Abstract
BACKGROUND
Prostate cancer is the second leading cause of cancer death in men, and early detection is essential to reduce mortality and increase survival. δ-Catenin is a unique β-catenin superfamily protein primarily expressed in the brain but is upregulated in human prostatic adenocarcinomas. Despite its close correlation with the disease, it is unclear whether δ-catenin presents the potential in prostate cancer screening because it is an intracellular protein. In this study, we investigated the hypothesis of δ-catenin accumulation in the urine of prostate cancer patients and its potential pathways of excretion into extracellular milieu.
METHODS
Prostate cancer cell cultures, human tissue biopsies, and voided urines were characterized to determine extracellular δ-catenin accumulation and co-isolation with exosomes/prostasomes.
RESULTS
We identified δ-catenin in culture media and in the stroma of human prostate cancer tissues. In PC-3 cells in culture, δ-catenin was partially co-localized and co-isolated with raft-associated membrane protein caveolin-1 and glycosylphosphatidylinositol-anchored protein CD59, suggesting its potential excretion into extracellular milieu through exosome/prostasome associated pathways. Interference with endocytic pathway using wortmannin did not block prostasome excretion, but δ-catenin overexpression promoted the extracellular accumulation of caveolin-1. δ-Catenin, caveolin-1, and CD59 were all detected in cell-free human voided urine prostasomes. δ-Catenin immunoreactivity was significantly increased in the urine of prostate cancer patients (p<0.0005).
CONCLUSIONS
This study demonstrated, for the first time, the extracellular accumulation of δ-catenin in urine supporting its potential utility for non-invasive prostate cancer detection.
Keywords: prostate cancer detection, δ-catenin, caveolin-1, CD59, prostate specific antigen
INTRODUCTION
Prostate cancer is the most prevalent noncutaneous cancer and the second leading cause of cancer death in men in the United States [1]. Over the past 20 years, the 5-year survival rate for prostate cancer has increased significantly due in large part to the availability of serum screening marker prostate specific antigen (PSA). This has led to the early detection of many prostate cancers at the local and regional stage. However, accumulating data has shown that serum PSA levels could be elevated as a result of conditions other than prostate cancer, such as benign prostatic hyperplasia (BPH). Consequently, false positives can be a significant problem for the PSA test and can lead to unnecessary biopsies and other interventions [for review, see Ref. 2]. In addition, preoperative serum PSA levels do not always correlate with cancer volume or the Gleason score of radical prostatectomy specimens [3]. Therefore, additional biomarkers, if proven better, are needed to either assist or replace PSA-based detection as more reliable prostate cancer indicators.
δ-Catenin is an adhesive junction associated protein [4, 5] in the β-catenin superfamily [6]. While β-catenin is ubiquitously expressed in the body, δ-catenin was initially identified as a neural specific protein in the brain [7, 8]. δ-Catenin distribution pattern is closely associated with F-actin [9, 10] and interacts with actin directly [10]. Although δ-catenin distribution is principally restricted to the brain in healthy individuals, it has become clear that δ-catenin is expressed in a variety of cancers of peripheral tissues, including breast, prostate, and esophageal tumors [11, 12]. Using quantitative PCR analysis, Burger et al [13] showed that δ-catenin mRNA is overexpressed in prostate cancer compared to BPH. At the protein level, we employed tissue microarray (TMA) and demonstrated that δ-catenin is upregulated in over 80% of prostatic adenocarcinomas, and its expression is correlated with increasing Gleason scores [12]. Our study showed that an increased expression of δ-catenin is accompanied by a reduction of tumor suppressor E-cadherin in primary prostatic adenocarcinomas, and the forced overexpression of δ-catenin in culture induced the redistribution of E-cadherin [12], supporting the potential roles of δ-catenin in interfering epithelial cell junctions and prostate cancer development.
Despite its close correlation with the disease, it is unclear whether δ-catenin can be useful in prostate cancer screening and detection since δ-catenin is predicted to be a cytoplasmic, cell junction associated protein. In this study, we demonstrate, for the first time, that δ-catenin was detected in the culture medium when it was overexpressed and was detectable in the stroma of human prostate cancer tissues. δ-Catenin was partially co-localized and co-isolated with caveolin-1 and CD59 and promoted the extracellular accumulation of caveolin-1. Significantly, δ-catenin immunoreactivity in cell-free human voided urines was increased in prostate cancer patients.
MATERIALS AND METHODS
Materials
Human voided urine specimens were collected at the Leo Jenkins Cancer Center of East Carolina University Brody School of Medicine and at the Vanderbilt University Medical Center. The clinical and pathological records were analyzed to determine the presence or absence of prostate cancer. Sample collection and analysis were performed according to the approved Institutional Research Board (IRB) protocols of both institutions. PSA scores and Gleason grades of patients were recorded. Mouse anti-δ-catenin and anti-caveolin-1 were from BD Biosciences. Mouse monoclonal anti-δ-catenin (J19) was a kind gift from Dr. Werner Franke (German Cancer Research Center, Heidelberg, Germany). Rabbit anti-δ-catenin was produced as described [4] while rabbit anti-caveolin-1 was from Cell Signaling. Mouse and rabbit anti-CD59 were kind gifts of Dr V. Horejsi (Institute of Molecular Genetics, Prague, Czech Republic). Mouse anti-actin was from Calbiochem. Unless otherwise indicated, all other chemicals were from Sigma.
Cell Culture and Transfection
Human prostate cancer cell lines CWR22Rv-1 [14] and PC-3 [15] were obtained from ATCC and cultured in RPMI 1640 medium (Invitrogen) supplemented with 10 % fetal bovine serum (FBS). Rat PC12 cells were cultured as described [16]. NIH3T3 fibroblast cells were cultured in DMEM F12 medium with 10% FBS. Full-length δ-catenin cDNA [10] was transfected using FuGENE 6 (Roche). Cells transfected with pEGFP (Clontech) were used as a vector control. For selection of pEGFP-δ-catenin transfected cells, cells were first incubated in G418 containing medium. Then, they were further selected by GFP-based cell sorting using a FACS Vantage (BD Biosciences). The stable cell lines were maintained in the medium containing G418.
Isolation of Prostasomes in Cultured Cells and Voided Human Urines
Prostasomes were isolated from the culture medium of cells incubated without serum for overnight. Cell death, as determined by trypan blue staining, was not observed under these conditions. The medium was centrifuged to remove cell debris first at 400 g and then at 10,000 g. For isolation of urine prostasomes, voided urines from human prostate cancer and benign subjects were obtained and were similarly centrifuged to remove aggregates. The supernatants were then centrifuged at 100,000 g to sediment prostasomes essentially as described [17, 18]. Isolated prostasome pellets and the supernatants were analyzed by SDS-PAGE and Western blotting.
Immunoprecipitation and Western Blots
Cultured cells were lysed in 10 mM HEPES, pH 7.3, 150 mM NaCl, 2mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 0.2% SDS with protease inhibitor cocktails. For human benign and prostate tumor tissues, cell lysates were prepared from the needle biopsies of frozen peripheral zones of prostatic acinar tissues as described [12]. The lysates were either analyzed directly by SDS-PAGE or by immunoprecipitation with anti-δ-catenin. The immunoprecipitates captured by protein G beads were subject to SDS-PAGE followed by transfer to nitrocellulose membranes (PGC Scientifics). Western blotting was performed using enhanced chemiluminescence (Amersham) for detection, and the results were analyzed by semi-quantification using ChemiDoc (BioRad).
Immunofluorescent Light Microscopy
Cultured cells were fixed in 4 % paraformaldehyde and permeabilized with 0.2% Triton X-100. They were stained with rhodamine phalloidin (Molecular Probes) to detect F-actin or immunostained with anti-caveolin-1 or anti-CD59 to compare their distributions with that of EGFP-δ-catenin. The cells were analyzed with a Zeiss Axiovert inverted fluorescent light microscope (Carl Zeiss) equipped with MetaMorph software (Molecular Devices).
Electron Microscopy
Cultured 3T3 cells overexpressing vector alone or pEGFP-δ-catenin were fixed in 4% paraformaldehyde and 2% glutaraldehyde. The cells showing green fluorescence were identified under the light microscope and the area was circled. The cells were then immunostained using rabbit anti-δ-catenin followed by HRP-conjugated secondary antibody labeling. The fluorescent cells showing positive HRP were marked and embedded in Epon. After the block was trimmed to retain only the areas containing a single fluorescent and HRP positive cell, ultrathin sections were cut and examined under the JEOL 100CX II transmission electron microscope. Isolated prostasomes were placed on the formvar-carbon coated grids and negatively stained before being analyzed by whole-mount electron microscopy.
Statistic Analysis
The comparisons of immunoscores for δ-catenin Western blot of urine samples from prostate cancer patients and control subjects were performed using SigmaPlot (SPSS Science). Student t-tests or one-way ANOVA were conducted and p-values were assigned. True positive, true negative, false positive, and false negative were defined as described previously [12].
RESULTS
Identification of δ-catenin in culture medium in vitro and in extracellular milieu of prostate cancer tissues in vivo
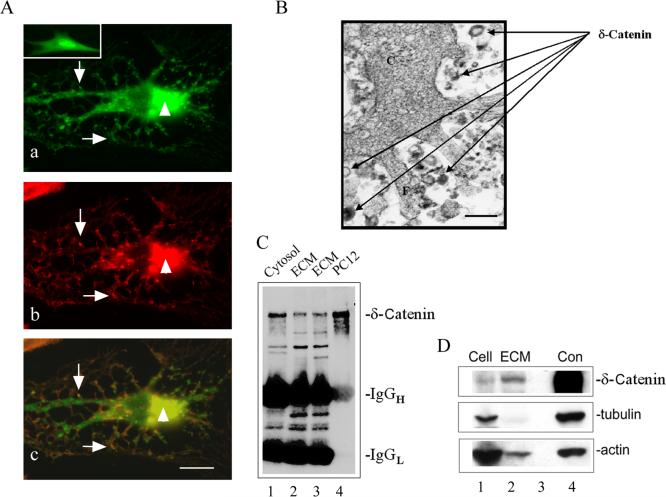
When 3T3 cells overexpressing δ-catenin were examined, two striking morphological features were observed. First, the cell shape was dramatically changed, and the cells formed many protrusions with branches (Fig 1A, a) when compared to control untransfected cells (Fig 1A, a. see insert). This result was consistent with our previous report [10]. Second, both δ-catenin and F-actin showed strong clustering (Fig 1A, a and b), and they were co-localized at many subcellular locations (Fig 1A, a, b and c, arrows and arrowheads). Because δ-catenin sequence indicated its ability for lipid interaction [10], these observations prompted the hypothesis that δ-catenin overexpression may lead to its close interaction with the plasma membrane and excretion from the cells. We first tested this possibility by examining the ultrastructural features of δ-catenin overexpressing cells fixed in culture. Immunohistochemistry combined with thin section electron microscopy showed that anti-δ-catenin immunoreactivities were associated with multilaminated vesicles shedding from the cells (Fig 1B, arrows, see also Ref.10). To confirm that δ-catenin was present extracellularly, culture medium of PC12 cells overexpressing δ-catenin was immunoprecipitated and Western blotted with anti-δ-catenin. Figure 1C showed that δ-catenin was clearly detected in the cells (lane 1: Cytosol) as well as in the culture medium (lane 2 and 3: ECM). Our previous studies showed that δ-catenin is overexpressed in prostatic adenocarcinomas [12]. To determine if δ-catenin can also be detected in the extracellular space in vivo, samples of needle biopsy of prostate cancer patients were examined. Western blots showed that δ-catenin was detected not only in the cell-enriched tumor biopsies (Fig 1D, lane 1), but also in the biopsies lacking cell mass (Fig 1D, lane 2), indicating that δ-catenin is present in the stroma of prostatic tissues.
Figure 1.
δ-Catenin accumulation in culture medium and in the stroma of prostate cancer tissue. A. Co-distribution of overexpressed δ-catenin with F-actin in NIH3T3 cells. a. Localization of δ-catenin to perinuclear area (arrowhead) and to the cellular protrusions (arrows). Insert: Morphology of a vector transfected cell. b. Localization of F-actin was similar to that of δ-catenin. c. Merged image of a and b showing partial co-localization of δ-catenin with F-actin (arrowheads and arrows). Bar: 10 μm. B. Thin section immunoelectron microscopy showing the shedding of vesicles immunoreactive for anti-δ-catenin staining in NIH3T3 cells. C: cytoplasm. F: filapodium. Arrows: multilaminated vesicles immunoreactive for anti-δ-catenin. Bar: 100 nm. C. Immunoprecipitated δ-catenin in the cytosol (lane 1) as well as in the culture medium (lane 2 and lane 3) of PC12 cells overexpressing δ-catenin. ECM: extracellular medium. Lane 4: Western blot of whole cell lysates of PC12 cells overexpressing δ-catenin. D. Western blot showing δ-catenin expression in prostate cancer cells and stroma in vivo. Note δ-catenin immunoreactivities in cells (Lane 1) and extracellular matrix (ECM, lane 2). Lane 3: Empty lane to separate lane 2 and 4. Lane 4: Neuronal lysate δ-catenin immunoreactivity shown as a positive control. Upper panel: anti-δ-catenin; middle panel: anti-tubulin; lower panel: anti-actin. Diminished tubulin and actin indicated the lack of cell mass in lane 2.
δ-Catenin partially co-distributed and co-isolated with caveolin-1 and CD59 in prostate cancer PC-3 cells
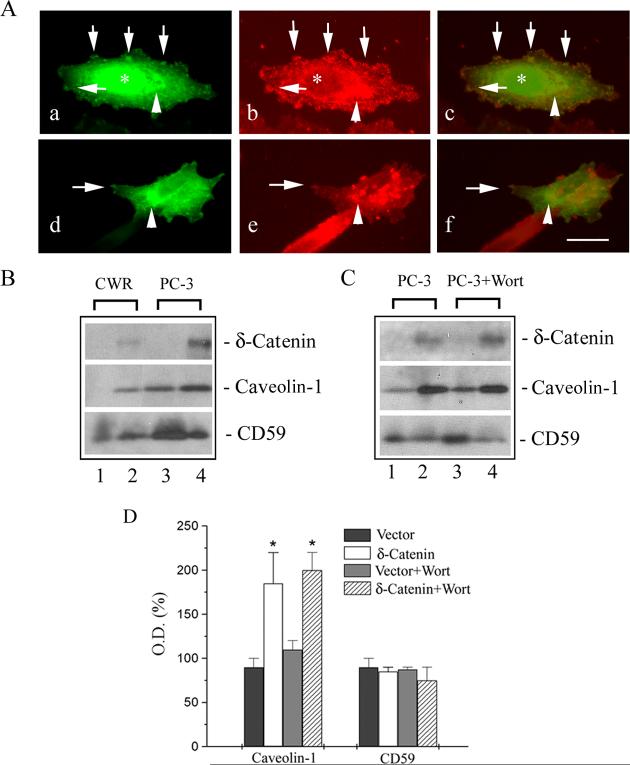
Prostasomes are the extracellular vesicles similar to the intensely studied exosomes and are excreted from prostate cancer cell lines such as PC-3 [18]. To test if δ-catenin is released into extracellular space through exosomal excretion, we first compared the distribution of δ-catenin with caveolin-1 and CD59, both of which are components of prostasomes in PC-3 cell culture medium [18]. Immunofluorescent light microscopy showed that δ-catenin was distributed throughout the PC-3 cells, but was more intensely localized at the perinuclear regions (Fig 2A, a and c, arrowheads). δ-Catenin was also labeled as small clusters at the cell periphery (Fig 2A, a and c, arrows). In addition, some cells displayed nuclear distribution of δ-catenin (Fig 2A, a and c, asterisk). Caveolin-1 was also localized at the perinuclear region (Fig 2A, b and c, arrowheads). In addition, it was labeled as many patchy, small clusters like that of δ-catenin (Fig 2A, b and c, arrows), albeit they were more widely distributed throughout the cell. Nuclear distribution of caveolin-1 was lacking. Merged fluorescent images showed that δ-catenin and caveolin-1 were partially co-localized at the perinuclear region and to the small clusters at the cell periphery (Fig 2A, c, arrowheads and arrows). CD59 distribution was quite distinct and was highly concentrated in clusters (Fig 2A, e, arrows). Some of the small clusters were co-localized with that of δ-catenin (Fig 2A, d and f, arrows), although their co-localization was not as extensive as compared to caveolin-1 with δ-catenin. There was also some perinuclear co-localization (Fig 2A, d, e and f, arrowheads).
Figure 2.
δ-Catenin co-distribution and co-isolation with caveolin-1 and CD59. A. Partial co-distribution of δ-catenin with caveolin-1 and CD59 in PC-3 prostate cancer cells in culture. a: δ-catenin; b: caveolin-1; c: merged image of a and b. d: δ-catenin; e: CD59; f: merged image of d and e. Arrows point to co-localization of δ-catenin with that of caveolin-1 and CD59. Arrowheads point to perinuclear region. Asterisks indicate nucleus. Bar: 15 μm. B. Western blot showing accumulation of δ-catenin, caveolin-1, and CD59 in prostasomes isolated from the culture media of prostate cancer cells. Upper panel: anti-δ-catenin. Middle panel: anti-caveolin-1. Lower panel: anti-CD59. Lane 1: CWR22Rv-1 cell expressing vector control; lane 2: CWR22Rv-1 cell overexpressing δ-catenin; lane 3: PC-3 cell expressing vector control; lane 4: PC-3 cell overexpressing δ-catenin. C. δ-Catenin overexpression associated with increased secretion of caveolin-1. Upper panel: anti-δ-catenin. Middle panel: anti-caveolin-1. Lower panel: anti-CD59. Lane 1: PC-3 cell expressing vector control; lane 2: PC-3 cell overexpressing δ-catenin; lane 3: Control PC-3 cells treated with wortmannin; lane 4: δ-Catenin overexpressing PC-3 cells treated with wortmannin. D. Densitometrical semi-quantification of caveolin-1 and CD59 accumulation with or without δ-catenin expression, and with or without treatment of wortmannin. Wort: Wortmannin. *: p<0.05.
We then examined if δ-catenin can be recovered from the prostasomes in prostate cancer CWR22Rv-1 and PC-3 cell culture medium. Endogenous δ-catenin expression level was moderate in CWR22Rv-1 and PC-3 cells [19] and was not readily detectable in the culture medium (Fig 2B, upper panel: lane 1 and 3). Overexpressed δ-catenin, however, can be detected in the prostasomes isolated from the culture medium (Fig 2B, upper panel: lane 2 and 4). Caveolin-1 expression was low in CWR22 prostate tumor derived cell lines and was not detectable in the medium [20]. Indeed, we were not able to detect caveolin-1 in the culture medium of CWR22Rv-1 cells (Fig 2B, middle panel: lane 1). However, δ-catenin overexpression in CWR22Rv-1 cells resulted in the detectable extracellular caveolin-1 (Fig 2B, middle panel: lane 2). Caveolin-1 was reported to be present in the prostasomes isolated from the culture medium of PC-3 cells [18]. Certainly, caveolin-1 was detected under this condition (Fig 2B, middle panel: lane 3). Here, δ-catenin overexpression further increased caveolin-1 detection (Fig 2B, upper panel: lane 4 and Fig 2D). CD59 was detectable in both CWR22Rv-1 cells (Fig 2B, lower panel: lane 1) and PC-3 cells (Fig 2B, lower panel: lane 3), although the effects of δ-catenin overexpression on CD59 accumulation in the prostasomes were quite variable (Fig 2B, lower panel: compare lane 1 with 2, and lane 3 with 4, respectively) and were not statistically significant (Fig 2D).
Wortmannin is an inhibitor of phosphatidylinositol-3 kinase (PI3K) and inhibits the formation of multivesicular bodies/late endosomes [21]. Wortmannin was reported to reduce caveolin-1 released in prostasome-like granules [18]. However, in our experiments, wortmannin did not reduce δ-catenin excretion (Fig 2C, upper panel: compare lane 2 with lane 4; Fig 2D). Caveolin-1 did not show reductions in excretion, either, (Fig 2C, middle panel: compare lane 1 with lane 3, and lane 2 with lane 4; Fig 2D). δ-Catenin overexpression resulted in a moderately reduced CD59 when treated with wortmannin (Fig 2C, lower panel: compare lane 2 and lane 4; Fig 2D). δ-Catenin overexpression, on the other hand, increased caveolin-1 but not CD59 accumulation in prostasomes (Fig 2C, and Fig 2D), confirming the results of Fig 2B in the independent experiments.
Identification of δ-catenin, caveolin-1, and CD59 in human voided urines
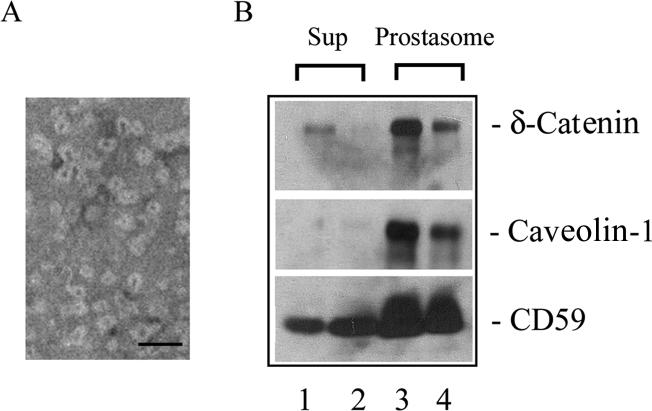
Caveolin-1 and CD59 were previously reported to be present in prostasomes in vivo [18], and exosomes were detected in human urines [22]. We therefore prepared prostasomal fractions from the voided urine of prostate cancer patients. Negative staining whole mount electron microscopy confirmed the near homogenous isolation of prostasomal vesicles of 35∼70 nm in size (Fig 3A), as reported [22]. The immunoreactivities of δ-catenin and CD59, but not caveolin-1, were detected in the cell free supernatant of voided urine (Fig 3B, lane 1 and 2). However, the immunoreactivities of δ-catenin, as well as that of caveolin-1 and CD59, were all detected in the prostasomal fractions of the urines (Fig 3B, lane 3 and 4), raising intriguing possibilities that all of them may be potential urine indicators of prostate cancer.
Figure 3.
Detection of δ-catenin, caveolin-1, and CD59 in human voided urines. A. Negative staining electron microscopy showing prostasomal vesicles isolated from prostate cancer patient voided urines. Bar: 100 nm. B. Detection of δ-catenin, caveolin-1, and CD59 in urine supernatants and prostasomes from prostate cancer patients. Lane 1 and 2, supernatants from two separate urine specimens after centrifugation at 100,000 g; lane 3 and 4: prostasomes isolated from the above two separate urine specimens. Sub: 100, 000 g supernatant.
Increased δ-catenin accumulation in urines is correlated with prostate cancer
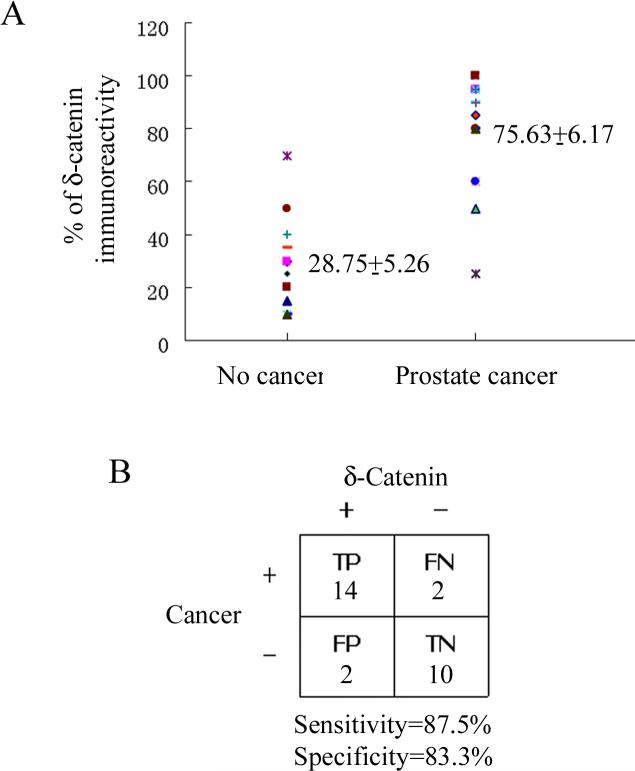
We investigated 31 cases of patients with or without active prostate cancer (Table I). Gleason scores were used to confirm prostatic adenocarcinomas in all but one case. We analyzed 28 cases of voided urines using immunoscore to determine whether δ-catenin expression is increased in prostate cancer (Table I.). Active disease referred to the clinically and pathologically confirmed prostate cancer. Western blots of anti-δ-catenin immunoprecipitated urine samples showed an immunoscore of 28.75±5.26 for the group without the disease and 75.63±6.17 for the presence of disease (Fig 4A, p<0.0005). This result indicated that δ-catenin was not only detectable in voided urine but also showed an increased expression in prostate cancer patients compared to control subjects without active disease. In this patient group, when we applied δ-catenin immunoscore 45 (Table I) as a reference point, 87.5% sensitivity and 83.3% specificity were obtained (Fig 4B). These results indicated that given the variability of PSA scores in this patient group, including δ-catenin immunoscores would present a notable improvement (Table I).
Table I.
Comparison of test values of PSA, Gleason score and δ-catenin immunoscore to the clinically and pathologically confirmed presence or absence of active prostate cancer.
| Case # | Prostate Cancer | Gleason Score | PSA | δ-Catenin immunoscore |
|---|---|---|---|---|
| 1 | Active | 3+3=6 | 5.4 | 85 |
| 2 | Active | 4+3=7 | 667 | 95 |
| 3 | Active | Poorly differentiated | 4.9 | 50 |
| 4 | Active | 3+4=7 | 0.9 | 95 |
| 5 | Active | 3+3=6 | 3.1 | 25 |
| 6 | Active | 5+3=8 | 0.3 | 80 |
| 7 | Active | 3+3=6 | N/A | 95 |
| 8 | Active | 3+3=6 | N/A | 80 |
| 9 | Active | 4+4=8 | 0.4 | 90 |
| 10 | Active | 4+3=7 | 3.4 | 100 |
| 11 | Active | 3+3=6 | 4.3 | 100 |
| 12 | Active | 3+3=6 | 4.8 | 80 |
| 13 | Active | 3+3=6 | 6.0 | 25 |
| 14 | Active | 3+4=7 | 16.1 | 60 |
| 15 | Active | 4+3=7 | 8.9 | 60 |
| 16 | Active | 3+3=6 | 16.2 | 90 |
| 17 | Inactive | 3.6 | 30 | |
| 18 | Inactive | 6.6 | 30 | |
| 19 | Inactive | 0.2 | 15 | |
| 20 | Inactive | 0.1 | 10 | |
| 21 | Inactive | 0.1 | 70 | |
| 22 | Inactive | N/A | 50 | |
| 23 | Inactive | 5.5 | N/A | |
| 24 | Inactive | 5.3 | N/A | |
| 25 | Inactive | 127.7 | N/A | |
| 26 | Inactive | N/A | 40 | |
| 27 | Inactive | 0.6 | 10 | |
| 28 | Inactive | 1.9 | 35 | |
| 29 | Inactive | N/A | 25 | |
| 30 | Inactive | N/A | 20 | |
| 31 | Inactive | 0.2 | 10 |
Note: N/A-not available
Figure 4.
Increased δ-catenin accumulation in urines correlated with prostate cancer. A. Scatter plot comparing δ-catenin immunoscore of prostate cancer urine specimens with that of control subjects. δ-Catenin immunoscores were determined by semi-quantitatively measuring the anti-δ-catenin immunoreactivities of Western blots. The strongest immuno-intensity of δ-catenin was designated as 100%. Total of 28 cases of voided urines from patients with or without active disease were analyzed. P<0.0005. B. The 2-by-2 table showing the relationship between δ-catenin immunoscores and the presence of prostate cancer. The immunoscore 45 was used as reference point to provide the optimal differentiation of presence or absence of prostate cancer. TP: true positive. FP: false positive. TN: true negative. FN: false negative. Specimens showing anti-δ-catenin immunoscores above 45 were considered true positive for cancer when clinical and pathological data indicated the active disease. They were considered false positive when the disease was excluded. The specimens showing anti-δ-catenin immunoscores equal or below 45 were considered as true negative if clinical and pathological date documented no disease, while they were considered as false negative if the disease was documented.
DISCUSSION
δ-Catenin is a neural specific protein that initially was found only in brain with a trace amount in the pancreas [8]. The completion of human genome project provided access to EST database, which indicated δ-catenin expression in cancer of peripheral tissues. Burger et al. [13] found δ-catenin transcription upregulation in prostate cancer compared to BPH. Lu et al. [12] employed TMA and demonstrated that δ-catenin protein was overexpressed in prostatic adenocarcinoma. These studies established the close relationship between δ-catenin overexpression and the disease, although its potential utility for prostate cancer screening and detection was unclear. Our current study demonstrated that δ-catenin can be detected in the culture medium when it is overexpressed, is detectable in human prostate cancer stroma tissues, and is present in the voided urines in a notable association with the disease occurrence.
It is possible that δ-catenin is excreted into the extracellular milieu via active mechanism(s). δ-Catenin is partially co-distributed with F-actin and directly binds to G-actin [10]. Actin interacting proteins are known to be released into extracellular space. For example, thymosin beta 15 (Tb15) regulates prostate cancer cell movement. While normal cells and early cancer cells do not express Tb15, its levels begin to rise, and the cells are able to move faster as cells become metastatic [23]. Tb15 was reported as a potential urine biomarker for prostate cancer [24]. Another study pointed out that a Tb15 homologue called TbNB has higher affinity for actin than that of Tb15 and is upregulated in prostate cancer [25]. Therefore, an array of proteins interacting with the actin network may be subject to excretion from prostate cancer cells and can be detected in the urine.
It would be important in the future to determine how these actin binding proteins enter the extracellular space. We showed that one possible route is through exosomal/prostasomal release. Caveolin-1 and CD59 are known to be secreted by this mechanism and are associated with prostasomes [18]. Caveolin is a 21−24 kDa integral membrane protein present in caveolae with its primary function in membrane trafficking and cholesterol transport. Caveolin-1 promotes IGF-I-induced cancer cell migration through actin cross-linking protein filamin A [26]. Interestingly, the elevated caveolin-1 expression and its secretion stimulate prostate cancer cell survival and contribute to metastasis [27]. Relevant to our findings, serum caveolin-1 was shown to be a potential biomarker for prostate cancer [28].
Supporting the literature, the partial co-localization and co-isolation of δ-catenin with caveolin-1 may indicate their potential interactions, which should be addressed in the future. δ-Catenin also promoted the accumulation of caveolin-1 in prostasomes in PC-3 cell culture medium, suggesting that they could enter the extracellular space via exosomal pathway. However, wortmannin, postulated to inhibit endocytosis and perhaps exosomal release of caveolin-1 [18], did not block δ-catenin and caveolin-1 accumulation in prostasomes. While CD59 was reduced moderately upon treatment with wortmannin, the co-distribution of δ-catenin with CD59 was not as extensive as compared to that of δ-catenin with caveolin-1. This result indicated that some δ-catenin and caveolin-1 may be excreted into the medium using other routes in addition to exosomal pathway. Our future studies will investigate these potential possibilities.
Prostate cancer screening has been greatly improved in recent years largely because of the discovery that elevated PSA values indicate the disease. However, due to the clinically high false positive and false negative detection with PSA, additional biomarkers for prostate cancer detection and screening would improve the accuracy of diagnosis. Our current study provided the first report that, like Tb15 and caveolin-1, δ-catenin may be another potential prostate cancer biomarker with actin interacting capability.
ACKNOWLEDGEMENT
We thank Dr. Robert Matusik for constructive suggestions, Melissa Clark and Christi Boykin for excellent technical assistance, and Lu laboratory members for many helpful discussions. This study was supported by NIH/NCI (CA111891) and the Department of Defense (PC040569) grants (Q.L.).
The abbreviation used are
- PSA
prostate specific antigen
- BPH
benign prostate hyperplasia
- TMA
tissue microarray
- TP
true positive
- FP
false positive
- TN
true negative
- FN
false negative
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res. 2004;10(12 Pt 1):3943–53. doi: 10.1158/1078-0432.CCR-03-0200. Review. [DOI] [PubMed] [Google Scholar]
- 3.Stamey TA, Johnstone IM, McNeal JE, Lu AY, Yemoto CM. Preoperative serum prostate specific antigen levels between 2 and 22 ng./ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng./ml. J Urol. 2002;167(1):103–11. [PubMed] [Google Scholar]
- 4.Lu Q, Paredes M, Medina M, et al. Delta-catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol. 1999;144:519–32. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho C, Zhou J, Medina M, et al. Delta-catenin is a nervous system-specific adherens junction protein which undergoes dynamic relocalization during development. J Comp Neurol. 2000;420:261–76. [PubMed] [Google Scholar]
- 6.Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76(5):789–91. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 7.Paffenholz R, Franke WW. Identification and localization of a neurally expressed member of the plakoglobin/armadillo multigene family. Differentiation. 1997;61:293–304. doi: 10.1046/j.1432-0436.1997.6150293.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Liyanage U, Medina M, et al. Presenilin interaction in the brain with a novel member of the armadillo family. Neuroreport. 1997;8:2085–90. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]
- 9.Jones SB, Lanford GW, Chen YH, Morabito M, Kim K, Lu Q. Glutamate-induced delta-catenin redistribution and dissociation from postsynaptic receptor complexes. Neuroscience. 2002;115(4):1009–21. doi: 10.1016/s0306-4522(02)00532-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Sirota A, Chen YH, et al. Dendrite-like process formation and cytoskeletal remodeling regulated by delta-catenin expression. Exp Cell Res. 2002;275(2):171–84. doi: 10.1006/excr.2002.5503. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Abdul A, Chen YH, et al. δ-Catenin has the potential to promote the proliferation/survival and invasiveness of human cancer cells. Mol Biol Cell. 2003;14:341a. [Google Scholar]
- 12.Lu Q, Dobbs LJ, Gregory CW, et al. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn in human prostate cancer. Hum Pathol. 2005;36:1037–48. doi: 10.1016/j.humpath.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Burger MJ, Tebay MA, Keith PA, et al. Expression analysis of delta-catenin and prostate-specific membrane antigen: their potential as diagnostic markers for prostate cancer. Int J Cancer. 2002;100(2):228–37. doi: 10.1002/ijc.10468. [DOI] [PubMed] [Google Scholar]
- 14.Sramkoski RM, Pretlow TG, Giaconia JM, et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–9. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 15.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 16.Lu Q, Mukhopadhyay NK, Griffin JD, Paredes M, Medina M, Kosik KS. Brain armadillo protein delta-catenin interacts with Abl tyrosine kinase and modulates cellular morphogenesis in response to growth factors. J Neurosci Res. 2002;67:618–24. doi: 10.1002/jnr.10151. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Lundqvist M, Carlsson L, Nilsson O, Lundkvist O, Ronquist G. Prostasome-like granules from the PC-3 prostate cancer cell line increase the motility of washed human spermatozoa and adhere to the sperm. Eur J Obstet Gynecol Reprod Biol. 2001;96(1):88–97. doi: 10.1016/s0301-2115(00)00382-1. [DOI] [PubMed] [Google Scholar]
- 18.Llorente A, Marco MC, Alonso MA. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci. 2004;117(Pt 22):5343–51. doi: 10.1242/jcs.01420. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Oh M, Ki H, et al. Identification of E2F1 as a positive transcriptional regulator for delta-catenin. Biochem Biophys Res Commun. 2008;369(2):414–20. doi: 10.1016/j.bbrc.2008.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D, Terrian DM. Regulation of caveolin-1 expression and secretion by a protein kinase cepsilon signaling pathway in human prostate cancer cells. J Biol Chem. 2002;277(43):40449–55. doi: 10.1074/jbc.M206270200. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Borja M, Wubbolts R, Calafat J, et al. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol. 1999;9(1):55–8. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- 22.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101(36):13368–73. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao L, Loda M, Janmey PA, Stewart R, Anand-Apte B, Zetter BR. Thymosin beta 15: a novel regulator of tumor cell motility upregulated in metastatic prostate cancer. Nat Med. 1996;2(12):1322–8. doi: 10.1038/nm1296-1322. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson LM, Chang EL, Becker CM, et al. Development of a sensitive and specific enzyme-linked immunosorbent assay for thymosin beta15, a urinary biomarker of human prostate cancer. Clin Biochem. 2005;38(6):558–71. doi: 10.1016/j.clinbiochem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Dhaese S, Jonckheere V, Goethals M, et al. Functional and profiling studies prove that prostate cancer upregulated neuroblastoma thymosin beta is the true human homologue of rat thymosin beta15. FEBS Lett. 2007;581(25):4809–15. doi: 10.1016/j.febslet.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Ravid D, Chuderland D, Landsman L, Lavie Y, Reich R, Liscovitch M. Filamin A is a novel caveolin-1-dependent target in IGF-I-stimulated cancer cell migration. Exp Cell Res. 2008;314(15):2762–73. doi: 10.1016/j.yexcr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Tahir SA, Yang G, Ebara S, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61(10):3882–5. [PubMed] [Google Scholar]
- 28.Tahir SA, Ren C, Timme TL, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 2003;9(10 Pt 1):3653–9. [PubMed] [Google Scholar]