Abstract
Congenital heart diseases are traditionally considered to be multifactorial in pathogenesis resulting from environmental and genetic interactions that determine penetrance and expressivity within a genetically predisposed family. Recent evidence suggests that genetic contributions have been significantly underestimated. However, single gene defects occur only in a minority of cases, and multigenetic causes of congenital heart diseases have not been fully demonstrated. Here, we show that interactions between alleles of 3 Pbx genes, which encode homeodomain transcription factors, are sufficient to determine the phenotypic presentation of congenital heart diseases in mice. A major role is served by Pbx1, whose inactivation results in persistent truncus arteriosus. Reduction or absence of Pbx2 or Pbx3 leads to Pbx1 haploinsufficiency and specific malformations that resemble tetralogy of Fallot, overriding aorta with ventricular septal defect, and bicuspid aortic valves. Disruption of Meis1, which encodes a Pbx DNA-binding partner, results in cardiac anomalies that resemble those caused by Pbx mutations. Each of the observed cardiac defects represents developmental abnormalities affecting distinct stages of cardiac outflow tract development and corresponds to specific types of human congenital heart disease. Thus, varied deficiencies in the Pbx gene family produce a full spectrum of cardiac defects involving the outflow tract, providing a framework for determining multigenetic causes of congenital heart anomalies.
Keywords: Pbx, Meis, Hox, heart development, neural crest cell, cardiac outflow tract, persistent truncus arteriosus, tetralogy of Fallot, overriding aorta, bicuspid aortic valve
Congenital anomalies of the heart occur in up to 5% of live births1 and are the leading cause of birth defect–related death in the United States.1 Defects in cardiac outflow tract (OFT) formation account for 20% to 30% of congenital heart defects (CHDs).2 Isolated malformations of the semilunar valves of the OFT, including bicuspid aortic valve, occur in an additional 2% to 3% of the population.3 Formation of the OFT is a complex developmental process that involves the division of the arterial trunk into aortic and pulmonic arteries, alignment of these 2 arteries with the cardiac chambers, and development of the aortic and pulmonic valves.4 Failures of arterial division, alignment, or valve formation lead to specific types of congenital heart disease in humans.4 For example, the absence of arterial septation results in persistent truncus arteriosus (PTA), whereas unequal septation of the OFT may lead to narrowing of the right ventricular OFT accompanied by ventricular septal defect and other associated defects (tetralogy of Fallot). Furthermore, cardiac defects may arise from misalignment of the aorta to the left ventricle, resulting in an overriding aorta straddling both right and left ventricles. Malformations of semilunar valves can cause bicuspid aortic valve or pulmonic valve stenosis. These different forms of cardiac OFT defects have distinct clinical manifestations and implications for treatment and long-term care.5
A long standing clinical view holds that CHDs are determined by individual genetic predisposition interacting with influential environmental factors.1,6–8 This traditional model, although underestimating genetic contributions,1,9 has been used to explain a low coincidence rate (2% to 3%) for most cardiac anomalies among siblings and to explain the variety of anomalies observed within a single pedigree. Although single gene or chromosomal abnormalities cause certain cardiac lesions or syndromes,1,9–11 they represent only a minority of cases and do not account for the complex inheritance of most CHDs. As alternative explanations to environmental effects or single gene inheritance, the display of CHDs may be determined by multiple alleles of a single major gene, by interaction of a major gene with several minor genes, or by the interactions of several minor genes acting together. Distinguishing among these scenarios, however, is challenging to study in the human population. The availability of targeted mouse mutations provides an experimental approach to study multigenetic etiology of CHDs.
Here, we describe how interactions between alleles of a new class of transcriptional regulators of heart development, the Pbx gene family, provide a mouse model for the complex inheritance of CHD. The Pbx genes, including Pbx1, -2, and -3, which encode TALE class homeodomain transcription factors,12–14 form heterooligomeric complexes with Hox and Meis proteins.15,16 We have examined the cardiac phenotypes of all possible combinations of null alleles of the Pbx genes. Embryos with different combinations of Pbx mutations display a spectrum of cardiac malformations in the OFT that include PTA, tetralogy of Fallot, overriding aorta, and bicuspid aortic valves. Each of the phenotypes produced by specific Pbx compound mutations represent developmental aberrations at distinct stages during OFT development, correlate with Pbx gene dosage, and correspond to specific types of CHDs in humans. Our studies also suggest that Pbx-governed OFT patterning requires interactions with their DNA-binding partner Meis proteins16 because Meis1 disruption results in cardiac defects that resemble Pbx mutations. These results provide a framework to consider the complex inheritance of human CHD as multigenetic interactions between paralogous genes.
Materials and Methods
Pbx-Deficient and Meis1-Deficient Mice
Targeted disruption of the Pbx and Meis1 genes and generation of the respective knockout mice have been described previously.17–19 Heterozygous knockout parental lines were backcrossed for at least 8 generations onto a C57BL/6 genetic background before intercrossing to obtain homozygous or compound mutant embryos. Phenotypes were generally analyzed in embryos (embryonic day [E]10.5 to 15.5) or neonates. Gestational age was determined by the date of observing a vaginal plug (set as E0.5) and by ultrasonography before harvesting embryos.20 For embryonic/perinatal lethality determination, 4 to 5 littermate controls were observed per mutant mouse. For cardiac structure analysis, 1 to 2 littermate controls were analyzed per mutant mouse.
Vascular Casting
The chest wall of mice was opened under microscopic visualization. A 33-gauge needle (Hamilton) mounted on a 1-mL tuberculin syringe was used to inject an acrylic resin (Batson no. 17) containing blue dye (Methyl Methacrylate Casting Kit, Polyscience Inc) into the right ventricle to perfuse the great arteries. Mice were then held at 4°C for 2 to 6 hours to allow the resin to polymerize and cast the vasculature.
Histology
Paraffin sections of mouse embryos were prepared as described previously.21,22 Consecutive sections through the chest (5 to 7 μmol/L) were collected, stained with hematoxylin/eosin, and analyzed by light microscopy.
Immunohistochemistry
Immunohistochemistry using anti-Pbx1b,16,23 anti-Pbx2 (clone 2.1), or anti-Pbx3a19 monoclonal antibodies was performed on paraffin sections of embryonic tissues. Paraffin sections (7 μM) from E9.5 and E12.5 mouse embryos were prepared and rehydrated. For antigen retrieval, the slides were immersed in citric acid–based antigen unmasking solution (Vector Laboratories) and boiled in a pressure cooker for 10 minutes, then cooled down at 4°C for 20 minutes. Endogenous peroxidase activities were blocked by treating in 3% H2O2 for 10 minutes. The slides were then blocked in 5% normal goat serum for 30 minutes. The primary mouse monoclonal antibodies were used at the following dilutions: anti-Pbx1b, 1:200; anti-Pbx2, 1:400; and anti-Pbx3a, 1:400. The antibodies were then incubated for 2 hours at room temperature. The biotinylated anti-mouse IgG secondary antibody (Vector Laboratories) was used at 1:250 for 30 minutes at room temperature. The signal was amplified using the VECTASTAIN Elite ABC kit (Vector Laboratories) for 30 minutes and developed with DAB (Vector Laboratories). Finally, the slides were counterstained with hematoxylin for nuclei and mounted with Permount (ThermoFisher).
Results
Pbx Proteins Are Widely Present in Tissues Essential for Heart Development
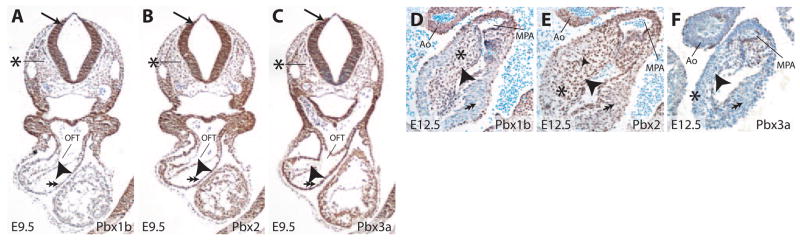
The Pbx genes (Pbx1, −2, and −3) regulate a variety of developmental processes,17,24–31 suggesting that they may also be important for heart development. To investigate this hypothesis, we first examined the distribution of Pbx proteins in tissues involved in heart development. In the heart, Pbx1b, the predominant isoform of Pbx1 during mouse embryogenesis,23 was present in both endocardial and myocardial cells of the OFT at E9.5 (Figure 1A). At E12.5, Pbx1b was present in endocardial cells and the mesenchyme of endocardial cushions that septate the OFT and give rise to the semilunar valves (Figure 1D). Pbx1b was also detected in endothelial and vascular smooth muscle cells of the aorta and main pulmonary arteries (Figure 1D). Furthermore, at E8.75 and E9.5, Pbx1b was widely present in cells within the neural tube, where the premigratory and newly delaminated cardiac neural crest cells (CNCCs) are located (Figure 1A and Figure I in the online data supplement, available at http://circres.ahajournals.org). CNCCs migrate to the OFT, form the aorticopulmonary septum, and contribute to OFT septation.32 Pbx1b was also present in mesenchymal cells that surround CNCCs and influence their migration (Figure 1A).33,34 Similarly to Pbx1, Pbx2 was broadly present at E9.5 in cells that included premigratory CNCCs within the neural tube and branchial mesenchyme (Figure 1B). Within the heart, Pbx2 was present in endocardial, myocardial, and cushion mesenchymal cells (Figure 1B and 1E). In the aorta and main pulmonary arteries, Pbx2 was detected in both endothelial and vascular smooth muscle cells (Figure 1E). Pbx3a was widely present at E9.5, including in premigratory CNCCs as well as all cell types of the heart. However, by E12.5, Pbx3a was downregulated in myocardial cells but remained present in endocardial cells and mesenchymal cells of the endocardial cushions (Figure 1C and 1F). Additionally, Pbx3 was present in endothelial but not vascular smooth muscle cells of the great arteries (Figure 1F), distinguishing it from Pbx1b and Pbx2. The widespread and overlapping presence of Pbx proteins in embryonic sites of cardiac development prompted us to investigate heart development in mice lacking individual or multiple Pbx genes.
Figure 1.
Pbx proteins are widely present in tissues relevant to heart development. A through C, Immunohistochemical analysis of Pbx proteins at E9.5. Pbx1b (A) is present in neural crest cells (arrow), mesenchymal cells of the branchial arch region (asterisk), endocardial cells (arrowhead), and myocardial cells (double arrows) of the cardiac OFT. Pbx2 (B) and Pbx3 (C) display similar expression patterns at E9.5. D through F, Presence of Pbx proteins in the cardiac OFT at E12.5. Pbx1b (D) is present in endocardial (arrowhead) and mesenchymal cells (asterisk) of the endocardial cushions of the OFT. Pbx1b is downregulated in myocardial cells (double arrow) at E12.5. Pbx1b is present in the endothelial and vascular smooth muscle cells of the aorta (Ao) and main pulmonary artery (MPA). Pbx2 (E) is present in endocardial (arrowhead), myocardial (double arrow), and mesenchymal cells (asterisk) of the OFT. Pbx2 is also present in endothelial and vascular smooth muscle cells of the aorta and main pulmonary artery. Pbx3a (F) is present in endocardial (arrowhead) and mesenchymal cells (asterisk) of the cushion but downregulated in myocardial cells (double arrow). Pbx3a is present in endothelial but not vascular smooth muscle cells of the great arteries.
Pbx1 Is Required for Septation of the Cardiac OFT
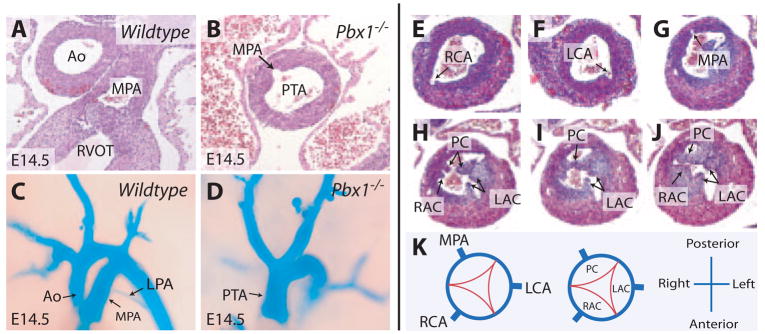
Embryos deficient for Pbx1 manifested a failure in septation of the cardiac OFT, resulting in a single arterial trunk (termed persistent truncus arteriosus or PTA) that emerged from the right ventricle (N=28) (Figure 2A through 2D). The PTA in Pbx1-null embryos gave rise to left and right coronary arteries anteriorly and a main pulmonary artery posteriorly (Figure 2B, 2E through 2G, and 2K). The main pulmonary artery branched into left and right pulmonary arteries as in wild-type embryos. A large ventricular septal defect was present in Pbx1−/− hearts (data not shown). Furthermore, a single heart valve was present at the base of the truncus (Figure 2H to 2K) and contained 3 valve leaflets and cusps: the right anterior, left anterior, and posterior cusps, representing the most common valve configuration seen in human PTA.5 Overall, the cardiac abnormalities of Pbx1−/− embryos resemble type II PTA in humans (Collett and Edwards classification),5 a severe form of cardiac OFT developmental failure. Besides cardiac defects, Pbx1−/− embryos exhibited anomalous patterning of the great arteries, including cervical aortic arch, absent or residual common carotid artery, aberrant origins of the subclavian arteries, and occasional right-sided aorta (C.-P.C. et al, manuscript in preparation). Vascular patterning defects were not observed in mice with any of the compound Pbx mutations described below.
Figure 2.
Pbx1−/− embryos display PTA. A and B, Transverse sections show a normal aorta and main pulmonary artery in a wild-type embryo (A) vs a common arterial trunk in a Pbx1−/− embryo (B). Ao indicates aorta; MPA, main pulmonary artery. C and D, Vascular casting of the great arteries emerging from the embryonic heart in wild-type (C) and Pbx1−/− (D) embryos at E14.5. LPA indicates left pulmonary artery. E through G, Serial histological sections show origins of the coronary and main pulmonary arteries in Pbx1−/− embryos at E14.5. RCA indicates right coronary artery; LCA, left coronary artery. H through J, Serial histological sections show the 3 truncal valve leaflets and cusps in Pbx1−/− embryos at E14.5. RAC indicates right anterior cusp; LAC, left anterior cusp; PC, posterior cusp. K, Schematic representation of the origins of coronary and pulmonary arteries and the truncal valve leaflets and cusps in Pbx1−/− embryos.
Pbx2 Contributes to the Alignment of Cardiac OFTs and Semilunar Valve Morphogenesis
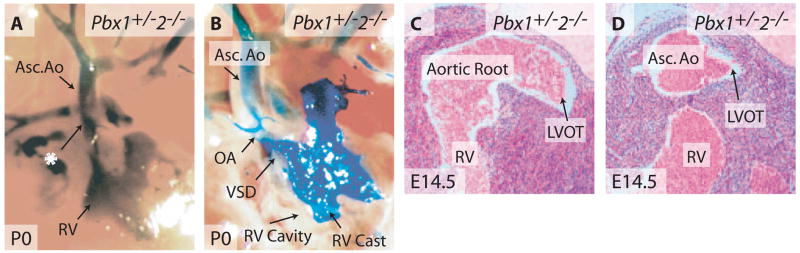
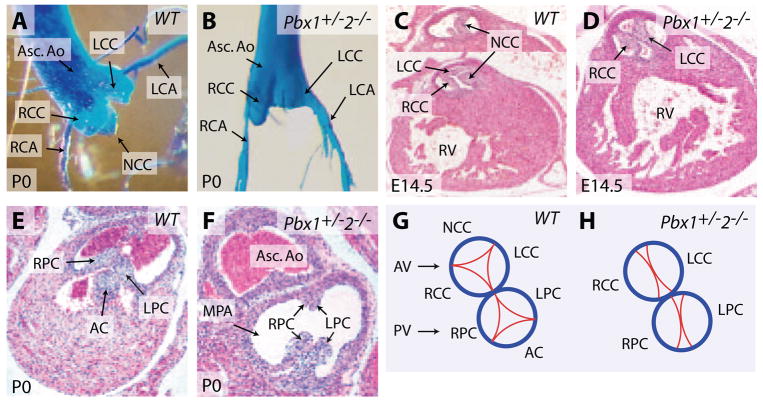
To investigate potential multigenic Pbx contributions to OFT development, mice bearing null alleles17–19 for Pbx1, Pbx2, and Pbx3 were intercrossed to produce embryos with 10 distinct Pbx allelotypes. These 10 allelic Pbx combinations represented all possible genotypes from the 27 theoretical combinations of 3 Pbx genes because of early lethality of mice of certain genotypes. For example, Pbx1+/−;Pbx3−/− mouse embryos could not be generated because the required parental Pbx1+/−;Pbx3+/− mice died neonatally (Table). Furthermore, to circumvent strain background effects, phenotypes were analyzed in embryos or neonates derived from parental mice that had been backcrossed onto the C57BL/6 genetic background for at least 8 generations. Most combinations of Pbx mutations yielded no detectable cardiac abnormalities, including mice homozygous for Pbx2- or Pbx3-null alleles (Table).18 However, when Pbx1 gene dosage was reduced by half, Pbx2 nullizygosity resulted in cardiac defects in the alignment of the aorta to the left ventricle in addition to specific semilunar valve malformations. Pbx1+/−;Pbx2−/− mice died within 24 hours after birth. Angiography of newborn mice showed an abnormal connection of the right ventricle with the ascending aorta (Figure 3A) and an overriding aorta that straddled both the right and left ventricles (Figure 3B), defects that were also evident by histological analysis (N=17) (Figure 3C and 3D). A ventricular septal defect underlying the aortic valve was present in Pbx1+/−;Pbx2−/− hearts (Figures 3C and 5H). Furthermore, in contrast to the normal trileaflet valves (Figure 4A, 4C, and 4G), the aortic valve was bicuspid in Pbx1+/−;Pbx2−/− mice, containing only the right and left coronary cusps (Figures 4B, 4D, 4H, and 5K) that gave rise to the right and left coronary arteries, respectively (Figure 4B). The bicuspid valve leaflets did not form commissures at their anchoring points along the aortic wall (Figure 4D), indicating that these valves could not properly occlude the aortic lumen and thus would lead to significant aortic regurgitation. Similarly, the pulmonic valve was also bicuspid containing right and left posterior leaflets in the absence of an anterior leaflet (Figure 4F and 4H). These cardiac defects resemble anomalies in human patients with overriding aorta and bicuspid aortic valve, functionally important anomalies of less severity than PTA. Thus, heterozygosity of Pbx1 reveals roles for Pbx2 in the alignment of the left ventricular OFT and semilunar valve morphogenesis.
Table.
Summary of Cardiac Anomalies Present With Different Combinations of Pbx1-, Pbx2-, and Pbx3-null Alleles
| Genotype | Lethality | Cardiac Phenotype |
|---|---|---|
| Pbx3−/− | Neonatal | None |
| Pbx2−/− | Increased | None |
| Pbx2−/−;Pbx3+/− | Increased | None |
| Pbx2−/−;Pbx3−/− | Embryonic | None |
| Pbx1+/− | None | None |
| Pbx1+/−;Pbx3+/− | Neonatal | None |
| Pbx1+/−;Pbx2+/− | Increased | None |
| Pbx1+/−;Pbx2+/−;Pbx3+/− | Neonatal | Bicuspid aortic valve |
| Pbx1+/−;Pbx2−/− | Neonatal | Overriding aorta; VSD; bicuspid aortic and pulmonic valves |
| Pbx1+/−;Pbx2−/−;Pbx3+/− | Embryonic | Tetralogy of Fallot (RVOT obstruction, VSD, right ventricular hypertrophy, overriding aorta) |
| Pbx1−/− | E15 to E16 | Truncus arteriosus originating from the right ventricle; VSD |
VSD indicates ventricular septal defect.
Figure 3.
Pbx2 makes subsidiary contributions to the alignment of cardiac OFTs. A, Right ventricular angiography of a newborn Pbx1+/−2−/− mouse. Asterisk indicates a direct connection between the right ventricle (RV) and the ascending aorta (Asc. Ao). B, Exposure of the right ventricular cavity in A reveals a high ventricular septal defect (VSD) and an overriding aorta (OA). C and D, Serial sections through the subaortic region in newborn Pbx1+/−2−/− mice demonstrate an aorta that overrides both ventricles. Section plane in C is caudal to the plane in D. LVOT indicates left ventricular OFT.
Figure 5.
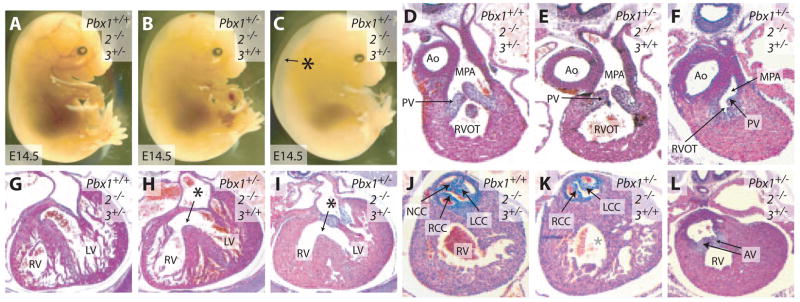
Both Pbx2 and Pbx3 contribute to OFT septation and semilunar valve formation. A though C, Gross appearance of Pbx2−/−3+/− (A), Pbx1+/−2−/− (B), and Pbx1+/−2−/−3+/− (C) embryos at E14.5. Asterisk indicates generalized edema. D through F, Transverse sections of the RVOT at E14.5. Pbx2−/−3+/− (D) and Pbx1+/−2−/− (E) embryos display patent RVOTs with slender pulmonic valve (PV) leaflets. The RVOT of Pbx1+/−2−/−3+/− embryos (F) is narrowed and associated with malformed pulmonic valve leaflets. G though I, Transverse sections of the right (RV) and left (LV) ventricles at E14.5. Both Pbx2−/−3+/− (G) and Pbx1+/−2−/− (H) mice have normal right and left ventricular walls. Pbx1+/−2−/− mice (H) exhibit a ventricular septal defect (asterisk). Pbx1+/−2−/−3+/− embryos (i) have both a ventricular septal defect (asterisk) and right ventricular hypertrophy. J through L, Transverse sections of the aortic valve at E14.5. Pbx2−/−3+/− mice (J) have a normal trileaflet aortic valve. Pbx1+/−2−/− mice (K) have a bicuspid aortic valve. Pbx1+/−2−/−3+/− embryos (L) have malformed aortic valves (AV). NCC indicates noncoronary cusp; RCC, right coronary cusp; LCC, left coronary cusp.
Figure 4.
Pbx1+/−2−/− mice have bicuspid semilunar valves. A and B, Casting of the aortic valves in newborn wild-type (A) and Pbx1+/−2−/− (B) mice. Asc. Ao indicates ascending aorta; LCA, left coronary artery; RCA, right coronary artery; LCC, left coronary cusp; RCC, right coronary cusp; NCC, noncoronary cusp. C and D, Histological sections show the tricuspid aortic valve in a wild-type (C) and bicuspid aortic valve in a Pbx1+/−2−/− embryo (D) at E14.5. RV indicates right ventricle. E and F, Histological sections show a tricuspid pulmonic valve in a newborn wild-type (E) mouse vs a bicuspid aortic valve in a newborn Pbx1+/−2−/− mouse (F). MPA indicates main pulmonary artery. G, Schematic representation of the trileaflet aortic and pulmonic valves. AV indicates aortic valve; PV, pulmonic valve; AC, anterior cusp; LPC, left posterior cusp; RPC, right posterior cusp. H, Schematic representation of the bicuspid aortic and pulmonic valves in Pbx1+/−2−/− mice. The noncoronary cusp (NCC) of the aortic valve and the anterior cusp (AC) of the pulmonic valve fail to develop.
Pbx3 Participates in the Formation of Cardiac OFT and Semilunar Valves
To further investigate the roles of Pbx3 in heart development, we intercrossed a Pbx3-null allele19 onto Pbx1- and/or Pbx2-deficient backgrounds. We found that embryos with compound Pbx1+/−;Pbx2−/−;Pbx3+/− mutations exhibited serious cardiac anomalies characteristic of tetralogy of Fallot. Pbx1+/−; Pbx2−/−;Pbx3+/− embryos, unlike Pbx1+/−;Pbx2−/− embryos (Figure 5B), displayed generalized edema (Figure 5C) similar to that reported previously in Pbx1−/− embryos.17 Pbx1+/−; Pbx2−/−;Pbx3+/− embryos showed unequal division of the truncus arteriosus at the expense of the right ventricular OFT (RVOT), leading to narrowing of RVOT and malformations of pulmonic valves (N=8) (Figure 5F). This RVOT obstruction, together with pulmonic valve malformations (Figure 5F), resulted in hypertrophy of the right ventricular wall (Figure 5I). Mild left ventricular hypertrophy was also present, likely reflecting volume overload of the left ventricle as a result of increased right to left cardiac shunting caused by RVOT obstruction and the presence of a large ventricular septal defect (Figure 5I), as well as possible pressure overload caused by the presence of malformed aortic valves (Figure 5L). The aorta of Pbx1+/−;Pbx2−/−;Pbx3+/− embryos arose mainly from the right ventricle, but it overrode both right and left ventricles (Figure 5L and data not shown). Malformed aortic valves were present between the misaligned aorta and ventricles (Figure 5L). The constellation of RVOT obstruction, right ventricular hypertrophy, ventricular septal defect, and overriding aorta in Pbx1+/−;Pbx2−/−;Pbx3+/− embryos mimics tetralogy of Fallot in humans. Thus, reduction of Pbx1 gene dosage uncovers the contributions of Pbx2 and Pbx3 toward equal division of truncus arteriosus into the left and right outflow channels.
Semilunar valve formation depends on both Pbx2 and Pbx3. Although Pbx1+/−;Pbx2+/− mice displayed no valve defects, Pbx1+/−;Pbx2+/−;Pbx3+/− mice had isolated bicuspid aortic and pulmonic valves as their only cardiac malformations (N=11) (data not shown), indicating that Pbx3 participates in trileaflet valve formation. Similarly, comparison of Pbx1+/−;Pbx2+/−;Pbx3+/− embryos, which had distinct bicuspid semilunar valves, with the normal valves in Pbx1+/−; Pbx3+/− mutants, further indicates that Pbx2 contributes to semilunar valve development. In addition, the necessity of Pbx1 haploinsufficiency for the development of semilunar valve malformations in Pbx2 and Pbx3 mutants, because Pbx2−/−;Pbx3+/− mice had no semilunar valve defects (Table), demonstrates that all 3 Pbx genes contribute to semilunar valve morphogenesis.
Meis1 Is Essential for Cardiac OFT Development
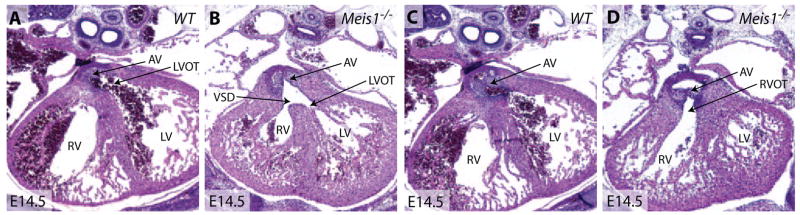
Given that Meis proteins are major in vivo DNA-binding partners of Pbx proteins,16 we hypothesized that development of the cardiac OFT would require Pbx and Meis interactions. To test this idea, we examined cardiac development in mice lacking Meis1.35 Meis1−/− embryos displayed subcutaneous hemorrhage and died between E14.5 and E15.5 (supplemental Figure IIB). A further analysis of Meis1−/− embryos revealed that the aorta was septated from the main pulmonary artery (supplemental Figure IID) but overrode both right and left ventricles accompanied by a ventricular septal defect (N=9) (Figure 6B and 6D and supplemental Figure IIE and IIF). This phenotype resembles Pbx1+/−Pbx2−/− heart defects and falls within the spectrum of OFT anomalies defined by Pbx deficiencies (Figure 7). This suggests that Pbx proteins function in concert with Meis1 partners during heart development. Because Meis1 is also part of a multigene family,36 compound mutations of Meis genes may also recapitulate a broad spectrum of cardiac defects similar to those induced by Pbx deficiencies.
Figure 6.
Meis1-null embryos display an overriding aorta and ventricular septal defect. A through D, Hematoxylin/eosin-stained sections from littermate E14.5 wild-type and Meis1−/− embryos. The aorta connects to the left ventricular OFT (LVOT) (A) but not the RVOT (C) in a wild-type embryo. In Meis1−/− embryos, the aorta connects to both the left ventricular OFT (B) and RVOT (D). AV indicates aortic valve; VSD, ventricular septal defect.
Figure 7.
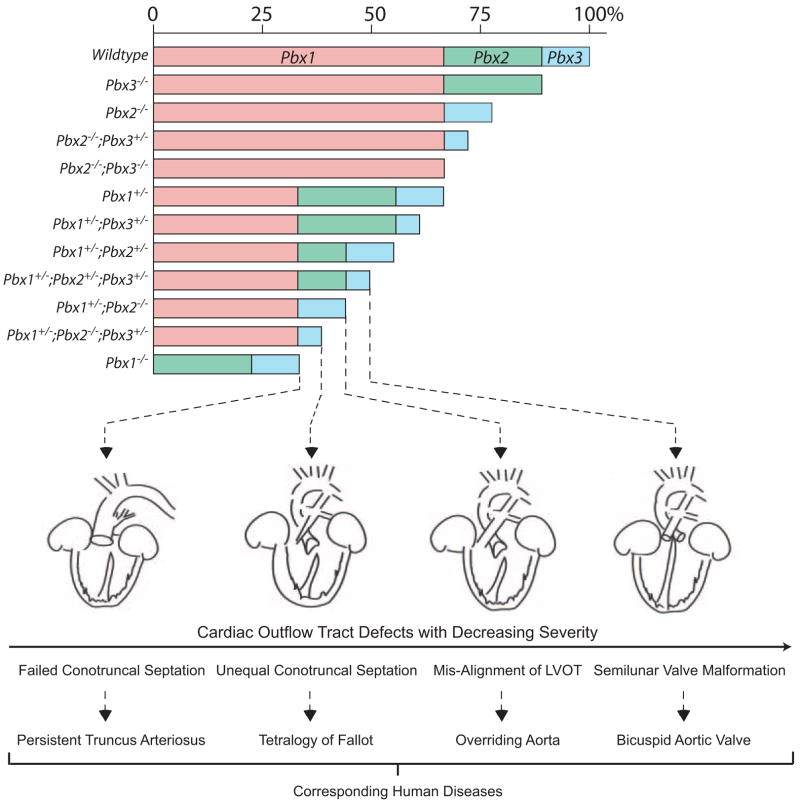
A semiquantitative model for the interactive roles of Pbx proteins in cardiac OFT morphogenesis. Pbx1 serves a major role in dictating conotruncal and semilunar valve development. Pbx2 and Pbx3 function to refine cardiac outflow morphogenesis. The bar chart indicates the proposed functional relative weight of Pbx proteins correlated with Pbx genotypes in cardiac development. Below, the cardiac phenotypes associated with specific Pbx genotypes are schematically depicted in decreasing order of severity, left to right. At the bottom, the human diseases that correspond to each developmental stage defined by Pbx deficiencies are listed.
Discussion
Many CHDs arise from malformations of the cardiac OFT and failure at specific steps in its formation results in distinctive cardiac anomalies of varying severity. These steps involve the division of the OFT into aorta and pulmonary arteries, alignment of these arteries to cardiac chambers, and formation of heart valves.4 Our results demonstrate how multigenetic interactions between alleles of related genes can determine the specific type of heart defect that develops.
The different cardiac phenotypes observed in Pbx allelic combinations suggest Pbx genes contribute to multiple steps of OFT development (Figure 7). Reduction of total Pbx1 to −3 gene dosage to half of wild-type levels reached a threshold where Pbx availability became limiting for heart development and manifested by isolated bicuspid semilunar valves in triple heterozygous Pbx1+/−;Pbx2+/−;Pbx3+/− mice. More severe phenotypes were observed in Pbx1+/−;Pbx2−/− mice, which displayed defects in the alignment of the left ventricular OFT, with resultant overriding aorta. Even more dramatic phenotypes occurred with additional compound alleles in Pbx1+/−; Pbx2−/−;Pbx3+/− mutants, where the cardiac OFT was divided unequally between the right and left hearts, leading to an obstruction in the right ventricular OFT. These biased divisions, compared with equal divisions in Pbx1+/−;Pbx2+/−; Pbx3+/− and Pbx1+/−;Pbx2−/− embryos, suggest that both Pbx2 and Pbx3 are essential, but functionally overlap, in fine-tuning the septation of the OFT and alignment of its left and right sides. The most severe phenotype was observed in Pbx1-null embryos, in which the OFT failed to complete the initial septation phase, suggesting that Pbx1 provides the greatest amount of combined Pbx function among the 3 proteins. Our results are most consistent with overlapping contributions of the 3 Pbx family members to common downstream molecular pathways that ultimately are required for OFT septation and alignment, as well as heart valve development. In support of an overlapping function, Pbx1, Pbx2, and Pbx3 have similar biochemical activities in forming transcriptional complexes with Hox and Meis proteins.15,16 The observations that certain members of these gene families, such as Hoxa337 and Meis1 (present study), are required for cardiac OFT development suggest that Pbx proteins likely heterodimerize with Hox and/or Meis proteins to control a subset of target genes to regulate OFT formation.
A functional-overlap model for Pbx genes in OFT development suggests that Pbx proteins act in cells in which the 3 proteins are simultaneously present. The roles of Pbx genes could occur early in development to establish correct differentiation or localization of relevant cells that later contribute to cardiac septation. Alternatively, Pbx proteins may function during active septation of the OFT. Pbx1, Pbx2, and Pbx3 are broadly present in E9.5 embryos, when the various cell types involved in OFT septation are being established. All 3 Pbx proteins are present in CNCCs before their delamination from the neural tube and during their migration to the heart to form the aortopulmonary septum.32 Besides, Pbx proteins are present in branchial mesenchymal cells, surface ectoderm and endoderm cells that neighbor the migrating CNCCs. Pbx proteins may function in these cells to regulate CNCC differentiation and/or migration.4,38–41 Furthermore, all Pbx proteins are present in endocardial and myocardial cells of the OFT at E9.5. Derived from secondary heart fields,42 these OFT cells are sites of action for genes, such as endothelin43 and semaphorin 3C,44 that regulate OFT development. Later, at E12.5, during the active process of OFT septation, Pbx1, Pbx2, and Pbx3 are commonly present in endocardial and cushion mesenchymal cells but not in myocardial or smooth muscle cells. Thus, the partially redundant functions of Pbx genes are, in part, a result of their overlapping expression in cells that contribute to cardiac OFT development.
The Pbx-defined cardiac malformations support a model in which the total level of Pbx gene function correlates with phenotype severity (Figure 7). A major gene (Pbx1) contributes to OFT development in the context of subsidiary roles for paralogous genes (Pbx2 and Pbx3). The spectrum of OFT defects observed in different combinations of Pbx gene mutations indicates that progressive reductions of Pbx gene dosage below a critical threshold correlate with increasingly severe anomalies, each corresponding to specific heart defects in humans. Conversely, there is no manifestation of congenital heart disease once combined Pbx expression is above a minimum level. Thus, the cardiac malformations observed in 10 distinct combinations of loss-of-function alleles of Pbx1, Pbx2, and Pbx3 demonstrate that genetic interactions and gene dosage are sufficient to determine the penetrance and expression of a range of specific cardiac lesions. Analogously in humans, environmental factors may not always be necessary to account for the penetrance or variable degrees of severity of cardiac defects within a genetically predisposed family. Multigenetic factors may play crucial roles in the manifestation of human congenital heart disease. An examination for polymorphic alleles of paralogous or interacting genes of an identified predisposing gene within human pedigrees may contribute to understanding the complex inheritance of congenital heart disease and ultimately improve the accuracy of genetic counseling.
Supplementary Material
Acknowledgments
We thank M. Ambrus and C. Nicolas for excellent technical assistance. We are indebted to M. Rabinovitch, D. Bernstein, and members of the laboratory of C.-P.C. for support and advice.
Sources of Funding
This work was supported by National Cancer Institute grants CA90735 and CA42971 (to M.L.C.); and National Heart, Lung, and Blood Institute grant HL085345, the American Heart Association, the Children’s Heart Foundation, the March of Dimes Birth Defects Foundation, the Baxter Foundation, and a Tobacco-Related Disease Research Program award (to C.P.C.). K.S. is supported by an American Heart Association Postdoctoral Fellowship; C.S. is supported by an NIH National Research Service Award fellowship; and K.Y.T. is supported by Stanford University Dean’s, McCormick, and NIH National Research Service Award fellowships.
Footnotes
Disclosures
None.
References
- 1.Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 2.Sandler T. Langman’s Medical Embryology. 10. Baltimore: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 3.Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med. 2000;342:334–342. doi: 10.1056/NEJM200002033420507. [DOI] [PubMed] [Google Scholar]
- 4.Kirby ML. Cardiac Development. 2007 [Google Scholar]
- 5.Rudolph AM. Congenital Diseases of the Heart: Clinical-Physiological Considerations. 2. New York: Futura Publishing Company; 2001. [Google Scholar]
- 6.Nora JJ. Multifactorial inheritance hypothesis for the etiology of congenital heart diseases. The genetic-environmental interaction. Circulation. 1968;38:604–617. doi: 10.1161/01.cir.38.3.604. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 8.Calcagni G, Digilio MC, Sarkozy A, Dallapiccola B, Marino B. Familial recurrence of congenital heart disease: an overview and review of the literature. Eur J Pediatr. 2007;166:111–116. doi: 10.1007/s00431-006-0295-9. [DOI] [PubMed] [Google Scholar]
- 9.Gelb BD. Recent advances in the understanding of genetic causes of congenital heart defects. Front Biosci. 2000;5:D321–D333. doi: 10.2741/gelb. [DOI] [PubMed] [Google Scholar]
- 10.Satoda M, Zhao F, Diaz GA, Burn J, Goodship J, Davidson HR, Pierpont ME, Gelb BD. Mutations in TFAP2B cause Char syndrome, a familial form of patent ductus arteriosus. Nat Genet. 2000;25:42–46. doi: 10.1038/75578. [DOI] [PubMed] [Google Scholar]
- 11.Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 12.Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monica K, Galili N, Nourse J, Saltman D, Cleary ML. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol. 1991;11:6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nourse J, Mellentin JD, Galili N, Wilkinson J, Stanbridge E, Smith SD, Cleary ML. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990;60:535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- 15.Chang CP, Shen WF, Rozenfeld S, Lawrence HJ, Largman C, Cleary ML. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 16.Chang CP, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol Cell Biol. 1997;17:5679–5687. doi: 10.1128/mcb.17.10.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selleri L, Depew MJ, Jacobs Y, Chanda SK, Tsang KY, Cheah KS, Rubenstein JL, O’Gorman S, Cleary ML. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001;128:3543–3557. doi: 10.1242/dev.128.18.3543. [DOI] [PubMed] [Google Scholar]
- 18.Selleri L, DiMartino J, van Deursen J, Brendolan A, Sanyal M, Boon E, Capellini T, Smith KS, Rhee J, Popperl H, Grosveld G, Cleary ML. The TALE homeodomain protein Pbx2 is not essential for development and long-term survival. Mol Cell Biol. 2004;24:5324–5331. doi: 10.1128/MCB.24.12.5324-5331.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee JW, Arata A, Selleri L, Jacobs Y, Arata S, Onimaru H, Cleary ML. Pbx3 deficiency results in central hypoventilation. Am J Pathol. 2004;165:1343–1350. doi: 10.1016/S0002-9440(10)63392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CP, Chen L, Crabtree GR. Sonographic staging of the developmental status of mouse embryos in utero. Genesis. 2003;36:7–11. doi: 10.1002/gene.10186. [DOI] [PubMed] [Google Scholar]
- 21.Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel CA, Selleri L, Jacobs Y, Warnke R, Cleary ML. Expression of Pbx1b during mammalian organogenesis. Mech Dev. 2001;100:131–135. doi: 10.1016/s0925-4773(00)00516-5. [DOI] [PubMed] [Google Scholar]
- 24.Capellini TD, Di Giacomo G, Salsi V, Brendolan A, Ferretti E, Srivastava D, Zappavigna V, Selleri L. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- 25.Schnabel CA, Selleri L, Cleary ML. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis. 2003;37:123–130. doi: 10.1002/gene.10235. [DOI] [PubMed] [Google Scholar]
- 26.DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, O’Gorman S, Weissman IL, Cleary ML. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98:618–626. doi: 10.1182/blood.v98.3.618. [DOI] [PubMed] [Google Scholar]
- 27.Manley NR, Selleri L, Brendolan A, Gordon J, Cleary ML. Abnormalities of caudal pharyngeal pouch development in Pbx1 knockout mice mimic loss of Hox3 paralogs. Dev Biol. 2004;276:301–312. doi: 10.1016/j.ydbio.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Schnabel CA, Godin RE, Cleary ML. Pbx1 regulates nephrogenesis and ureteric branching in the developing kidney. Dev Biol. 2003;254:262–276. doi: 10.1016/s0012-1606(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 29.Sanyal M, Tung JW, Karsunky H, Zeng H, Selleri L, Weissman IL, Herzenberg LA, Cleary ML. B-cell development fails in the absence of the Pbx1 proto-oncogene. Blood. 2007;109:4191–4199. doi: 10.1182/blood-2006-10-054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waskiewicz AJ, Rikhof HA, Moens CB. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev Cell. 2002;3:723–733. doi: 10.1016/s1534-5807(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Rowan S, Yue Y, Heaney S, Pan Y, Brendolan A, Selleri L, Maas RL. Pax6 is regulated by Meis and Pbx homeoproteins during pancreatic development. Dev Biol. 2006;300:748–757. doi: 10.1016/j.ydbio.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 33.Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 34.Thomas T, Kurihara H, Yamagishi H, Kurihara Y, Yazaki Y, Olson EN, Srivastava D. A signaling cascade involving endothelin-1, dHAND and msx1 regulates development of neural-crest-derived branchial arch mesenchyme. Development. 1998;125:3005–3014. doi: 10.1242/dev.125.16.3005. [DOI] [PubMed] [Google Scholar]
- 35.Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, Jenkins NA, Copeland NG. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J. 2004;23:450–459. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura T, Jenkins NA, Copeland NG. Identification of a new family of Pbx-related homeobox genes. Oncogene. 1996;13:2235–2242. [PubMed] [Google Scholar]
- 37.Chisaka O, Capecchi MR. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- 38.Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–987. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- 39.Brown CB, Wenning JM, Lu MM, Epstein DJ, Meyers EN, Epstein JA. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol. 2004;267:190–202. doi: 10.1016/j.ydbio.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin JF, Baldini A, Lindsay E. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- 42.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest. 1995;96:293–300. doi: 10.1172/JCI118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.