Abstract
The present study sought to determine the interaction between the novelty-seeking trait and cocaine treatment on gene expression in the fibroblast growth factor (FGF) system. Specifically, we assessed the regulation of FGFR1 in response to cocaine in animals that were selectively bred on the basis of their locomotor response to a novel environment. High-responder (HR) rats are those that exhibit increased locomotor response and exploratory behavior in a novel environment and low-responder (LR) rats are those that exhibit lower levels of exploratory behavior and are less active. Both phenotypes received daily injections of either cocaine (15mg/kg, i.p.) or saline for seven consecutive days. Animals were sacrificed 45 minutes following their last injection and FGFR1 gene expression was assessed in the hippocampus and prefrontal cortex by mRNA in situ hybridization. HR-bred rats exhibited increased FGFR1 mRNA in the hippocampus compared to LR-bred rats. Furthermore, cocaine decreased FGFR1 mRNA in the hippocampus and increased FGFR1 mRNA in the prefrontal cortex. Finally, HR and LR rats differed in their response to cocaine between brain regions. In the hippocampus, cocaine decreased gene expression in HR-bred rats without affecting LR-bred rats, whereas in the prefrontal cortex cocaine increased gene expression in LR-bred rats without affecting HR-bred rats. These results suggest that cocaine interacts with the novelty-seeking trait to alter gene expression. Thus, the FGF system may contribute to individual differences in the response to drugs of abuse.
Keywords: Individual differences, fibroblast growth factor, hippocampus
The fibroblast growth factor system has previously been shown to be altered following cocaine and amphetamine exposure. Specifically, FGF2 was upregulated in several brain regions following both acute and chronic exposure [8, 9, 11]. Acute and chronic stress has also been shown to interact with cocaine to alter FGF2 gene expression and this interaction appears to be regionally selective [10]. Furthermore, FGF2 has been found to be required for the development of amphetamine sensitization [8]. These results suggest that FGF2 may be directly involved in the development of sensitization and may be a key factor underlying the molecular mechanisms of addiction.
Regulation of the FGF system at the receptor level has not yet been studied in response to drugs of abuse or in animals that differ in their drug-taking behavior. In order to address this question, we utilized an animal model that differs in novelty-seeking behavior. We have generated selectively bred rat lines that exhibit individual differences in response to a novel environment with some animals exhibiting high levels of locomotor activity and other animals exhibiting low levels of locomotor activity [17]. In outbred rats, high-responder animals exhibit an increased propensity to self-administer drugs of abuse, whereas low-responder animals exhibit a decreased propensity to self-administer drugs of abuse [13, 15, 16]. Furthermore, we have preliminary evidence suggesting that selectively bred HRs and LRs differ in cocaine sensitization and self-administration (Flagel et al., personal communication).
HR-bred animals also differ in their anxiety-like behavior and stress-responsiveness [3, 15]. Furthermore, the FGF system has been implicated in response to both acute and chronic stress [8]. Thus, the purpose of this study was to determine whether selectively bred HR and LR rats exhibit differences in FGFR1 gene expression. We also sought to determine whether cocaine treatment alters FGFR1 gene expression. To determine whether cocaine treatment interacts with the novelty-seeking trait to modulate gene expression, we assessed FGFR1 gene expression in selectively bred HR and LR rats following a regime of repeated cocaine treatment known to elicit behavioral sensitization.
Male Sprague-Dawley rats from generation nine of our HR/LR breeding colony (305–490g; University of Michigan, Ann Arbor, MI.) were housed under a 12 hr light/dark cycle with food and water available ad libitum. Sixteen HR and sixteen LR rats (approximately 6 months old) were used in this study. All rats were maintained at the University of Michigan animal facilities in accordance with the University Committee Use and Care of Animals. The experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). All efforts were made to minimize the number of animals used for this study.
HR and LR rats were screened for locomotor activity ten days prior to the initial drug injection as previously described [4]. HR and LR rats were administered either cocaine hydrochloride dissolved in 0.9% saline (15mg/kg, i.p.; n=8/group) or saline (i.p.; n=8/group) every 24 h for seven days. The cocaine sensitization paradigm used has been previously described and the dose and treatment regimen was appropriate to study individual differences in response to the drug [6, 7]. Rats were sacrificed 45 minutes following the last injection of either saline or cocaine.
For mRNA in situ hybridization, tissue was sectioned at −20°C at 10μm, sliced in series, mounted on SuperFrost Plus slides (FisherScientific) and stored at −80°C until processed. In situ hybridization methodology and analysis has been previously described elsewhere [13]. Sections were taken every 200μm. The sequence of rat mRNA used for generating the probe was complementary to the following RefSeq database no. FGFR1 (NM_024146, 320– 977). The probe was synthesized in our laboratory. All cDNA segments were extracted (Qiaquick Gel Extraction Kit, Qiagen, Valencia, CA), subcloned in Bluescript SK (Stratagene, LA Jolla,CA ) and confirmed by nucleotide sequencing. After seven days of exposure, the films were developed (Kodak D-19; Eastman Kodak, Rochester, NY, USA). Brain section images were captured from film with a CCD camera (TM-745, Pulnix) using MCID. Radioactive signals were quantified using computer-assisted optical densitometry software (Scion Image). Optical densities were determined by outlining the region of interest for the dorsal hippocampus. For the medial prefrontal cortex, a 20 pixel X 20 pixel box was placed over the region. Optical density measurements were corrected for background, and the signal threshold was defined as the mean gray value of background plus 3.5X its standard deviation. Only pixels with gray values exceeding the above-defined threshold were included in the analysis. Data from multiple sections per animal were averaged resulting in a mean integrated optical density value for each animal and then averaged for each group. Differences in FGFR1 gene expression were analyzed in each region by a two-way ANOVA followed by posthoc comparisons. A Student’s t-test was performed for locomotor activity in a novel environment. Data are presented as mean ± SEM.
As expected from previous generations, the selectively bred HR and LR rats exhibited significant differences in locomotor activity in response to a novel environment. Specifically, HR-bred rats (654 ± 40.5) exhibited significantly higher locomotor scores than LR-bred rats (324 ± 11.6) (p < 0.001). Although not assessed in this study, the selectively bred HR and LR rats would be expected to differ on the novelty-seeking dimension.
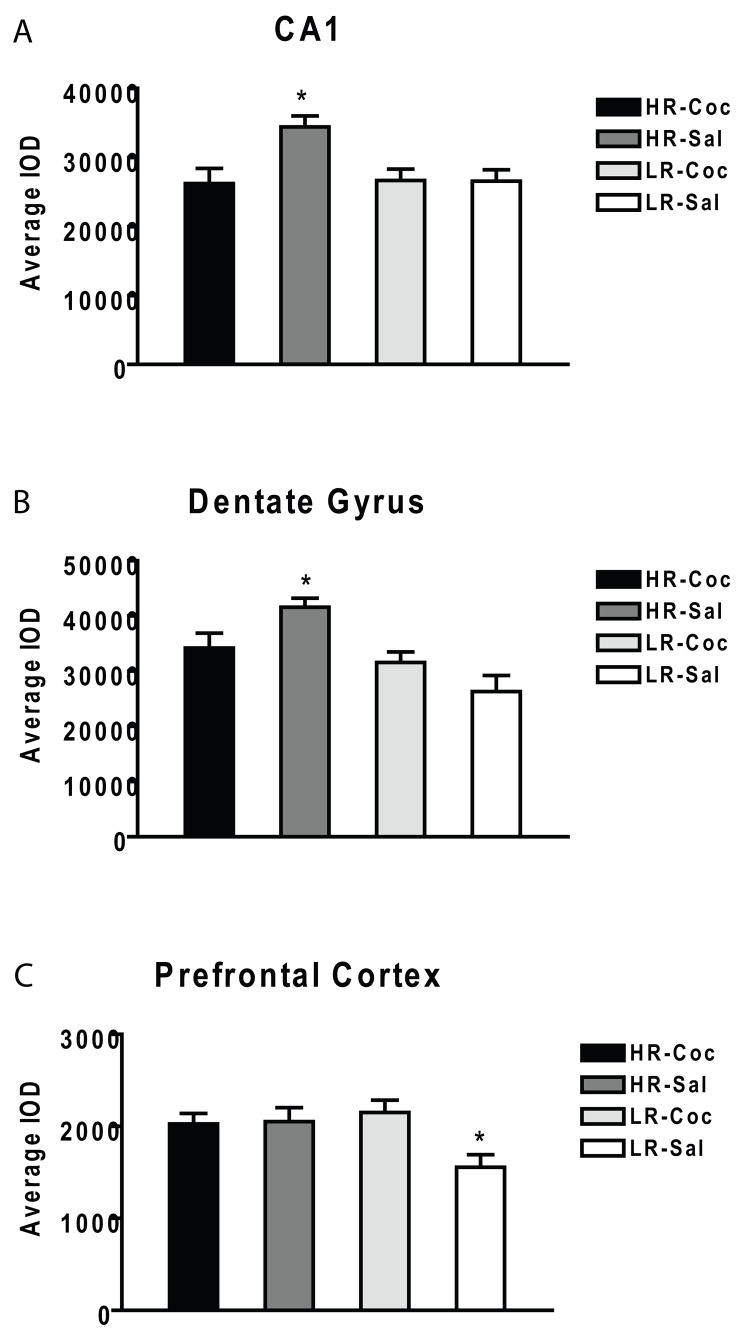
Figure 1 illustrates FGFR1 gene expression for the HR-bred and LR-bred rats by treatment in the hippocampal subfields. In CA1 of the hippocampus, there was a significant main effect of group, with the HR animals exhibiting higher expression of FGFR1 mRNA than LR animals (F1,31 = 4.31, p < 0.05). Furthermore, there was a significant main effect of treatment, with the cocaine animals exhibiting lower expression of FGFR1 mRNA than vehicle controls (F1,31 = 5.11, p < 0.05). Finally, there was a significant interaction between group and treatment (F1,31 = 5.36, p < 0.05). Here, the HR-Saline animals exhibited higher expression of FGFR1 mRNA than HR-Cocaine (p < 0.05), LR-Saline (p < 0.05) and LR-Cocaine (p = 0.005) animals, see Figure 2a.
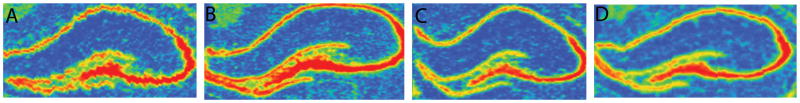
Figure 1. Representative FGFR1 mRNA in situ hybridization images in the hippocampus.
Red denotes a high level of gene expression in (A) HR-Cocaine (B) HR-Saline (C) LR-Cocaine (D) LR-Saline rats. Note that the highest gene expression is in CA2 and CA3 of the hippocampus.
Figure 2. Average integrated optical density of FGFR1 gene expression in various brain regions.
(A) Cocaine decreased FGFR1 mRNA in CA1 of the hippocampus in HR rats without affecting LR rats. (B) Cocaine decreased FGFR1 mRNA in the dentate gyrus in HR rats without affecting LR rats. (C) Cocaine increased FGFR1 mRNA in the prefrontal cortex in LR rats without affecting HR rats.
In the dentate gyrus of the hippocampus, there was a significant main effect of group, with the HR animals exhibiting higher expression of FGFR1 mRNA than LR animals (F1,31 = 14.31, p < 0.001). There was no significant main effect of treatment (F1,31 = 0.21, p = 0.65). However, there was a significant interaction between group and treatment (F1,31 = 7.22, p < 0.05). Here, the HR-Saline animals exhibited higher expression of FGFR1 mRNA than HR-Cocaine (p < 0.05), LR-Saline (p < 0.001) and LR-Cocaine (p < 0.05) animals, see Figure 2b.
In the prefrontal cortex, there was a significant main effect of treatment (F1,31 = 4.39, p < 0.05), with the cocaine animals exhibiting higher expression of FGFR1 mRNA than vehicle controls. There was no significant main effect of group (F1,31 = 1.84, p = 0.19). However, there was a significant interaction between group and treatment (F1,31 = 5.17, p < 0.05). Here, the LR-Saline animals exhibited lower expression of FGFR1 mRNA than LR-Cocaine (p < 0.05), HR-Saline (p < 0.05) and HR-Cocaine (p < 0.05) animals, see Figure 2c.
These results suggest that animals selectively bred for differences in the novelty-seeking trait exhibit differences in FGFR1 gene expression. Furthermore, HR-bred animals exhibited higher FGFR1 mRNA expression in the hippocampus than LR-bred animals. In outbred HR and LR rats, several other studies have also shown alterations in gene expression. For example, corticotrophin-releasing hormone (CRH) gene expression was increased in the paraventricular nucleus of the hypothalamus and glucocorticoid receptor (GR) gene expression was decreased in the hippocampus of HRs compared to LRs [13]. Furthermore, gene expression differences have been characterized in HR and LR rats following psychosocial stress. For example, LR rats show increased Ca+/calmodulin kinase IIb (CAMKIIb) gene expression in the hippocampus following social defeat [14]. More recently, differences in serotonin (5-HT) receptors and the cholecystokinin (CCK) system have been shown to be altered in HR and LR rats. HR rats show increased CCK gene expression in the hippocampus compared to LR rats, whereas HR rats have decreased 5-HT7 receptor gene expression compared to LR rats [1, 2].
The results also suggest that cocaine alters FGFR1 mRNA differentially depending on the region. Cocaine increased FGFR1 mRNA in the prefrontal cortex and decreased FGFR1 mRNA in the hippocampus. Previous studies have found that both acute and chronic cocaine increased FGF2 gene expression in various brain regions [11]. Our study suggests that the receptor may respond differentially to cocaine treatment between regions. However, we cannot differentiate between the acute and chronic treatment effects on FGFR1 gene expression in the present study.
Finally, cocaine and novelty-seeking behavior interact to modulate FGFR1 gene expression. This modulation also differed depending on the region. In the hippocampus, cocaine decreased gene expression in HRs without affecting LRs, whereas in the prefrontal cortex cocaine increased gene expression in LRs without affecting HRs. A previous study showed stress and cocaine to interact and modulate FGF2 gene expression [10]. Similar to the above-mentioned study, the interaction between cocaine and the novelty-seeking trait was regionally selective.
The possibility exists that an increased tone in the FGF system may lead to an increase in drug-taking behavior. This has recently been demonstrated with another growth factor, brain-derived neurotrophic factor (BDNF) [12]. Rats that received infusions of BDNF into the nucleus accumbens exhibited an increase in cocaine self-administration. Moreover, the higher levels of FGFR1 in the hippocampus found in HR-bred rats relative to LR-bred rats in the current study may contribute to the enhanced propensity to self-administer drugs. Although recent evidence suggests that the HR/LR phenotype has little to do with the transition to addiction [3, 5], most would agree that there are distinct differences between these phenotypes in initial drug-taking behavior [3]. Thus, although it is likely a combination of traits (e.g. novelty-seeking, impulsivity) that contribute to substance abuse vulnerability, our results suggest that the FGF system plays a prominent role in mediating the initial propensity to take drugs and studies are currently underway to determine if this is indeed the case. Future studies should also determine whether FGFR1 gene expression is altered in the mesolimbic system (e.g. nucleus accumbens and ventral tegmental area) following a sensitizing regimen of cocaine.
In summary, cocaine altered FGFR1 gene expression in a regionally selective manner. Selectively bred rats that have increased novelty-seeking behavior also exhibited increased FGFR1 gene expression. Furthermore, cocaine and novelty-seeking behavior interact to regulate gene expression, and this regulation differed depending on the region. Thus, FGFR1 represents a novel therapeutic target in both the vulnerability to drug abuse and the response to drugs of abuse.
Acknowledgments
This work was supported by NIDA Program Project Grant # 5P01DA021633-02, NIDA Training Grant #5T32DA007268-14 and #5T32DA007267-13, NIH Conte Center Grant #L99MH60398 and NIMH Program Project Grant #MH42251-01. We would also like to acknowledge Sharon Burke, Jennifer Fitzpatrick, Tracy Simmons and James Stewart for their technical assistance and expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballaz SJ, Akil H, Watson SJ. Analysis of 5-HT6 and 5-HT7 receptor gene expression in rats showing differences in novelty-seeking behavior. Neuroscience. 2007;147:428–38. doi: 10.1016/j.neuroscience.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Ballaz SJ, Akil H, Watson SJ. The CCK-system underpins novelty-seeking behavior in the rat: Gene expression and pharmacological analyses. Neuropeptides. 2008;42:245–53. doi: 10.1016/j.npep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–64. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 6.Flagel SB, Robinson TE. Quantifying the psychomotor activating effects of cocaine in the rat. Behav Pharmacol. 2007;18:297–302. doi: 10.1097/FBP.0b013e3281f522a4. [DOI] [PubMed] [Google Scholar]
- 7.Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J Neurosci. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berlin) 2000;151:152–65. doi: 10.1007/s002130000417. [DOI] [PubMed] [Google Scholar]
- 10.Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Stress and cocaine interact to modulate basic fibroblast growth factor (FGF-2) expression in rat brain. Psychopharmacology (Berl) 2008;196:357–64. doi: 10.1007/s00213-007-0966-x. [DOI] [PubMed] [Google Scholar]
- 11.Fumagalli F, Pasquale L, Racagni G, Riva MA. Dynamic regulation of fibroblast growth factor 2 (FGF-2) gene expression in the rat brain following single and repeated cocaine administration. J Neurochem. 2006;96:996–1004. doi: 10.1111/j.1471-4159.2005.03627.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 13.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabbaj M, Evans S, Watson SJ, Akil H. The search for the neurobiological basis of vulnerability to drug abuse: using microarrays to investigate the role of stress and individual differences. Neuropharmacology 47 Suppl. 2004;1:111–22. doi: 10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berlin) 2001;158:382–7. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- 16.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 17.Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]