Abstract
Transplantation of stem cells or immune cells has shown promise for the treatment of a number of diseases. Monitoring magnetically labeled cells with MRI has furthered our understanding of cellular migration and the pathophysiology of diseases in experimental models. These studies should pave the way for guiding clinical trials using cell-based therapies. This review will briefly describe the various methods used to label and track cells with MRI and the potential for such methods to translate to human applications.
Introduction
Cellular therapies have garnered considerable interest as potential treatments of diseases and for regenerative medicine. However, ensuring that cells reach their intended targets will be necessary to maximize the efficacy of these treatments. This has motivated the development of noninvasive imaging techniques to monitor the temporal and spatial migration of cells to target tissues. Magnetic resonance imaging (MRI) is a well-suited tool for in vivo cell tracking due to its high spatial resolution. Numerous methods have been developed and refined in experimental models to track and monitor cells in vivo. Nearly all approaches require cells be manipulated in vitro, either through loading the cells with nanoparticles or through genetic modifications (1–3). Recently, the use of superparamagnetic iron oxide nanoparticles (SPION) to visualize cell migration has been applied clinically, demonstrating the potential capabilities of monitoring cellular therapies by MRI. This concise review will describe the methods used to label cells for tracking with in vivo MRI with an emphasis on potential clinical translation. The readers are encouraged to examine excellent reviews (1–3) for comprehensive coverage of the many aspects of labeling cells for detection with MRI.
Methods of Cell Labeling
Labeling mammalian cells for detection with MRI is achieved by loading the stem cells or immune cells in vitro with either paramagnetic contrast agents (ie., gadolinium chelates), experimental or clinically-approved SPIONs used off-label, or perfluorocarbon nanoparticles.
Gadolinium-based Compounds
Gadolinium (Gd) chelates are clinically approved contrast agents that have been used to label cells in experimental cellular MRI studies. When cells are loaded via electroporation, Gd locates to the cytoplasm, resulting in a decrease of the T1 relaxation time constant (4). In contrast, incubation of cells with Gd results in uptake into endosomes, which shortens the T2 relaxation time constant. Modo, et al.(5) tracked the migration of intracerebrally-injected stem cells with a combination gadolinium chelate-fluorescent label and were able to detect the presence and migration of cells at least 14 days post-injection on T2-weighted images with correlative findings by fluorescence microscopy. A transient negative effect of gadolinium-based agents on cell proliferation has been observed, and therefore, further evaluation will be needed to ensure that there is no long term toxicity and that the ability of the cells to repair damage has not been compromised (6). Recently, gadolinium-based agents with substantially greater T1 relaxivities have been developed to label cells. Anderson et al. (7) were able to label mesenchymal stromal cells (MSC) with gadolinium fullerenol, which has a T1 relaxivity of 10 fold greater than gadolinium chelates, and detected an increase in signal intensity on T1 weighted images following direct injection into the rat thigh at 7T. However, gadolinium fullerenol labeling initially decreased the stem cell proliferation, suggesting that the agent may be altering mitochondrial function. Thus, there remains a need to identify paramagnetic agents that exert strong T1 effects and allow sufficient detection of cells versus surrounding tissues in circumstances of low numbers of labeled cells or low concentrations of gadolinium.
Fluorinated Nanoparticles
The main advantage of labeling cells with 19F perfluorocarbon nanoparticles is the high specificity for labeled cells, since fluorine can be directly detected with MRI and there are no endogenous fluorine atoms in the body. Aherns, et al. (8) labeled dendritic cells with cationic perfluoropolyether agents and tracked the migration of the cells to regional lymph nodes following injection into the foot pad of mice using 19F MRI. Additionally, stem/progenitor cells loaded with perfluorocarbon nanoparticles have been tracked with MRI and MR spectroscopy at both clinical and high field strengths (9). Limitations of this cell labeling approach include the need for high concentrations of 19F to achieve a minimal detection threshold, relatively long scanning times, and separate 1H images for anatomical localization of 19F detected cells.
Superparamagnetic Iron Oxide Nanoparticles
Superparamagnetic iron oxide nanoparticles are a family of MRI contrast agents that have seen extensive use to efficiently label cells for cellular imaging. Various methods are used to prepare SPIONs, resulting in a wide range of physiochemical differences including core size, shape, mono or oligocrystalline composition, and outer coating, all of which alter their biological activity and ability to use these agents to label cells (2). In addition, micron sized iron oxide beads (MPIOs; 0.3 to >5 microns) incorporating a fluorescent marker are also being used to label cells for cellular MRI studies in experimental models (10). Iron perturbs the main magnetic field and can therefore be visualized as signal voids on T2 or T2*-weighted images. The detection threshold for SPIO labeled cells is affected by magnetic field strength and acquisition parameters. Importantly, the magnetic susceptibility effects of SPION labeled cells result in a blooming artifact that extends beyond the size of an individual particle, which makes the cells conspicuous enough to potentially detect individual cells. Incubating cells with experimental or clinically approved, coated SPION (i.e., ferumoxides, ferucarbotran or ferumoxtran-10) with or without transfection agents results in iron concentrations from 1 to >30 picograms iron per cell compared with unlabeled cells containing approximately <0.1 picograms iron(11). Magnetic labeling cells with SPIONs have little or no effects on proliferation, metabolic expression profiles, reactive oxygen species formation, apoptosis rates, or differentiation in the majority of cells studied(11, 12). Several techniques have been proposed to increase the sensitivity for iron-labeled cells by directly detecting the magnetic inhomogeniety caused by the iron, using off-resonance or refocusing techniques(13, 14), and these are termed “positive contrast” techniques. However, these same techniques can also be affected by intrinsic susceptibility differences, thereby limiting their utility.
Following direct injection of a mixture of ferumoxides and 111Indium-oxine labeled dendritic cells in the lymph node of patients with melanoma, de Vries et al. (15) were able to demonstrate the migration of labeled cells by single photon emission computerized tomography (SPECT) and MRI through inguinal lymph nodes (Figure 1). Multimodality imaging allowed for quantification of the number of cells in the lymph nodes resulting in MRI detection limit of approximately 2000 ferumoxides-labeled dendritic cells per voxel based on corresponding measurements by SPECT. Several groups have shown that it is possible to track single magnetically labeled cells by cellular MRI in experimental models(10, 16) although it remains to be seen whether these techniques will translate to the clinic.
Figure 1.
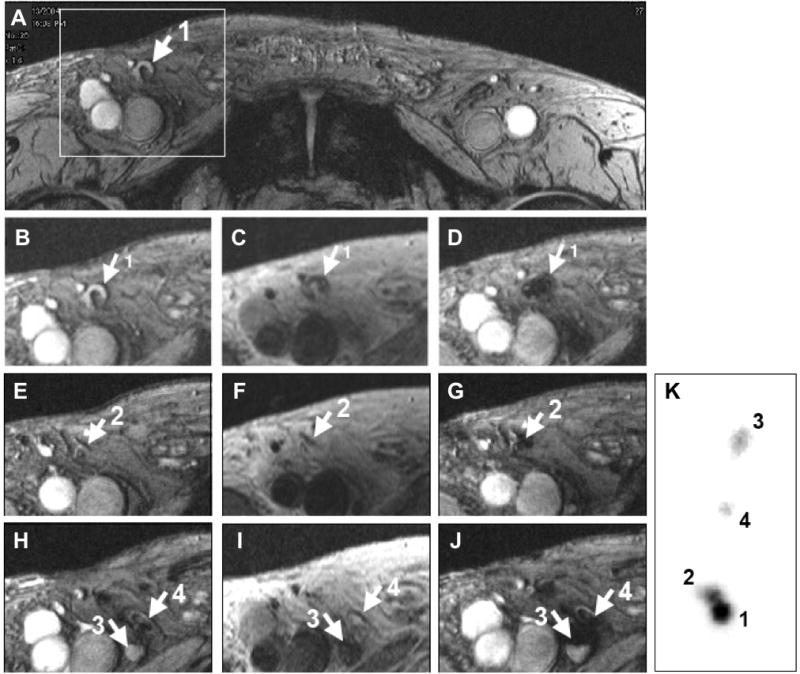
Monitoring of in vivo migration of SPIO and 111In-labeled dendritic cells (DC) with MRI and SPECT after intranodal injection. (A, B, E, and H) T2*-weighted gradient echo transverse images before vaccination showing right inguinal lymph nodes (LN) with a high signal intensity (arrows). (C, F, and I) T2-weighted spin-echo images after vaccination show LN with dark-gray signal intensity. (D, G, and J) On GE images after vaccination all these LNs show decreased signal intensity. (K) Ex vivo scintigraphy of the resected LN basin verified the MR findings. From this scintigraphy the percentage of migrated cells could be calculated (LN 1: 60%; LN2: 32%, LN3: 2%; LN4: 6% of the 7.5 ×106 injected DCs). Adapted from ref (15), with permission.
Magnetic Reporter Genes
Investigators have recently inserted MR reporter genes into cells that results in the expression of iron storage proteins, allowing stored iron to be detected by MRI. Genove, et al. (17) used an adenoviral vector carrying a transgene for light and heavy chain ferritin protein to transfect cells, permitting the detection of cells by MRI in both in vitro and in vivo models. Cohen, et al. (18) generated transgenic mice with tissue specific, inducible transcriptional regulation using the tetracycline transactivator gene to express heavy chain ferritin. These authors were able to demonstrate tetracycline-modulated ferritin concentration in liver and endothelial cells in brain as measured by T2 relaxation with MRI. Importantly, the sensitivity to ferritin-expressing cells is not lost due to dilution of the compound as the cells divide. Recently, Zurkiya, et al. (19) transfected cells with genes from magnetotactic bacteria (i.e., MagA) under doxycycline regulated gene expression, resulting in the intracellular production of iron oxide nanoparticles similar to synthetic SPION. MagA expressing cells could be visualized by MRI following transplantation in mouse brain following 5 day induction with doxycycline. The generalized implementation of these techniques in stem cells tracking as treatment strategies needs to be explored.
Applications
SPION have been used extensively to track the migration of stem cells(20–22), immune cells (i.e., dendritic cells, splenocytes, or lymphocytes) (23), and many other cell types (24, 25) in numerous animal models of malignancy, angiogenesis, ischemia and infarction, organ failure, autoimmune diseases, and transplantation rejection. Magnetically labeled neural stem cells (NSC) or neural progenitor cells have been implanted in normal brains of neonatal or adult animals, in stroke(5, 22), traumatic brain injury(26), or spinal cord injuries(27). Guzman et al (28) followed SPION-labeled NSC migration and site specific differentiation in neonatal and adult rodent brain and in a brain injury model. We have demonstrated the incorporation of SPION-labeled mouse endothelial progenitor cells into the growing neovasculature of tumors in intracerebral(29) and flank tumor models (Figure 2). Wu, et al. (23) demonstrated that the infiltration of labeled cells was a robust marker of cardiac transplant rejection. Kraitchman, et al. (20) infused dual labeled MSCs (111Indium-oxine and ferumoxides) and reported focal and diffuse uptake in the infarcted myocardium by SPECT/CT. However, magnetically-labeled MSCs detected around the infarction on histology were not observed on MRI. This study demonstrates the need to develop multimodal techniques to detect the presence of distributed labeled cells within target tissues.
Figure 2.
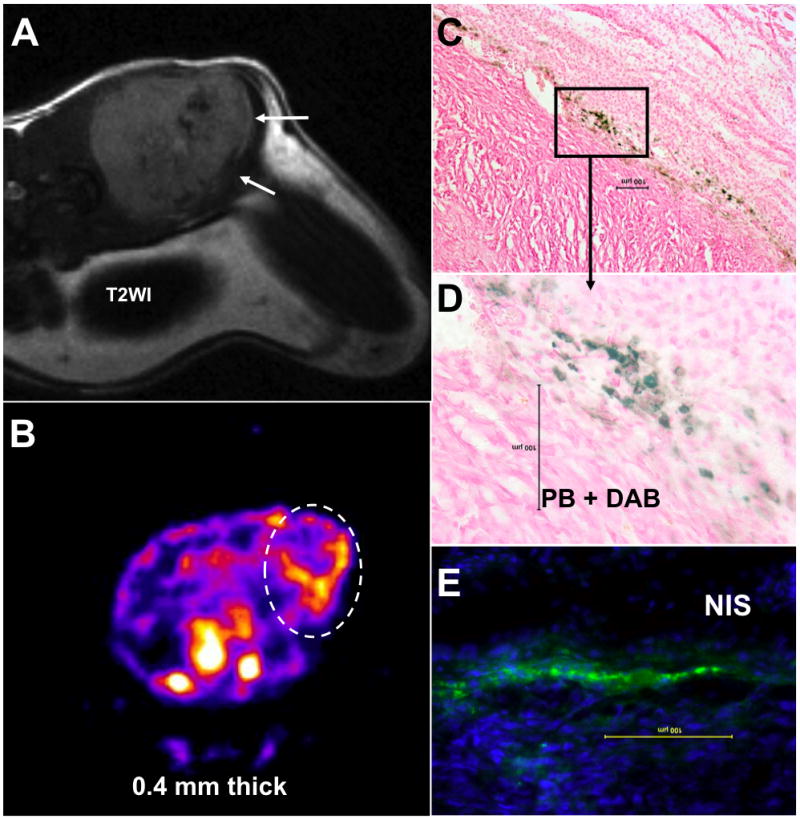
Accumulation of magnetically labeled, transgenic endothelial progenitor cells (EPCs) around implanted tumor. T2-weighted MRI shows low signal intensity areas at the margin of the tumor (A, white arrows), that correspond to sites of iron positive cells detected by Prussian blue staining (C and D). The central hypointense areas are due to hemorrhage within the tumor. SPECT image (B; trans-axial sections) indicates the accumulated transgenic EPCs (white dotted oval ROI) detected by T99m. The SPECT study also confirms the migration and homing of EPCs at the margin of the tumors seen on MRI. Immunohistochemistry depicts the accumulation of NIS-positive cells at the corresponding sites, detected by anti-hNIS antibody and FITC labeled secondary antibodies (E). The findings prove that EPCs can carry reporter or therapeutic genes to the site of interest and magnetically labeled EPCs act as a probe for cellular MRI. Images provided courtesy of Arbab Ali, M.D., Ph.D., Department of Radiology, Henry Ford Health System.
The incorporation of magnetically labeled islet cells under the kidney capsule or infused in the portal vein liver has allowed for the noninvasive monitoring of the treatment of type 1 diabetes in mouse models(30). Magnetically labeled islets maintained normoglycemia in the animals and MRI could detect these cells for up to 180 days post-implantation. Serial MRI of islet cell rejection in the liver of mice has been demonstrated by labeling cells with SPION (31) and demonstrates the potential value of this technique in monitoring allogenic transplants. These and many other preclinical studies have demonstrated the potential translation of SPION labeling and cellular MRI to the clinic.
There are four clinical studies in the literature involving magnetically labeled cells and MRI to monitor the migration of cells to target tissue. The first reported trial involved the injection of magnetically labeled dendritic cells into lymph nodes of patients with melanoma as part of a phase I vaccine study(32). MRI was used to visualize the migration of cells to other lymph nodes within 2 days (Figure 1). MRI also demonstrated that the injections of labeled cells missed targeted lymph nodes in 4 patients indicating the utility and importance of high-resolution noninvasive imaging in treatment planning. Zhu, et al. (26) cultured and labeled neuronal stem cells (NSC) obtained from patients with open head trauma. Patients received intracerebral injections of either ferumoxides-labeled or unlabeled cells around the injured tissue. Serial MRI up to 7 weeks demonstrated the migration of magnetically labeled NSCs from injections sites as hypointense voxels. Neurological complications were not reported in patients receiving labeled cells. In patients with spinal cord injury, autologous bone marrow CD34+ cells labeled with magnetic beads could be monitored homing to the injury site for several weeks after infusion into the cerebral spinal fluid (27). MRI was also used to track cadaveric magnetically labeled pancreatic islet cells infused into the portal vein in patients with type 1 diabetes(25), and hypointense regions on T2 weighted MRI of the liver were observed for at least 6 weeks. Despite the limited applications in human patients, these studies nonetheless provide strong evidence that tracking cells with MRI could have important clinical implications.
Cell tracking with MRI suffers from some common limitations observed with all exogenous cell tagging including: sensitivity of the imaging technique, dilution of the exogenous label with cell division, and potential transfer of the magnetic label to tissue macrophages or bystander cells. Although single cells can be detected in vivo in experimental models, the improvement of MRI hardware and detection techniques may improve the sensitivity to reliably detect the presence of labeled cells in tissue. The concentration of the label dilutes as cells divide, limiting the ability to track cells from a few days to several weeks. However, changes in different MRI parameters over time may indicate the engraftment and functional improvement of the target organs/tissues. The transfer of the SPION to activated macrophages or bystander cells in vivo can potentially complicate the interpretation of in vivo MRI results. However, it has been recently reported that the transfer of SPION or genetic label BrdU from MSC to activated macrophages represented a relatively small amount compared to total label injected(33).
Future Directions
Although the most promising agents for use in clinical applications are those that have proven to have little or no effects on cell behavior, the functional status of cells is difficult to monitor by in vivo MRI. For cell-therapy, the mere presence of labeled cells detected with MRI does not reflect the viability, proliferation, or differential capacity of the cells. Newer “smart” MRI agents hold promise in this regard, as the functional status of the cell can be probed in addition to its location(34). The advantages afforded by other noninvasive imaging modalities, such as positron emission tomography (PET), single photon emission computerized tomography (SPECT), ultrasound, and optical imaging, could be utilized in conjunction with MRI(35). In this regard, multi-functional agents may also serve to provide detailed information of cell function and viability. Tracking labeled cells with MRI has clearly demonstrated its utility in evaluating promising cell-based therapies in preclinical models. While the techniques for labeling cells using magnetic particles continue to be advanced, the translation to the clinic is expected to occur in the near future and will provide researchers with a valuable tool to monitor cell therapies.
Acknowledgments
This work was supported by the Intramural Research Program of the Clinical Center at the National Institutes of Health.
References
- 1.Arbab AS, Frank JA. Cellular MRI and its role in stem cell therapy. Regen Med. 2008 Mar;3(2):199–215. doi: 10.2217/17460751.3.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm C, Gazeau F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials. 2008 Aug;29(22):3161–3174. doi: 10.1016/j.biomaterials.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Modo M, Hoehn M, Bulte JW. Cellular MR imaging. Mol Imaging. 2005;4:143–164. doi: 10.1162/15353500200505145. [DOI] [PubMed] [Google Scholar]
- 4.Terreno E, Geninatti Crich S, Belfiore S, et al. Effect of the intracellular localization of a Gd-based imaging probe on the relaxation enhancement of water protons. Magn Reson Med. 2006 Mar;55(3):491–497. doi: 10.1002/mrm.20793. [DOI] [PubMed] [Google Scholar]
- 5.Modo M, Mellodew K, Cash D, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311–317. doi: 10.1016/j.neuroimage.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Brekke C, Morgan SC, Lowe AS, et al. The in vitro effects of a bimodal contrast agent on cellular functions and relaxometry. NMR Biomed. 2007 Apr;20(2):77–89. doi: 10.1002/nbm.1077. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SA, Lee KK, Frank JA. Gadolinium-fullerenol as a paramagnetic contrast agent for cellular imaging. Invest Radiol. 2006;41(3):332–338. doi: 10.1097/01.rli.0000192420.94038.9e. [DOI] [PubMed] [Google Scholar]
- 8.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 9.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. Faseb J. 2007 Jun;21(8):1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 11.Arbab AS, Yocum GT, Rad AM, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 12.Pawelczyk E, Arbab AS, Pandit S, Hu E, Frank JA. Expression of transferrin receptor and ferritin following ferumoxides-protamine sulfate labeling of cells: implications for cellular magnetic resonance imaging. NMR Biomed. 2006 Aug;19(5):581–592. doi: 10.1002/nbm.1038. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med. 2005;53:999–1005. doi: 10.1002/mrm.20477. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Dahnke H, Jordan EK, Schaeffter T, Frank JA. In vivo MRI using positive-contrast techniques in detection of cells labeled with superparamagnetic iron oxide nanoparticles. NMR Biomed. 2008;21:242–250. doi: 10.1002/nbm.1187. [DOI] [PubMed] [Google Scholar]
- 15.De Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 16.Heyn C, Ronald JA, Ramadan SS, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56:1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 17.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005 Apr;11(4):450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 18.Cohen B, Ziv K, Plaks V, et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007 Apr;13(4):498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 19.Zurkiya O, Chan AW, Hu X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn Reson Med. 2008 Jun;59(6):1225–1231. doi: 10.1002/mrm.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005 Sep 6;112(10):1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman R, Uchida N, Bliss TM, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008 May;39(5):1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu YL, Ye Q, Foley LM, et al. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster PJ, Dunn EA, Karl KE, et al. Cellular magnetic resonance imaging: in vivo imaging of melanoma cells in lymph nodes of mice. Neoplasia. 2008;10:207–216. doi: 10.1593/neo.07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toso C, Vallee JP, Morel P, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8:701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 27.Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007 Jun;16(3):461–466. doi: 10.1089/scd.2007.0083. [DOI] [PubMed] [Google Scholar]
- 28.Guzman R, Uchida N, Bliss TM, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson SA, Glod J, Arbab AS, et al. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005 Jan 1;105(1):420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 30.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006 Jan;12(1):144–148. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 31.Evgenov NV, Medarova Z, Pratt J, et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55:2419–2428. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 32.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 33.Pawelczyk E, Arbab AS, Chaudhry A, Balakumaran A, Robey PG, Frank JA. In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells. 2008 May;26(5):1366–1375. doi: 10.1634/stemcells.2007-0707. [DOI] [PubMed] [Google Scholar]
- 34.Gilad AA, McMahon MT, Walczak P, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007 Feb;25(2):217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 35.Ly HQ, Frangioni JV, Hajjar RJ. Imaging in cardiac cell-based therapy: in vivo tracking of the biological fate of therapeutic cells. Nature clinical practice. 2008 Aug;5 Suppl 2:S96–102. doi: 10.1038/ncpcardio1159. [DOI] [PubMed] [Google Scholar]


