Abstract
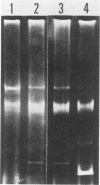
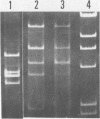
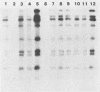
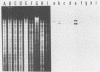
Plasmid profiles, the location of cholera toxin subunit A genes, and the presence of the defective VcA1 prophage genome in classical Vibrio cholerae isolated from patients in Bangladesh in 1982 were compared with those in older classical strains isolated during the sixth pandemic and with those in selected eltor and nontoxigenic O1 isolates. Classical strains typically had two plasmids (21 and 3 megadaltons), eltor strains typically had no plasmids, and nontoxigenic O1 strains had zero to three plasmids. The old and new isolates of classical V. cholerae had two HindIII chromosomal digest fragments containing cholera toxin subunit A genes, whereas the eltor strains from Eastern countries had one fragment. The eltor strains from areas surrounding the Gulf of Mexico also had two subunit A gene fragments, which were smaller and easily distinguished from the classical pattern. All classical strains had 8 to 10 HindIII fragments containing the defective VcA1 prophage genome; none of the Eastern eltor strains had these genes, and the Gulf Coast eltor strains contained a different array of weakly hybridizing genes. These data suggest that the recent isolates of classical cholera in Bangladesh are closely related to the bacterial strain(s) which caused classical cholera during the sixth pandemic. These data do not support hypotheses that either the eltor or the nontoxigenic O1 strains are precursors of the new classical strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Wachsmuth K., Davis B. R., Bopp C. A., Chaiken B. P., Lee J. V. Toxigenic Vibrio cholerae O1 strain from Mexico identical to United States isolates. Lancet. 1983 Oct 15;2(8355):912–912. doi: 10.1016/s0140-6736(83)90894-2. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges M. C., Wachsmuth I. K., Birkness K. A., Moseley S. L., Georges A. J. Genetic probes for enterotoxigenic Escherichia coli isolated from childhood diarrhea in the Central African Republic. J Clin Microbiol. 1983 Jul;18(1):199–202. doi: 10.1128/jcm.18.1.199-202.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Romig W. R. Complete and Defective Bacteriophages of Classical Vibrio cholerae: Relationship to the Kappa Type Bacteriophage. J Virol. 1975 May;15(5):1231–1238. doi: 10.1128/jvi.15.5.1231-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Romig W. R. Genetic basis of toxin production and pathogenesis in Vibrio cholerae: evidence against phage conversion. Infect Immun. 1975 Mar;11(3):445–452. doi: 10.1128/iai.11.3.445-452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Becker S., Huq M. I., Stoll B. J., Khan M. U., Merson M. H., Lee J. V., Black R. E. Endemic cholera in rural Bangladesh, 1966-1980. Am J Epidemiol. 1982 Dec;116(6):959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Murphy J. R. Molecular epidemiological studies of United States Gulf Coast Vibrio cholerae strains: integration site of mutator vibriophage VcA-3. Infect Immun. 1983 Oct;42(1):224–230. doi: 10.1128/iai.42.1.224-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Levine M. M. Cloned cholera enterotoxin genes in study and prevention of cholera. Lancet. 1981 Nov 21;2(8256):1162–1163. doi: 10.1016/s0140-6736(81)90605-x. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Moseley S. L., Falkow S. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect Immun. 1981 May;32(2):661–667. doi: 10.1128/iai.32.2.661-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Samadi A. R., Huq M. I., Shahid N., Khan M. U., Eusof A., Rahman A. S., Yunus M., Faruque A. S. Classical Vibrio cholerae biotype displaces EL tor in Bangladesh. Lancet. 1983 Apr 9;1(8328):805–807. doi: 10.1016/s0140-6736(83)91860-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Wachsmuth I. K., Shangkuan Y. H., Schmidt E. V., Barrett T. J., Schrader J. S., Scherach C. S., McGee H. B., Feldman R. A., Brenner D. J. Salmonellosis associated with marijuana: a multistate outbreak traced by plasmid fingerprinting. N Engl J Med. 1982 May 27;306(21):1249–1253. doi: 10.1056/NEJM198205273062101. [DOI] [PubMed] [Google Scholar]