et al, 1995; Iribarne et al., 1998b; DeMaria et al., 1999 |
| Anti-fungal agent | |||
| Ketoconazole | Increased effect due to decreasing opioid metabolism; sedation is a problem | Increased effect due to decreasing opioid metabolism | Ibrahim et al, 2000; Kosten et al, 2002 |
| Anxiolytic | |||
| Benzodiazepines (e.g., Flunitrazepam) | Increased opioid effect | Increased opioid effect | Reynaud et al 1998; Singh et al, 1992; Kintz, 2002; Ernst et al, 2002 Kilicarslan et al., 2000 |
| Gastrointestinal | |||
| Omeprazole | Increased effect of methadone reduced respiration in the rat | No change in effect | Kilicarslan et al., 2000 |
| Selected HIV | |||
| Protease inhibitors | |||
| Atazanavir or atazanavir/ritonavir | No change in effect of methadone, no effect of methadone on AZT | Increase buprenorphine and buprenorphine metabolite concentrations and might require a decreased buprenorphine dose. | Friedland et al., 2005; McCance-Katz et al., 2007. |
| Indinavir | Increased effect due to decreasing opioid metabolism | Increased effect due to decreasing opioid metabolism | Iribarne et al, 1998a; Fornataro, 1999 |
| Ritonavir | Alone has no clinical effect on methadone; combination lopinavir-ritonavir, showed methadone withdrawal & need for dose adjustment/ effects on methadone metabolism varied | Increased effect due to decreasing opioid metabolism; increasing buprenorphine levels my not be clinically meaningful | Iribarne et al, 1998a; McCance-Katz et al, 2003; Stevens et al., 2003 |
| Saquinavir | Increased effect due to decreasing opioid metabolism | Increased effect due to decreasing opioid metabolism | Iribarne et al, 1998b |
| Non-nucleoside reverse transcriptase inhibitor | |||
| Efavirenz | Decreased methadone effect, dose increase needed | Decreased buprenorphine levels but not enough to result in withdrawal | McCance-Katz et al., 2002; McCance-Katz et al., 2006. |
| Zidovudine (AZT) | Increased AZT effect (toxicity is possible but rare); no effect on methadone | No clinically meaningful change in AZT; no change in effect in buprenorphine | Iribarne et al, 1998a; McCance-Katz et al., 2001; McCance-Katz et al., 1998 |
| Pain | |||
| Partial Opioid Agonists | Risk of opioid withdrawal | Theoretical risk of withdrawal depending on type of partial agonist medication | Strain et al, 1993 |
| Parkinson’s treatment | |||
| Amantidine | No change in effect | No change in effect | Oliveto et al., 1995; Kosten et al, 1992 |
This table provides examples of buprenorphine or methadone drug interactions and some selected references for these. The table is not intended to provide a comprehensive list of drug interactions with either medication. Please see CSAT TIP #43 (2005) for more information.
4. Induction
Guidelines for inducing pregnant patients to methadone have been well-established in several publications (CSAT, 1993, 2005; Kaltenbach et al., 1998). One must ensure the patient is not concurrently using other drugs that could increase the risk of over-sedation. Care should also be taken to avoid increasing the dose too quickly or slowly to minimize overdosing and to forestall potential premature termination from treatment due to the inability of the medication to alleviate withdrawal, respectively. The quality of the therapeutic alliance with the health care providers initially established during assessment can help with retention.
The initial daily dose of methadone should be 10 to 30 mg. The lower dose may be suitable for women who are primarily dependent on short acting oral opioids such as codeine, hydrocodone and oxycodone. If the patient should experience withdrawal within a few hours, additional doses of 5 to 10 mg every 4 to 6 hours while the patient is awake may be administered for breakthrough withdrawal. It is vital not to misinterpret nonspecific distress associated with life situation or pregnancy as withdrawal or withhold needed methadone if bone fide withdrawal is present. It takes 4–5 days to reach steady state for methadone. Thus, caution must be used to base dose increases on symptoms at peak methadone levels (i.e., 2–4 hours after administration) rather than how long the effects last; otherwise, as methadone accumulates over the first 3 to 7 days, overdose may occur (Srivastava & Kahan, 2006). Therefore, total daily doses should not be increased any more frequently than every 3 to 5 days. The dose should be titrated up to the optimal dose where the patient experiences no withdrawal for at least 24 hours after a dose, uses no other opioid and experiences minimal or no cravings. A standardized assessment of withdrawal such as the Clinical Opiate Withdrawal Scale (COWS) (Wesson & Ling, 2003) or Clinical Institute Narcotic Assessment Scale for Withdrawal Symptoms (CINA) (Peachey & Lei, 1988) is helpful to quantify withdrawal. However, some withdrawal symptoms (e.g., nausea, back ache etc.) overlap with pregnancy symptoms and no instruments are currently available to disentangle the two medical conditions. This is where clinical judgment supersedes assessment tools. Often a patient who has experienced opioid withdrawal previously when not pregnant can guide the prescribing care provider with whom an effective therapeutic alliance has been established.
For buprenorphine, the more complex pharmacology (mu partial agonist/kappa antagonist) may make induction more challenging, especially if the patient is actively using at the time of first assessment. If the first dose of buprenorphine is administered too soon after the last opioid intake (prior to manifestation of clinical symptoms of opioid withdrawal which can be numerous hours if long acting opioids were used) or in too high a dose, there is a potential for significant precipitated withdrawal. As with methadone, if the buprenorphine dose is too low, it may not relieve withdrawal symptoms completely or for 24 hours until the next dose is due (e.g., Lintzeris et al., 2001). Unlike methadone, buprenorphine has the added complexity of possibly precipitating withdrawal.
In opioid-dependent pregnant patients in the second trimester, transition from slow-release morphine or methadone to buprenorphine resulted in a “transient dysphoric mood status” that was observed for two days, similar to reports in non-pregnant patients (Eder et al., 1998). Similar to non-pregnant patients, allowing at least six hours between short-acting opioids and buprenorphine administration (i.e., a time when some objective signs of opioid withdrawal are present) was found to improve the tolerability of induction onto buprenorphine (Jones et al., 2005a).
Identifying the optimal timing of the initial buprenorphine dose for patients taking long-acting opioids (e.g., MS Contin®, OxyContin®, methadone) is more difficult. The few reports of transferring opioid-dependent pregnant women from methadone directly to buprenorphine (which we are not recommending at this time) indicate that it is possible (but not advised) to transition pregnant women in the second or third trimester from oral methadone (up to 85 mg) doses to sublingual buprenorphine (up to 12 mg). The major complaint due to this transition was dysphoric mood (Fischer et al., 1998, 2000) and “clear headed” status (Jones et al., 2006b).
Our own experience with pregnant women has shown that rapid induction onto 12–14 mg of buprenorphine in 2–3 days can be accomplished in pregnant women (e.g., Jones et al., 2005b; Fischer et al., 2006). Ideally, doses should be based upon the severity of opioid dependence. The safety of buprenorphine makes it less likely to result in sedation.
Regardless of whether patients are inducted onto methadone or buprenorphine, ancillary medications that are safe for use during pregnancy and may ease the common symptoms of withdrawal are listed. Acetaminophen (Tylenol®) is given for aches and pains. Antacids (Tums®) are given for indigestion. Loperamide (Imodium A–D®) may be given for diarrhea. Docusate sodium (Colace®) may be given for constipation. Hydroxyzine (Vistaril®) may be given for anxiety or restlessness. Diphenhydramine hydrochloride (Benadryl®) may also be given for anxiety and restlessness. Educating patients regarding behavioral methods to control some symptoms may minimize medication use.
5. Stabilization
The same dosing criteria can be used for both non-pregnant and pregnant patients. Methadone stabilization protocols and recommended dose adjustment guidelines are available in the CSAT TIP #43 (2005). Based on its long half-life, methadone is generally administered once daily. However, methadone-maintained pregnant women frequently complain of increasing withdrawal symptoms as pregnancy progresses, and thus may need elevations of their dose to maintain therapeutic plasma levels and prevent relapse and break-through withdrawal signs and symptoms. Kreek (1974; 1979; 1986) and others (e.g., Gazaway et al., 1993) have demonstrated that, for a given dose of methadone, plasma levels are significantly lower and withdrawal symptoms are increased during the third trimester compared to earlier trimesters. The reduced plasma levels coincide with increased methadone metabolism and faster clearance of methadone during the third trimester (Pond et al., 1985). A serum trough level of 0.24 mg/L or greater of methadone should be adequate to prevent withdrawal symptoms in pregnancy (Drozdick et al., 2002). Ideally blood methadone levels should be coupled with clinical response to determine dose changes.
If single daily doses fail to mitigate withdrawal, split-dosing is an option. The total daily dose is given in two divided doses separated by at least eight hours. Split-dosing may be advantageous in the third trimester of pregnancy when metabolism of methadone and clearance rates increase and steady-state methadone levels decline; DePetrillo and Rice (1995) found less third trimester illicit opioid and cocaine use in split-versus single-dosing groups (.5% and .3%, vs. 24% and 15%, respectively). The benefits of split-dosing may include less inhibition of fetal movement and breathing than is observed following a single daily methadone dose (Wittmann & Segal, 1991). Given buprenorphine’s long biological half-life and unique pharmacology it is anticipated that split dosing will not be necessary.
For buprenorphine stabilization, doses between 4mg to 24mg per day may be appropriate for non-pregnant patients (Chiang & Hawks, 2003); however, there is no recommended minimum or maximum dose in the buprenorphine product insert. This circumstance is due in part to variability in sublingual absorption of buprenorphine (Chiang & Hawks, 2003), its subsequent metabolism, and patient response. As with methadone, the primary goal in choosing a stable dose of buprenorphine for a given patient should be to attain a level that suppresses opioid withdrawal effects, and hence, provides the best opportunity to retain the patient in treatment.
In randomized double-blind studies, dose increases during the course of pregnancy for both methadone and buprenorphine were an average of 3 unit increases (totaling averages of 30 mg for methadone and 6 mg for buprenorphine) (Jones et al., 2005b). While there has been considerable research investigating the parameters of buprenorphine dosing in non-pregnant patients (e.g., Amass et al., 2001, 2000,1998,1994; Bickel et al., 1999; Greenwald et al., 2002; Petry et al., 2000, 2001), similar research has not yet been conducted in pregnant patients. Given the extended biological half-life buprenorphine by virtue of slow dissociation from the mu receptor, blood volume changes with pregnancy might be less problematic than with methadone maintenance.
6. Preventing and Managing Relapse
The best approach to relapse is to recognize the patient’s warning signs before drug use occurs and monitor urine drug screens regularly. The patient’s clinical presentation at each visit should be noted for changes that suggest precursors to relapse. Careful probing will often reveal a pattern to the patient’s drug use (e.g., related to specific recurrent stressors). Strategies to help the patient modify her patterned responses to stressors can be implemented. The use of a functional analysis with patients is especially beneficial for determining the where, when, why, and who of drug use (see Meyers & Smith, 1995 for guidelines for implementing this assessment method). Careful observation and review of patient behavior (e.g., changes in clinic attendance) can help prevent a lapse before it starts. If drug use is admitted or detected, acknowledge it in a nonjudgmental way, neither punishing nor condoning the behavior. Acknowledge the lapse and praise the patient for preventing it from becoming a relapse. Reviewing the functional analysis and then implementing treatment plan changes may prevent continued drug use by the patient. While any drug use is a matter of concern, which drug a patient is using is also a key to determining areas for treatment plan revision. If a patient is using additional opioids, a dose increase may be needed. If she does not appear in withdrawal and reports her dose is adequate, yet she continues to use opioids, it is important to determine what factors are maintaining her behavior is important (e.g., using opioids because her significant other does not want to use alone). If non-opioids are being used, the dose of her medication may not require an adjustment, as methadone and buprenorphine only treat opioid dependence; however, the role that opioid abstinence symptoms are playing in this behavior (e.g., benzodiazepines or alcohol used to self-medicate anxiety or other withdrawal symptoms) needs consideration. High doses of benzodiazepines are a risk for overdose when combined with opioids. In the case of alcohol dependence, inpatient medically-assisted withdrawal using benzodiazepines with frequent fetal monitoring may be needed. In the case of benzodiazepine dependence, these drugs should be tapered only very slowly if possible. Care should be taken to avoid precipitating hypno-sedative withdrawal as this is very detrimental to both mother and fetus (Einarson et al., 2001). Antabuse® and naltrexone must also be avoided during pregnancy. The former is due to its teratogenic effects and the latter is due to its ability to precipitate severe opioid withdrawal in patients taking methadone or buprenorphine. The concomitant abuse of non-opioid licit and illicit drugs is an issue to address clinically. Although agonist medication dose reductions may be required for patient safety in the case of chronic benzodiazepine abuse, decreasing a patient’s dose as a “punishment” for other drug use is not appropriate medical care and creates vulnerability for relapse to use of opioids. Promising behavioral treatments to address concomitant licit and illicit substance abuse include Cognitive behavioral therapy (CBT), (Carroll et al., 2006, Rawson et al., 2002) and contingency management (CM) (e.g., Lussier et al., 2006), with the combination producing the most optimal outcomes (Carroll et al., 2006, Rawson et al., 2002).
7. Ensuring Comprehensive Care
Methadone maintenance combined with prenatal care and a comprehensive drug treatment program can improve many of the detrimental maternal and neonatal outcomes associated with untreated heroin abuse (Connaughton et al., 1977; Kandall et al., 1977). While ethical concerns do not allow a random assignment to a no-treatment or an inadequate-treatment control group, the available literature suggests that buprenorphine given in a comprehensive care setting would be associated with benefits similar to those observed with methadone. Necessary elements that comprise comprehensive care for this patient population have been reviewed (see, for example, Finnegan, 1991; Kaltenbach et al., 1998). Since most research on opioid dependence during pregnancy includes low socio-economic patient samples needing multiple medical and psychosocial services, it is possible that those pregnant women entering office-based buprenorphine treatment may require different components in comprehensive care to optimize their treatment response.
8. Agonist Medication Management during Labor and Delivery
Clinical experience and limited case reports suggest that in order to avoid withdrawal, agonist medications should be continued without interruption (e.g., daily dosing) during labor and delivery and the immediate post-partum period (CSAT, 2005; Jones et al., 2006c). If there is a need to keep the patient ”nil per os” due to the likelihood of an operative delivery requiring anesthesia, the methadone can be administered in a very small volume of juice (i.e., 20 to 30ml). If there is a concern that the methadone dose may slow down the labor, observed split dosing is an option. The remaining dose should be administered at the first symptoms of withdrawal. Buprenorphine is associated with minimal spontaneous withdrawal, thus withholding a dose is possible if needed. Providing buprenorphine IM in a dose equivalent to that given sublingually is another alternative.
9. Pain Medication Management during Labor and Delivery and Recent Post-Partum
Buprenorphine dosing during labor and delivery is complicated by its pharmacological profile. The human mu opioid receptor occupancy is dose-related: 27–47% at 2 mg/day and up to 89–98% at 32 mg/day (Greenwald et al., 2007). The slow dissociation and low intrinsic activity of buprenorphine at the mu receptor (Cowan et al., 1995) results in an enhanced safety profile. These same pharmacological characteristics potentially complicate adequate maternal delivery and post-partum pain relief, because pain medications may not be able to adequately reach the target receptors. The approach to treating pain in non-pregnant buprenorphine maintained patients may include discontinuing buprenorphine and treating with full scheduled opioid agonist analgesics by titrating to effect to avoid withdrawal and then to achieve analgesia (e.g., sustained-release and immediate-release morphine or other opioid agonist analgesics) or if the pain is not severe, dividing the total buprenorphine dose into small doses given every 6 to 8 hours to take advantage of its analgesic properties. When using opioids, higher doses than typical doses may be required in order to displace buprenorphine from the mu opioid receptor (Alford et al., 2006).
The maintenance medication provided to treat opioid dependence is usually inadequate for pain management. Regional analgesia (e.g., epidural) can provide adequate pain relief in women receiving methadone or buprenorphine who choose to have analgesia for childbirth or require analgesia for cesarean delivery. Commonly used opiate agonist/antagonist medications such as nalbuphine (Nubain®) or butorphanol (Stadol®) are contraindicated as opiate withdrawal may be precipitated in the opiate dependent patient. NSAIDs or acetaminophen rather than opioids are recommended for post-partum management of moderate pain in buprenorphine-treated patients (Dunlop et al., 2003). Opioids in combination with acetaminophen and an NSAID are suggested for methadone-maintained patients (Dunlop et al., 2003). Women maintained on methadone required approximately 70% more opiate analgesic following cesarean delivery compared to non-opiate dependent control patients, particularly in the first 24 hours following delivery. Opiate utilization was similar following vaginal birth, despite higher pain scores in the methadone maintained patients. (Meyer et al., 2007a). Women maintained on buprenorphine required less opiate analgesia following vaginal birth, with a non-significant increase in opiate utilization and pain scores following cesarean delivery (Meyer et al., 2007b). The lack of increased opioid utilization may be due to a ceiling effect and suggest the need for prospective studies in this area. Case reports of buprenorphine or methadone-treated patients who each delivered via C-section suggested that routine opioid analgesic doses delivered via a patient-controlled analgesia (PCA) pump 24 hours after surgery are effective in reducing post-partum pain (Jones et al., 2006c). Since most opioid-dependent patients require greater-than-typical doses of opioid analgesics for pain management (Gunderson & Stimmel, 2004) individualized pain control is needed. Ideally, the patient and anesthesiologist should discuss pain management options well before delivery. Clear explanations and reassurance that their pain will be adequately managed likely reduces distress and opioid requirements.
10. Post-Delivery Phase
10.1 Agonist Medication Management
Following delivery, consensus statements recommend maintaining patients on methadone doses similar to levels received prior to pregnancy, but patients frequently wish to be medication free upon delivery of the child. Patients should be advised of the stresses of early motherhood, the likelihood of relapse during medication tapering and the desirability of continuing agonist treatment. Post-partum methadone doses are recommended to be reduced to half the dosage required in the third trimester (CSAT, 2005). These recommendations may depend on the amount of methadone received during pregnancy. One report of 10 patients found that, of those women maintained on doses between 25–100 mg, only one showed mild signs of over-medication within three days after delivery (Jones et al., in press). Given the large individual variability among patients, dose changes should be guided by signs and symptoms of over-or under-medication (Kaltenbach et al., 1998).
10.2 Evaluation and Treatment of Neonatal Abstinence Syndrome (NAS)
Neonates exposed to opioid agonists in utero are prone to display a NAS often requiring medical management. However, a discussion of the assessment and management of NAS is outside the scope of the paper.
10.3 Breast-Feeding
Breast milk is the most complete form of nutrition for infants, with a range of benefits for health, growth, immunity, development (USDHHS 2000a,b, Widstrom et al., 1990, Virden et al., 1988) and societal cost savings (Cohen et al., 1995). While the overall amount of methadone in breast milk appears to be low, ranging from 21 to 314 ng/mL, and not related to maternal methadone dose (Jansson et al., 2007), ingesting breast milk relative to formula has been found to be associated with less severe NAS (Abdel-Latif et al., 2005). Thus, breast-feeding is compatible with methadone and should be encouraged. Gradual weaning from the breast is recommended, as at least two infants appeared to develop NAS following abrupt discontinuation of breast-feeding by women receiving 70 mg and 130 mg of methadone, respectively (Malpas & Darlow, 1999). Incompatible conditions with breastfeeding include an HIV-positive status, and/or continued ingestion of illicit drugs and/or alcohol. Hepatitis C is not a contraindication for breast-feeding. Patients who smoke cigarettes should be counseled as to the morbidities associated with neonatal nicotine exposure as well as the negative effects of smoking on health.
Buprenorphine is excreted in breast milk and levels are similar or higher than levels observed in the blood and the apparent plasma to milk ratio is approximately 1. This ratio gives guidance to providers in estimating the total daily buprenorphine consumption of the infant. Given the low oral bioavailability of buprenorphine, infant exposure will be only 1/5 to 1/10 of the total amount of buprenorphine available. Thus, buprenorphine levels in breast milk may have little effects on NAS (Auriacombe & Loustauneau, 2000).
11. Other Clinical Issues
11.1 Methadone and Buprenorphine Interactions with Other Medications
To the best of our knowledge there have been no prospective drug interaction studies conducted in pregnant women maintained on either methadone or buprenorphine. In the absence of such data, it is logical to consider that the known drug interactions summarized in Table I may also occur in pregnant women. However, there are maternal physiological changes (e.g., gastroinstetinal motility slowing, tidal volume and pulmonary blood flow increases) as well as placental (e.g., metabolism and blood flow) and fetal (e.g., liver enzyme and albumin development) factors which could alter drug metabolism and drug interactions (Wunsch et al., 2003). Because drug interactions may occur at the site of aromatase formation and activity (Nanovskaya et al., 2004; Zharikova et al., 2006), an investigation of placenta exposure to methadone or buprenorphine did not appear to inhibit estrogen formation at a level that is likely to alter maternal or neonatal outcomes (Zharikova et al., 2007).
Because methadone has been a part of the treatment of opioid dependence for several decades, substantial information exists that documents its interaction with other medications. Depending on the medication, the dosing of either methadone or the concomitant medication will need to be adjusted.
In general, buprenorphine appears to have fewer significant drug interactions than methadone because it has low affinity for the 3A4 isoenzyme that is responsible for the metabolism of many drugs by the Cytochrome P-450 system (Iribarne et al., 1997; 1998a,b). When drug interactions do occur, they appear to increase the effects of buprenorphine (i.e., decreasing buprenorphine metabolism) and can be mitigated by a buprenorphine dose reduction. Similar to methadone (Ernst et al., 2002), concurrent intravenous or very high-dose use of buprenorphine and benzodiazepines is associated with overdose deaths (e.g., Kintz, 2002a,b; Reynaud et al., 1998; Singh et al., 1992). The interaction mechanism does not appear to be pharmacokinetic (Kilicarslan & Sellers, 2000). Finally, it should be noted that the interaction of sublingual buprenorphine and oral benzodiazepines is unclear.
Pharmacodynamic and pharmacokinetic interactions of buprenorphine with other medications are generally predicted and observed to be similar to those of methadone with other medications (e.g., increased sedation) though of lesser magnitude since buprenorphine as a partial agonist has lower maximal activity at the mu receptor (See Table I).
11.2 Managing Co-Occurring Psychiatric Disorders
There is a high prevalence (56%–73%) of co-occurring Axis I disorders in pregnant drug-dependent women (Burns et al., 1985; Fitzsimons et al., 2007; Haller et al., 1993; Regan et al., 1982). Mood disorders during pregnancy have been associated with adverse maternal health behaviors, a high risk of postpartum depression and behavioral effects on the offspring (Bonari et al., 2004; Cohen et al., 2004). Thus, opioid-dependent pregnant patients should be screened for and given appropriate medication and behavioral treatments for their disorders.
Pharmacotherapy use should be based on a positive risk/benefit ratio and not include drugs known to have teratogenic effects such as dilantin (Meador et al., 2006). SSRIs should be used as necessary with a caution that there might be a risk of pulmonary hypertension, an uncommon disorder in the neonate or frank SSRI toxicity that is rarely fatal (Kulin et al., 1998; Chambers et al., 2006). Fluvoxamine induces methadone metabolism and if not avoided, methadone doses should be modified. Benzodiazepines should not be administered due to their high risk of dependence in this population.
For patients with a sexual abuse history, flashbacks, intense emotions and negative memories may occur during key treatment times including medication induction and labor and delivery. Appropriately trained staff can help the patient identify and cope with these traumatic memories and increase her opportunities to remain in treatment and drug free (Records & Rice, 2002). Finally, the need for psychotropic medications may be lessened by appropriate use of opioid agonists because they may have significant antidepressant/anxiolytic effects.
12. Summary
In summary, our understanding and knowledge about the treatment of opioid dependence during pregnancy has grown in the last 40 years. In that time, data has supported the need for adequate methadone-dosing regimens. The understanding of the complexities of this disorder and that of poly-drug addiction during pregnancy has grown and informed treatment strategies have been established. No longer are pregnant women excluded from agonist maintenance treatment; – rather they often receive first priority because the benefits considerably outweigh the risks. The substance dependence treatment field is now converging to view the risks and benefits of treatment of opioid dependence with both the mother and child as equally important rather than competing against each other. Moreover, the advent of buprenorphine has brought both a new treatment option and unique challenges to treatment not only in terms of dose induction and pain management, but also the need for rational decisions about whether methadone or buprenorphine may be most appropriate for a given clinical situation. Finally, the MOTHER study represents a major advance towards using evidence based data to drive optimal treatment approaches tailored to the needs of each opioid-dependent pregnant patient.
Acknowledgements
All grants are from National Institute on Drug Abuse unless noted otherwise. Brown University, R01DA015778 and Dr. Barry Lester, Amy Salisbury, Michelle Zawatski, Kathy Hawes and Marissa Cerrone; Wayne State University, R01DA15832 and co-investigators Drs. Carl Christenson, Virginia Delaney-Black, Robert Sokol, Charles Schuster, Eugene Cepeda, and the assistance of Darlene Tansil and Mea Ebenbichler; Johns Hopkins University, R01 DA015764 and the staff Ave Childrey, Laetitia Lemoine, Heather Fitzsimons, Julia Shadur, Michelle Tuten, Cheryl Claire, Lori Barger, Behavioral Pharmacology Research Pharmacy and Nursing staff, Center for Addiction and Pregnancy staff, co-investigators Drs. Donald Jasinski, Lauren Jansson, Robert Dudas, Lorraine Milio, Vickie Walters, Eric Strain, and George Bigelow; Thomas Jefferson University, R01DA015738 and the current staff Priscilla Sepe, Amber Holbrook, Family Center staff, OB and Pediatric nursing staff, and co-investigators Drs. Vincenzo Berghella, Jason Baxter, Jay Greenspan and Laura McNicholas; University of Toronto, R01 DA015741, Toronto Centre for Substance Use in Pregnancy, Drs Alice Ordean and Bhushan Kapur as co-investigators, and Ms Alla Osadchy as research coordinator and the assistance of Ms. Lydia Pantea; Vanderbilt University, R01 DA 017513 and M01 RR00095 from the General Clinical Research Center and co-investigators Drs. Karen D'Apolito (co-PI), Paul Bodea-Barothi, Nancy Chescheir, Joseph Gigante, Barbara Engelhardt, nurse practitioners, Michelle Collins, Mavis Schorn, and Karen Starr, as well as the assistance of Cayce Watson and Mark Nickel; University of Vermont, R01 DA 018410, and Drs. John Brooklyn, Stephen Higgins, Anne Johnston, Marjorie Meyer, and Stacey Sigmon, as co-investigators; University of Vienna, R01 DA018417 co-investigators Drs. Kenneth Thau, Bernadette Winklbaur, Nina Ebner, Klaudia Rohrmeister, Inge Frech, Martin Langer, Manfred Weninger, Nina Kopf, (cand. med); Ingrid Kügler and nurses Doris Leopoldinger, and Burgi Gfrerer. We also gratefully thank Reckitt Benckiser Inc. for providing the buprenorphine medication and placebo product for the MOTHER trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Latif ME, Pinner J, Clews S, Cooke F, Lui K, Oei J. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics. 2006;117:e1163–e1169. doi: 10.1542/peds.2005-1561. [DOI] [PubMed] [Google Scholar]
- Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Annals of Internal Medicine. 2006;144:127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amass L, Bickel WK, Crean JP, Blake J, Higgins ST. Alternate-day buprenorphine dosing is preferred to daily dosing by opiate-dependent humans. Psychopharmacology. 1998;136:217–225. doi: 10.1007/s002130050559. [DOI] [PubMed] [Google Scholar]
- Amass L, Bickel WK, Higgins ST, Badger GJ. Alternate-day dosing during buprenorphine treatment of opiate dependence. Life Sciences. 1994;54:1215–1228. doi: 10.1016/0024-3205(94)00848-5. [DOI] [PubMed] [Google Scholar]
- Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug and Alcohol Dependence. 2000;58:143–152. doi: 10.1016/s0376-8716(99)00074-5. [DOI] [PubMed] [Google Scholar]
- Amass L, Kamien JB, Mikulich SK. Thrice-weekly supervised dosing with the combination buprenorphine-naloxone tablet is preferred to daily supervised dosing by opioid-dependent humans. Drug and Alcohol Dependence. 2001;61:173–181. doi: 10.1016/s0376-8716(00)00141-1. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Loustauneau A. Medical treatment of the pregnant heroin addict – Review of the literature. Pregnancy and drug misuse update 2000. Proceedings: Seminar organized by the Co-operation Group to Combat Drug Abuse and Illicit Trafficking in Drugs (Pompidou Group); 29–30 May 2000; Strasbourg, France: Drugs and Addiction. Council of Europe; 2000. Dec, pp. 39–74. [Google Scholar]
- Bertschy G, Baumann P. Vulnerability to fluoxetine-induced indifference syndrome among opiate addicts: a case report. Biological Psychiatry. 1995;38:404–406. doi: 10.1016/0006-3223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- Bertschy G, Eap CB, Powell K, Baumann P. Fluoxetine addition to methadone in addicts: pharmacokinetic aspects. Therapeutic Drug Monitoring. 1996;18:570–572. doi: 10.1097/00007691-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999;146:111–118. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Blinick G, Wallach RC, Jerez E. Pregnancy in narcotics addicts treated by medical withdrawal: The methadone detoxification program. American Journal of Obstetrics and Gynecology. 1969;105:997–1003. doi: 10.1016/0002-9378(69)90117-3. [DOI] [PubMed] [Google Scholar]
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Canadian Journal of Psychiatry. 2004;49:726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. Journal of Neuroscience. 2005;25:5117–5126. doi: 10.1523/JNEUROSCI.0866-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K, Melamed J, Burns W, Chasnoff I, Hatcher R. Chemical dependence and clinical depression in pregnancy. Journal of Clinical Psychology. 1985;41:851–854. doi: 10.1002/1097-4679(198511)41:6<851::aid-jclp2270410621>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, et al. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. State Methadone Treatment Guidelines. (Treatment Improvement Protocol Series 1) Rockville, MD: US Department of Health and Human Services; 1993. [Google Scholar]
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. (Treatment Improvement Protocol Series 40) Rockville, MD: US Department of Health and Human Services; 2004. [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. (Treatment Improvement Protocol Series 43) Rockville, MD: US Department of Health and Human Services; 2005. [PubMed] [Google Scholar]
- Chambers CD, Hernandez-Diaz S, Van Marter, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New England Journal of Medicine. 2006;354:579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug and Alcohol Dependence. 2003;70:S39–S47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Cohen R, Mrtek MB, Mrtek RG. Comparison of maternal absenteeism and infant illness rates among breast-feeding and formula-feeding women in two corporations. American Journal of Health Promotion. 1995;10:148–153. doi: 10.4278/0890-1171-10.2.148. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Nonacs R, Viguera AC, Reminick A. Diagnosis and treatment of depression during pregnancy. CNS Spectrums. 2004;9:209–216. doi: 10.1017/s1092852900009007. [DOI] [PubMed] [Google Scholar]
- Comfort M, Zanis DA, Whiteley MJ, Kelly-Tyler A, Kaltenbach KA. Assessing the needs of substance abusing women. Psychometric data on the psychosocial history. Journal of Substance Abuse Treatment. 1999;17:79–83. doi: 10.1016/s0740-5472(98)00048-8. [DOI] [PubMed] [Google Scholar]
- Connaughton JF, Resser D, Schut J, Finnegan LP. Perinatal addiction: Outcome and management. American Journal of Obstetrics and Gynecology. 1977;129:679–686. doi: 10.1016/0002-9378(77)90652-4. [DOI] [PubMed] [Google Scholar]
- Cowan A. Update of the general pharmacology of buprenorphine. In: Lewis J, Cowan A, editors. Buprenorphine: combating drug abuse with a unique opioid. New York: Wiley; 1995. pp. 31–47. [Google Scholar]
- DeMaria PA, Jr, Serota RD. Therapeutic use of the methadone fluvoxamine drug interaction. Journal of Addictive Diseases. 1999;18:5–12. doi: 10.1300/J069v18n04_02. [DOI] [PubMed] [Google Scholar]
- DePetrillo PB, Rice JM. Methadone dosing and pregnancy: Impact on program compliance. International Journal of Addiction. 1995;30:207–217. doi: 10.3109/10826089509060743. [DOI] [PubMed] [Google Scholar]
- Drozdick J, 3rd, Berghella V, Hill M, Kaltenbach K. Methadone trough levels in pregnancy. American Journal of Obstetrics and Gynecology. 2002;187:1184–1188. doi: 10.1067/mob.2002.127132. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Meddle SL, Toschi N, Bosch OJ, Neumann ID. Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. Journal of Neuroendocrinology. 2005;17:40–48. doi: 10.1111/j.1365-2826.2005.01272.x. [DOI] [PubMed] [Google Scholar]
- Dunlop A, Panjari M, O’Sullivan H, et al. Clinical Guidelines for the Use of Buprenorphine during Pregnancy. Fitzroy: Turning Point Alcohol and Drug Centre; 2003. [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clinical Pharmacokinetics. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- Eder H, Fischer G, Gombas W, Jagsch R, Stuhlinger G, Kasper S. Comparison of buprenorphine and methadone maintenance in opiate addicts. European Addiction Research. 1998;4 Suppl 1:3–7. doi: 10.1159/000052034. [DOI] [PubMed] [Google Scholar]
- Einarson A, Selby P, Koren G. Discontinuing antidepressants and benzodiazepines upon becoming pregnant. Beware of the risks of abrupt discontinuation. Canadian Family Physician. 2001;47:489–490. [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine's physical dependence potential: Antagonist-precipitated withdrawal in humans. Journal of Pharmacology and Experimental Therapeutics. 1996;276:449–459. [PubMed] [Google Scholar]
- Ernst E, Bartu A, Popescu A, Ileutt KF, Hansson R, Plumley N. Methadone-related deaths in Western Australia 1993–99. Australian and New Zealand Journal of Public Health. 2002;26:364–370. doi: 10.1111/j.1467-842x.2002.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Festinger DS, Lamb RJ, Kountz MR, Kirby KC, Marlowe D. Pretreatment dropout as a function of treatment delay and client variables. Addictive Behaviors. 1995;20:111–115. doi: 10.1016/0306-4603(94)00052-z. [DOI] [PubMed] [Google Scholar]
- Festinger DS, Lamb RJ, Kirby KC, Marlowe DB. The accelerated intake: A method for increasing initial attendance to outpatient cocaine treatment. Journal of Applied Behavior Analysis. 1996;29:387–389. doi: 10.1901/jaba.1996.29-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger DS, Lamb RJ, Marlowe DB, Kirby KC. From telephone to office: Intake attendance as a function of appointment delay. Addictive Behaviors. 2002;27:131–137. doi: 10.1016/s0306-4603(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Finnegan LP. Perinatal substance abuse: Comments and perspectives. Seminars in Perinatology. 1991;15:331–339. [PubMed] [Google Scholar]
- Fischer G, Etzersdorfer P, Eder H, et al. Buprenorphine maintenance in pregnant opiate addicts. European Addiction Research. 1998;4 Suppl 1:32–36. doi: 10.1159/000052040. [DOI] [PubMed] [Google Scholar]
- Fischer G, Johnson RE, Eder H, et al. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95:239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- Fitzsimons HE, Tuten M, Vaidya V, Jones HE. Mood disorders affect drug treatment success of drug-dependent pregnant women. Journal of Substance Abuse Treatment. 2007;32:19–25. doi: 10.1016/j.jsat.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Fornataro K. Methadone and anti-HIV drugs. The Body Positive. 1999;12:13. [PubMed] [Google Scholar]
- Friedland G, Andrews L, Schreibman T, et al. Lack of an effect of atazanavir on steady-state pharmacokinetics of methadone in patients chronically treated for opiate addiction. AIDS. 2005;19:1635–1641. doi: 10.1097/01.aids.0000183628.20041.f2. [DOI] [PubMed] [Google Scholar]
- Gazaway PM, Bigelow GE, Brooner RK. The influence of pregnancy upon trough plasma levels of methadone and its' opioid effects. NIDA Research Monograph. 1993;132:112. [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biological Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Gerstein DR. The effectiveness of drug treatment. Research Publications- Association for Research in Nervous and Mental Disease. 1992;70:253–282. [PubMed] [Google Scholar]
- Greenwald M, Johanson CE, Bueller J, et al. Buprenorphine duration of action: muopioid receptor availability and pharmacokinetic and behavioral indices. Biological Psychiatry. 2007;61:101–110. doi: 10.1016/j.biopsych.2006.04.043. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Schuh KJ, Hopper JA, Schuster CR, Johanson CE. Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans. Psychopharmacology (Berl) 2002;160:344–352. doi: 10.1007/s00213-001-0975-0. [DOI] [PubMed] [Google Scholar]
- Gunderson EW, Stimmel B. Treatment of pain in drug addicted persons. In: Galanter M, Kleber H, editors. Textbook of Substance Abuse Treatment. 3rd Ed. Washington DC: American Psychiatric Pub Inc.; 2004. pp. 563–574. [Google Scholar]
- Haller DL, Knisley JS, Dawson KS, et al. Perinatal substance abusers. Psychological and social characteristics. Journal of Nervous Mental Diseases. 1993;181:509–513. doi: 10.1097/00005053-199308000-00006. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Stein JA, Grella CE. Contrasting predictors of readiness for substance abuse treatment in adults and adolescents: a latent variable analysis of DATOS and DATOS-A participants. Drug and Alcohol Dependence. 2005;80:63–81. doi: 10.1016/j.drugalcdep.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Ibrahim RB, Wilson JG, Thorsby ME, Edwards DJ. Effect of buprenorphine on CYP3A activity in rat and human liver microsomes. Life Sciences. 2000;66:1293–1298. doi: 10.1016/s0024-3205(00)00436-7. [DOI] [PubMed] [Google Scholar]
- Iribarne C, Berthou F, Carlhant D, et al. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug Metabolism and Disposition. 1998a;26:257–260. [PubMed] [Google Scholar]
- Iribarne C, Picart D, Dréano Y, Bail J-P. Involvement of cytochrome P4503A4 in N-dealkylation of buprenorphine in human liver microsomes. Life Sciences. 1997;60:1953–1964. doi: 10.1016/s0024-3205(97)00160-4. [DOI] [PubMed] [Google Scholar]
- Iribarne C, Picart D, Dreano Y, Berthou F. In vitro interactions between fluoxetine or fluvoxamine and methadone or buprenorphine. Fundamental and Clinical Pharmacology. 1998b;12:194–199. doi: 10.1111/j.1472-8206.1998.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Dipietro J, Elko A. Fetal response to maternal methadone administration. American Journal of Obstetrics and Gynecology. 2005;193(3 Pt 1):611–617. doi: 10.1016/j.ajog.2005.02.075. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Dipietro JA, Elko A, Velez M. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. Journal of Maternal, Fetal and Neonatal Medicine. 2007;20:677–685. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- Jones HE. Drug addiction during pregnancy: Advances in maternal treatment and understanding child outcomes. Current Directions for Psychological Science. 2006a;15:126–130. [Google Scholar]
- Jones HE, Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug and Alcohol Dependence. 2005b;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, Milio L. Post-cesarean pain management of patients maintained on methadone or buprenorphine. American Journal on Addictions. 2006c;15:258–259. doi: 10.1080/10550490600626721. [DOI] [PubMed] [Google Scholar]
- Jones HE, Suess P, Jasinski DR, Johnson RE. Transferring methadone-stabilized pregnant patients to buprenorphine using an immediate release morphine transition: an open-label exploratory study. American Journal of Addiction. 2006b;15:61–70. doi: 10.1080/10550490500419094. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jasinski DR, Milio L. Randomized controlled study transitioning opioid-dependent pregnant women from short-acting morphine to buprenorphine or methadone. Drug and Alcohol Dependence. 2005a;78:33–38. doi: 10.1016/j.drugalcdep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Jones HE, O’Grady KE, Schlundt D, Tuten M. Methadone Maintenance vs. Methadone Taper: Maternal and Neonatal Outcomes. doi: 10.1080/10550490802266276. (Submitted a) [DOI] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, O’Grady KE, Jasinski DR, Milio L. Dosing Adjustments in Post-Partum Patients Maintained on Buprenorphine or Methadone. Journal of Addiction Medicine. doi: 10.1097/ADM.0b013e31815ca2c6. (in press) [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: Effects and management. Obstetrics and Gynecology Clinics of North America. 1998;25:139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Kandall SR, Albin S, Gartner LM, Lee KS, Eidelman A, Lowinson J. The narcotic-dependent mother: fetal and neonatal consequences. Early Human Development. 1977;1:159–169. doi: 10.1016/0378-3782(77)90017-2. [DOI] [PubMed] [Google Scholar]
- Kilicarslan T, Sellers EM. Lack of interaction of buprenorphine with flunitrazepam metabolism. American Journal of Psychiatry. 2000;157:1164–1166. doi: 10.1176/appi.ajp.157.7.1164. [DOI] [PubMed] [Google Scholar]
- Kintz P. A new series of 13 buprenorphine-related deaths. Clinical Biochemistry. 2002a;35:513–516. doi: 10.1016/s0009-9120(02)00304-1. [DOI] [PubMed] [Google Scholar]
- Kintz P. Buprenorphine-related deaths. In: Kintz P, Marquet P, editors. Buprenorphine Therapy of Opiate Addiction. Totowa: Humana Press; 2002b. pp. 109–118. [Google Scholar]
- Kissin WB, Svikis DS, Moylan P, Haug NA, Stitzer ML. Identifying pregnant women at risk for early attrition from substance abuse treatment. Journal of Substance Abuse Treatment. 2004;27:31–38. doi: 10.1016/j.jsat.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Krystal JH, Charney DS, Price LH, Morgan CH, Kleber HD. Opiate antagonist challenges in buprenorphine-maintained patients. Drug and Alcohol Dependence. 1990;25:73–78. doi: 10.1016/0376-8716(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Morgan CM, Falcione J, Schottenfeld RS. Pharmacotherapy for cocaine-abusing methadone-maintained patients using amantadine or desipramine. Archives of General Psychiatry. 1992;49:894–898. doi: 10.1001/archpsyc.1992.01820110058009. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Oliveto A, Sevarino KA, Gonsai K, Feingold A. Ketoconazole increases cocaine and opioid use in methadone maintained patients. Drug Alcohol Dependence. 2002;66:173–180. doi: 10.1016/s0376-8716(01)00198-3. [DOI] [PubMed] [Google Scholar]
- Kreek M. Drug interactions with methadone in humans. NIDA Research Monograph. 1986;68:193–225. [PubMed] [Google Scholar]
- Kreek M. Methadone disposition during the perinatal periods in humans. Pharmacology, Biochemistry and Behavior. 1979;11:7–13. [PubMed] [Google Scholar]
- Kreek M, Schecter A, Gutjahr C. Analyses of methadone and other drugs in maternal and neonatal body fluids: use in evaluation of symptoms in a neonate of mother maintained on methadone. American Journal of Drug Alcohol Abuse. 1974;1:409–419. doi: 10.3109/00952997409011033. [DOI] [PubMed] [Google Scholar]
- Kulin NA, Pastuszak A, Koren G. Are the new SSRIs safe for pregnant women? Canadian Family Physician. 1998;44:2081–2083. [PMC free article] [PubMed] [Google Scholar]
- Lejeune C, Simmat-Durand L, Gourarier L, et al. Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Dependence. 2006;82:250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Ritter A, Dunlop A, Muhleisen P. Training Primary Health Care Professionals to Provide Buprenorphine and LAAM Treatment. Substance Abuse. 2002;23:245–254. doi: 10.1080/08897070209511497. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Malpas TJ, Darlow BA. Neonatal abstinence syndrome following abrupt cessation of breastfeeding. New Zealand Medical Journal. 1999;112:12–13. [PubMed] [Google Scholar]
- McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. American Journal on Addictions. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse GD, et al. Interaction between buprenorphine and atazanavir or atazanavir/ritonavir. Drug and Alcohol Dependence. 2007 doi: 10.1016/j.drugalcdep.2007.06.007. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse GD, et al. Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clinical Infectious Diseases. 2006;43 Suppl 4:S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Friedland G, Jatlow P. The protease inhibitor lopinavir-ritonavir may produce opiate withdrawal in methadone-maintained patients. Clinical Infectious Diseases. 2003;37:476–482. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Friedland G, Kosten TR, Jatlow P. Effect of opioid dependence pharmacotherapies on zidovudine disposition. American Journal on Addictions. 2001;10:296–307. [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Jatlow P, Friedland G. Methadone effects on zidovudine disposition (AIDS Clinical Trials Group 262) Journal of Acquired Immune Deficency Syndromes and Hum Retrovirology. 1998;18:435–443. doi: 10.1097/00042560-199808150-00004. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Wagner K, Benvenuto A, Howard D, Plante D. Intrapartum and postpartum analgesia for women maintained on methadone during pregnancy. Obstetrics and Gynecology. 2007a;110:261–266. doi: 10.1097/01.AOG.0000275288.47258.e0. [DOI] [PubMed] [Google Scholar]
- Meyer M, Paranya G, Kristensen E, Plante D. Buprenorphine impairs intrapartum patient controlled epidural analgesia (PCEA) efficacy. Poster presentation presented at the annual meeting of the Society of Obstetric Anesthesia and Perinatology; Alberta, Canada. 2007b. [Google Scholar]
- Meyers RJ, Smith JE. Clinical Guide to Alcohol Treatment. The Community Reinforcement Approach. New York: Guilford Press; 1995. [Google Scholar]
- National Institutes of Health Consensus Development Panel. Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Journal of the American Medical Association. 1998;280:1936–1943. [PubMed] [Google Scholar]
- Nanovskaya TN, Deshmukh SV, Nekhayeva IA, Zharikova OL, Hankins GD, Ahmed MS. Methadone metabolism by human placenta. Biochemical Pharmacology. 2004;68:583–591. doi: 10.1016/j.bcp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Oliveto A, Kosten TR, Schottenfeld R, Falcioni J, Ziedonis D. Desipramine, amantadine, or fluoxetine in buprenorphine-maintained cocaine users. Journal of Substance Abuse Treatment. 1995;12:423–428. doi: 10.1016/0740-5472(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Paetzold W, Eronat V, Seifert J, Holze I, Emrich HM, Schneider U. Detoxification of poly-substance abusers with buprenorphine. Effects on affect, anxiety, and withdrawal symptoms. Nervenarzt. 2000;71:722–729. doi: 10.1007/s001150050656. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Lei H. Assessment of opioid dependence with naloxone. British Journal of Addiction. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Badger GJ. Examining the limits of the buprenorphine interdosing interval: daily, every-third-day and every-fifth-day dosing regimens. Addiction. 2001;96:823–834. doi: 10.1046/j.1360-0443.2001.9668234.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Badger GJ. A comparison of four buprenorphine dosing regimens using open-dosing procedures: Is twice-weekly dosing possible? Addiction. 2000;95:1069–1077. doi: 10.1046/j.1360-0443.2000.95710698.x. [DOI] [PubMed] [Google Scholar]
- Pond S, Kreek M, Tong T, et al. Changes in methadone pharmacokinetics during pregnancy. Journal of Pharmacology and Experimental Therapeutics. 1985;234:1–6. [Google Scholar]
- Ramirez-Cacho WA, Flores S, Schrader RM, McKay J, Rayburn WF. Effect of chronic maternal methadone therapy on intrapartum fetal heart rate patterns. Journal for the Society of Gynecological Investigation. 2006;13:108–111. doi: 10.1016/j.jsgi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Records K, Rice MJ. A Comparative Study of Postpartum Depression in Abused and Nonabused Women. Archives of Psychiatric Nursing. 2005;19:281–290. doi: 10.1016/j.apnu.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Regan D, Leifer B, Matteucci T, Finnegan L. Depression in Pregnant Drug-dependent women. NIDA Research Monograph. 1982;41:466–472. [PubMed] [Google Scholar]
- Rementeria JL, Nunag NN. Narcotic withdrawal in pregnancy: Stillbirth incidence with a case report. American Journal of Obstetrics and Gynecology. 1973;116:1152–1156. doi: 10.1016/0002-9378(73)90953-8. [DOI] [PubMed] [Google Scholar]
- Reynaud M, Petit G, Potard D, Courty P. Six deaths linked to concomitant use of buprenorphine and benzodiazepines. Addiction. 1998;93:1385–1392. doi: 10.1046/j.1360-0443.1998.93913859.x. [DOI] [PubMed] [Google Scholar]
- Schlatter J, Madras JL, Saulnier JL, Poujade F. Drug interactions with methadone. Presse Medicine. 1999;28:1381–1384. [PubMed] [Google Scholar]
- Singh RA, Mattoo SK, Malhotra A, Varma VK. Cases of buprenorphine abuse in India. Acta Psychiatry Scandinavia. 1992;86:46–48. doi: 10.1111/j.1600-0447.1992.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Kahan M. Methadone induction doses: are our current practices safe? Journal of Addictive Diseases. 2006;25:5–13. doi: 10.1300/J069v25n03_02. [DOI] [PubMed] [Google Scholar]
- Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. American Journal of Drug and Alcohol Abuse. 1990;16:67–76. doi: 10.3109/00952999009001573. [DOI] [PubMed] [Google Scholar]
- Stasiewicz PR, Stalker R. A comparison of three “interventions” on pretreatment dropout rates in an outpatient substance abuse clinic. Addictive Behaviors. 1999;24:579–582. doi: 10.1016/s0306-4603(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Stevens RC, Rapaport S, Maroldo-Connelly L, Patterson JB, Bertz R. Lack of methadone dose alterations or withdrawal symptoms during therapy with lopinavir/ritonavir. Journal of Acquired Immune Deficiency Syndrome. 2003;33:650–651. doi: 10.1097/00126334-200308150-00016. [DOI] [PubMed] [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Precipitated withdrawal by pentazocine in methadone-maintained volunteers. Journal of Pharmacology and Experimental Therapeutics. 1993;267:624–634. [PubMed] [Google Scholar]
- Tong TG, Benowitz NL, Kreek MJ. Methadone-disulfiram interaction during methadone maintenance. Journal of Clinical Pharmacology. 1980;20:506–513. doi: 10.1002/j.1552-4604.1980.tb02543.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. HHS Blueprint for Action on Breastfeeding. Washington, DC: U.S. Government Printing Office; 2000a. [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2010. Understanding and Improving Health. Chapter 16. 2nd ed. ed. Washington, DC: U.S. Government Printing Office; 2000b. [Google Scholar]
- Virden SF. The relationship between infant feeding method and maternal role adjustment. Journal of Nurse-Midwifery. 1988;33:31–35. doi: 10.1016/0091-2182(88)90246-7. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–972. [PubMed] [Google Scholar]
- Widstrom AM, Wahlberg V, Matthiesen AS, et al. Short-term effects of early suckling and touch of the nipple on maternal behaviour. Early Human Development. 1990;21:153–163. doi: 10.1016/0378-3782(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Wilson GS, Desmond MM, Wait RB. Follow-up of methadone-treated and untreated narcotic-dependent women and their infants: health, developmental, and social implications. Journal of Pediatrics. 1981;98:716–722. doi: 10.1016/s0022-3476(81)80830-x. [DOI] [PubMed] [Google Scholar]
- Wittmann BK, Segal S. A comparison of the effects of single- and split-dose methadone administration on the fetus: ultrasound evaluation. International Journal of Addiction. 1991;26:213–218. doi: 10.3109/10826089109053183. [DOI] [PubMed] [Google Scholar]
- Wunsch MJ, Standard V, Schnoll SH. Treatment of pain in pregnancy. Clinical Journal of Pain. 2003;19:148–155. doi: 10.1097/00002508-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Zharikova OL, Deshmukh SV, Kumar M, et al. The effect of opiates on the activity of human placental aromatase/CYP19. Biochemical Pharmacology. 2007;73:279–286. doi: 10.1016/j.bcp.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Zharikova OL, Deshmukh SV, Nanovskaya TN, Hankins GD, Ahmed MS. The effect of methadone and buprenorphine on human placental aromatase. Biochemical Pharmacology. 2006;71:1255–1264. doi: 10.1016/j.bcp.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Zuspan FP, Gumpel JA, Mejia-Zelaya A, Madden J, Davis R. A. Fetal stress from methadone withdrawal. American Journal of Obstetrics and Gynecology. 1975;122:43. doi: 10.1016/0002-9378(75)90613-4. [DOI] [PubMed] [Google Scholar]