Abstract
Rationale: It is well established that sleep-disordered breathing (SDB) is independently associated with insulin resistance, glucose intolerance, and type 2 diabetes mellitus. However, data on whether SDB alters in vivo kinetics of glucose and insulin are lacking.
Objectives: The primary goal of this study was to use the frequently sampled intravenous glucose tolerance test (FSIVGTT) in subjects with and without SDB to model the in vivo kinetics of glucose and insulin. Minimal model analysis of the FSIVGTT data was used to derive parameters of insulin sensitivity, glucose effectiveness (a measure of the ability of glucose to mediate its own disposal), and pancreatic β-cell function.
Results: A total of 118 nondiabetic subjects underwent polysomnography, the FSIVGTT, and body composition measurements including determination of percent body fat. Compared with normal subjects (apnea-hypopnea index < 5 events/h), those with mild, moderate, and severe SDB displayed a 26.7, 36.5 and 43.7% reduction in insulin sensitivity, respectively, independent of age, sex, race, and percent body fat. The disposition index, an integrated measure of pancreatic β-cell function, was also reduced in patients with moderate to severe SDB. The decrease in insulin sensitivity and the disposition index were correlated with the average degree of oxyhemoglobin desaturation. In contrast, glucose effectiveness was negatively correlated with the frequency of respiratory event–related arousals.
Conclusions: The results of this study suggest that, independent of adiposity, SDB is associated with impairments in insulin sensitivity, glucose effectiveness, and pancreatic β-cell function. Collectively, these defects may increase the risk of glucose intolerance and type 2 diabetes mellitus in SDB.
Keywords: sleep-disordered breathing, sleep apnea, glucose metabolism, insulin resistance, beta-cell function
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Sleep-disordered breathing (SDB) is associated with insulin resistance and type 2 diabetes mellitus. Whether SDB is also associated with alterations in glucose disposal independent of insulin action and in pancreatic insulin secretion is not known.
What This Study Adds to the Field
Independent of obesity, patients with SDB exhibit impairments in insulin sensitivity, glucose disposal independent of insulin action, and pancreatic insulin secretion.
Sleep-disordered breathing (SDB) is a relatively common condition that has increased dramatically in the general population in tandem with obesity. Characterized by recurrent collapse of the upper airway, SDB results in episodic drops in oxyhemoglobin saturation and transient arousals from sleep. Epidemiologic studies have shown that SDB is a risk factor for hypertension (1), cardiovascular disease (2), and all-cause mortality (3, 4). Independent of obesity, SDB has also been associated with insulin resistance, glucose intolerance, and type 2 diabetes mellitus (5, 6). Although much of the evidence on SDB and glucose metabolism has been relatively consistent, almost all of the published reports have used body mass index (BMI) to account for the confounding effects of adiposity. It is well established that BMI does not discriminate between muscle and adipose tissue and cannot provide an assessment of body fat distribution. Moreover, age, sex, and ethnicity modify the relation between BMI and body fat, indicating that BMI may not accurately reflect adiposity across different population subsets (7, 8). In contrast, techniques such as dual-energy X-ray absorptiometry (DEXA) provide a more precise estimate of body fat mass and thus would allow for a more rigorous assessment of whether SDB and metabolic dysfunction are independently associated.
In addition to the issue of residual confounding due to adiposity, it is important to recognize that a majority of available studies on SDB and metabolic function have used steady-state measurements to assess glucose metabolism. Simple surrogate metrics, such as the homeostasis model of insulin resistance (9), have been used to correlate the severity of SDB with the degree of insulin resistance. Although such studies have been highly informative and paved the way for additional research, a major drawback with the use of steady-state measures is their inability to characterize the dynamic relation between insulin sensitivity and insulin secretion. A decrease in peripheral insulin sensitivity is fed back to the pancreatic β-cell, which increases insulin output to maintain normal glucose tolerance (10, 11). A defect in this compensatory response in the face of insulin resistance is central to the pathogenesis of glucose intolerance and type 2 diabetes mellitus (12). Thus, although SDB may lead to deterioration in insulin sensitivity, information on SDB-related alterations in pancreatic β-cell function is lacking.
In light of the above issues, the aim of the current study was to quantify the pathophysiologic alterations responsible for altered glucose metabolism in patients with SDB. To provide new insights into potential mechanisms, the frequently sampled intravenous glucose tolerance test (FSIVGTT) was used along with the minimal model analysis of the resulting data. The minimal model describes the in vivo kinetics of glucose and insulin and provides estimates of peripheral insulin sensitivity and pancreatic β-cell function. The minimal model also provides an assessment of glucose effectiveness, which represents the ability of glucose to facilitate its own disposal independent of an insulin response. DEXA-based body fat mass was determined and used in conjunction with BMI and waist circumference to account for the confounding effects of adiposity. It was hypothesized that, independent of adiposity, the frequency of apneas and hypopneas, the severity of nocturnal hypoxemia, and the degree of sleep fragmentation would be negatively correlated with insulin sensitivity, insulin secretion, and glucose effectiveness.
METHODS
Study Sample and Protocol
The study sample consisted of normal subjects and patients with newly diagnosed but untreated SDB. Normal subjects were recruited from the general community. Patients undergoing polysomnography and confirmed to have SDB were also recruited. Subjects with a fasting glucose greater than 125 mg/dl or those with a history of type 2 diabetes mellitus, angina, myocardial infarction, coronary revascularization, congestive heart failure, or stroke were excluded. In addition, history of a circadian rhythm disorder, chronic insufficient sleep (< 7 hours per night), obstructive lung disease, renal or hepatic dysfunction, upper airway surgery, cancer, or any chronic inflammatory condition also excluded participation. Finally, subjects using anti-inflammatory agents (e.g., steroids), supplemental oxygen, or positive airway pressure therapy were ineligible. After the initial screening visit, those enrolled were counseled on maintaining at least 7 hours of sleep per night and asked to consume at least 250 g of carbohydrates per day. Usual sleep habits were objectively assessed with an ambulatory wrist activity monitor that was worn for at least 5 days before completion of the metabolic tests. Informed consent was obtained from all subjects, and the protocol was approved by the local Institutional Review Board.
Polysomnography
The polysomnogram included recordings of C3/M2 and C4/M1 electroencephalograms (EEG), right and left electrooculograms, submental and bilateral anterior tibialis electromyograms, and body position. Respiration was monitored with a nasal pressure transducer, with thermocouples at the nose and mouth, and with thoracic and abdominal strain gauges. Continuous recording of SaO2 was obtained with an oximeter (Ohmeda 3700; Ohmeda, Englewood, CO). All physiologic signals were digitized for off-line analysis of sleep and breathing patterns (Embla recordings systems; Medcare, Buffalo, NY). Sleep-stage scoring was performed on 30-second epochs according to the criteria of Rechtschaffen and Kales (13). Apneas were identified if airflow was absent in the themistor and nasal cannula channels for at least 10 seconds. Hypopneas were identified if there was at least 30% reduction in airflow lasting at least 10 seconds and associated with a 4% oyxhemoglobin desaturation or an EEG arousal. The apnea-hypopnea index (AHI) was defined as the number of apneas or hypopneas per hour of sleep. The degree of oxyhemoglobin desaturation (ΔSaO2) associated with each disordered breathing event was determined, and the overall average was used along with the percentage of total sleep time below an oxyhemoglobin saturation of 90% as measures of sleep-related hypoxemia. Arousals were identified as abrupt shifts of at least 3 seconds duration in EEG frequency according to standard criteria (14).
Anthropometrics and Assessment of Body Fat
Weight was measured to the nearest 0.1 kg, and height was measured using a portable stadiometer to the nearest 0.5 cm. Waist circumference was measured midway between the lower rib margin and the iliac crest with the subject in the standing position at the end of a normal expiration. Fat mass and percent body fat were determined using a Hologic QDR-4500A (Hologic, Inc., Bedford, MA) DEXA scanner. Percent body fat was determined as the ratio of total fat mass to body weight. All body composition measurements were obtained on the same day as the intravenous glucose tolerance test.
Frequently Sampled Intravenous Glucose Tolerance Test
Subjects were admitted to a clinical research unit (∼ 9 am) after an overnight fast for the insulin-modified FSIVGTT (15, 16). An intravenous line was inserted in the antecubital vein for glucose and insulin administration with a second line in the contralateral antecubital vein for blood sampling. Baseline blood samples were obtained at −15, −10, −5, and −1 minute before the glucose injection. At time 0 minutes, a weight-adjusted dose of glucose (50% dextrose, 0.3 g/kg) was administered intravenously over 1 minute followed by a continuous infusion of normal saline. Twenty minutes after the glucose injection, a weight-adjusted dose of regular insulin (0.03 U/kg) was administered. Blood samples were collected after the glucose injection at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 minutes. Glucose and insulin concentrations for each sample were determined in duplicate. The resulting values were subjected to the minimal model analysis using the MINMOD software (15–17).
The minimal model provides a mathematical representation of glucose disposal during the FSIVGTT using two coupled differential equations (see online supplement). The first equation represents the physiologic factors that facilitate the return of glucose to basal levels after the glucose injection. These include the effects of insulin, which normalize plasma glucose through the glucose transporter system, and the effects of glucose, which can normalize its concentration independent of an insulin response. The second equation describes the movement of insulin from plasma into the interstitial space where it exerts its physiologic actions. By using the measured insulin profile during the FSIVGTT as the input to the model, the glucose concentration profile is determined by nonlinear least-squares fitting, and parameters of insulin-sensitivity (SI) and glucose effectiveness (SG) are numerically estimated. SI quantifies the effect of insulin in enhancing glucose disposal. SG quantifies the effects of glucose on its own disposal independent of any insulin response. SG is further divided into two components: the contribution of hyperglycemia per se to increase glucose disposal and the effect of basal insulin levels. The basal component of SG is referred to as the basal insulin effect and is the product of basal insulin levels (Ib) and SI. The contribution of non-insulin–dependent glucose uptake (glucose effectiveness at zero insulin [GEZI]) to glucose disposal is the difference between total SG and the basal insulin effect: GEZI = SG − (Ib × SI). In addition to estimating SI, SG, and GEZI, the acute insulin response to glucose (AIRg), an index of pancreatic β-cell response, was determined as the area under the insulin curve between 0 and 10 minutes. Finally, the disposition index (DI), which is an integrated measure of pancreatic β-cell function, was calculated as the crosss-product of SI and AIRg. Additional details regarding the minimal model are provided in the online supplement.
Statistical Analysis
The dependent variables derived from the FSIVGTT were SI, SG, GEZI, AIRg, and DI. Each was examined for normality and log-transformed if necessary. Results are reported as arithmetic means along with the standard errors. Bivariate analyses were conducted to describe the association between each FSIVGTT parameter and SDB status. To account for differences in adiposity and other factors across groups of SDB severity, multivariable linear regression models were used. Covariates considered in these multivariable models included age, gender, race, BMI, waist circumference, and percent body fat. To minimize the influence of outlying values, SI and DI were log-transformed before modeling. For variables that remained skewed despite transformation (SG and GEZI), median regression analyses (18) were used. Continuous and categorical forms of each independent variable (e.g., AHI, ΔSaO2) were considered. Results from categorical analyses are presented for ease of interpretation. All statistical analyses were conducted using the SAS 9.0 or Stata 7.0 statistical software packages.
RESULTS
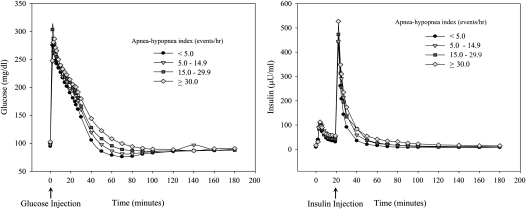
A total of 118 subjects met the enrollment criteria and completed overnight polysomnography and the FSIVGTT. Eleven subjects had missing actigraphy data on habitual sleep patterns. Sensitivity analysis that excluded these subjects showed no major differences in the overall inferences regarding the association between SDB and glucose disposal during the FSIVGTT. Thus, the analysis included all 118 subjects that were grouped as follows: 39 subjects had no SDB (AHI < 5 events/h), 34 had mild SDB (AHI, 5.0–14.9 events/h), 22 had moderate SDB (AHI, 15.0–29.9 events/h), and 23 had severe SDB (AHI ≥ 30.0 events/h). Table 1 summarizes the characteristics of the study sample by SDB status and shows that older age, male sex, and increasing adiposity were associated with greater severity of SDB. Actigraphy monitoring of usual sleep habits showed little difference in the average time spent in bed across the four groups. Estimated habitual sleep duration was similar and was at least 7 hours except in subjects with severe SDB. Finally, compared with normal subjects, those with moderate to severe SDB displayed typical alterations in sleep architecture, including frequent arousals, more stage 1 sleep, and less slow-wave and REM sleep. During the FSIVGTT, patients with SDB had a slower rate of decline in glucose levels after the glucose injection than normal subjects (Figure 1, left). In contrast, the first-phase insulin response or the area under the insulin curve during the first 10 minutes was comparable across the four groups (Figure 1, right). Table 2 shows the unadjusted parameters derived from the minimal model analysis of the glucose and insulin profiles during the FSIVGTT. Insulin sensitivity (SI), glucose effectiveness (SG), and glucose effectiveness at zero insulin (GEZI) progressively decreased with increasing severity of SDB. The acute insulin response to glucose (AIRg) did not vary despite a progressive decline in SI. The disposition index (DI = SI × AIRg) was also lower in patients with moderate to severe SDB.
TABLE 1.
CHARACTERISTIC OF THE STUDY SAMPLE BY SLEEP-DISORDERED BREATHING SEVERITY
| AHI < 5.0 events/h (n = 39) | AHI 5.0–14.9 events/h (n = 34) | AHI 15.0–29.9 events/h (n = 22) | AHI ≥ 30.0 events/h (n = 23) | |
|---|---|---|---|---|
| Demographics | ||||
| Male sex, % | 43.6* | 61.8 | 63.6 | 82.6‡ |
| White race, % | 92.3 | 79.4 | 95.5 | 78.3 |
| Age, yr | 37.7 (1.9) | 46.5 (2.0) | 51.6 (2.0) | 52.4 (2.0)§ |
| Body composition | ||||
| BMI, kg/m2 | 26.5 (0.8) | 29.6 (1.1) | 30.7 (0.8) | 32.8 (1.3)§ |
| Waist circumference, cm | 84.1 (1.8) | 91.8 (2.3) | 96.1 (2.3) | 106.4 (3.2)§ |
| Percent body fat, % | 30.3 (1.7) | 31.9 (1.8) | 32.6 (1.6) | 34.9 (1.6)† |
| Actigraphy monitoring | ||||
| Usual time in bed, h | 7.8 (0.2) | 7.6 (0.2) | 7.8 (0.1) | 7.5 (0.2) |
| Sleep duration, h | 7.2 (0.2) | 7.0 (0.2) | 7.3 (0.1) | 6.5 (0.2) |
| Polysomnography | ||||
| Total sleep time, h | 6.8 (0.2) | 6.9 (0.2) | 6.7 (0.2) | 6.4 (0.2) |
| Stage 1 sleep, % | 8.1 (0.8) | 10.4 (0.9) | 11.1 (1.5) | 15.8 (2.0)§ |
| Stage 2 sleep, % | 55.7 (1.3) | 60.0 (1.4) | 62.7 (1.6) | 62.4 (2.5)§ |
| Slow wave sleep, % | 13.7 (1.2) | 9.8 (1.0) | 7.6 (1.1) | 5.0 (1.4)§ |
| REM sleep, % | 22.5 (0.8) | 19.8 (1.1) | 18.6 (1.6) | 16.7 (1.2)§ |
| Arousal index, n/h | 8.1 (0.4) | 13.3 (1.0) | 19.7 (1.3) | 38.3 (4.3)§ |
| ΔSaO2, % | 3.0 (0.2) | 3.8 (0.2) | 3.8 (0.2) | 7.1 (0.9)§ |
| Fasting serum measurements | ||||
| Glucose, mg/dl | 94.7 (1.2) | 97.9 (1.4) | 100.9 (1.9) | 102.5 (2.2)§ |
| Insulin, μU/ml | 9.0 (0.7) | 12.9 (1.0) | 11.9 (1.2) | 16.4 (1.7)§ |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; REM = rapid eye movement; ΔSaO2 = average oxyhemoglobin desaturation.
Values reported are either percentages or means (SE).
P < 0.05 for comparisons across AHI groups (P values determined by χ2 for categorical variables or Cuzick's nonparametric test for trend for continuous variables).
P < 0.02 for comparisons across AHI groups (P values determined by χ2 for categorical variables or Cuzick's nonparametric test for trend for continuous variables).
P < 0.001 for comparisons across AHI groups (P values determined by χ2 for categorical variables or Cuzick's nonparametric test for trend for continuous variables).
Figure 1.
Glucose and insulin profiles from the frequently sampled intravenous glucose tolerance test in subjects with and without sleep-disordered breathing. Values are average concentrations of glucose (left) and insulin (right) versus time.
TABLE 2.
MINIMAL MODEL ANALYSIS OF THE FREQUENTLY SAMPLED INTRAVENOUS GLUCOSE TOLERANCE TEST DATA
| FSIVGTT parameter | AHI < 5.0 events/h (n = 39) | AHI 5.0–14.9 events/h (n = 34) | AHI 15.0–29.9 events/h (n = 22) | AHI ≥ 30.0 events/h (n = 23) |
|---|---|---|---|---|
| SI, (mU/L)−1 min−1 | 3.7 (0.3)* | 2.5 (0.2) | 2.5 (0.4) | 1.8 (0.3)† |
| AIRg, (mU/L) min−1 | 510.9 (80.1) | 601.0 (69.9) | 518.7 (80.7) | 527.8 (85.6) |
| DI, dimensionless | 1,592.0 (215.3) | 1,332.1 (176.4) | 1,054.5 (219.8) | 845.0 (148.2)† |
| SG × 10−2, min−1 | 2.1 (0.1) | 1.7 (0.1) | 1.6 (0.3) | 1.5 (0.1)† |
| GEZI × 10−3, min−1 | 1.8 (0.1) | 1.4 (0.1) | 1.4 (0.1) | 1.3 (0.1)† |
Definition of abbreviations: AIRg = acute insulin response to glucose; DI = disposition index (SI × AIRg); FSIVGTT = frequently sampled intravenous glucose tolerance test; GEZI = glucose effectiveness at zero insulin; SG = glucose effectiveness; SI = insulin sensitivity.
Values reported as means (standard error).
P < 0.001 for comparisons across AHI groups (P values determined by Cuzick's nonparametric test for trend).
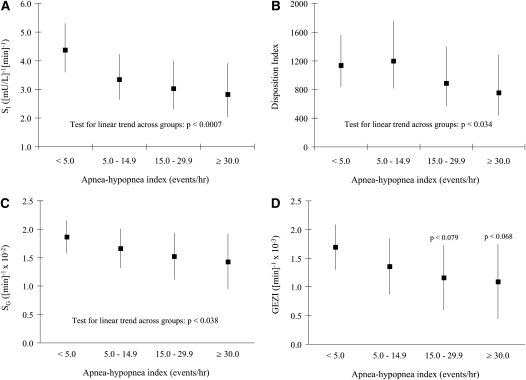
To characterize the independent associations between measures of SDB severity and the FSIVGTT-derived parameters, multivariable regression methods were used. Models were initially constructed with all three measures of adiposity (i.e., BMI, percent fat, and waist circumference) as covariates. However, given the modest to strong correlation between percent body fat and BMI (r = 0.63; P < 0.0001) and between BMI and waist circumference (r = 0.78; P < 0.0001), sensitivity analyses were conducted by including each measure individually and in all possible pairwise combinations. Irrespective of the variables used in the multivariable models, parameter estimates remained essentially unchanged after the inclusion of percent body fat. Thus, to minimize collinearity, percent body fat was used as the single adiposity measure along with age, sex, and race. Despite multivariable adjustments, SDB severity (i.e., AHI) remained inversely associated with SI, DI, SG, and GEZI (Figure 1). Compared with normal subjects, the adjusted mean SI was 26.7, 36.5, and 43.7% lower in patients with mild, moderate, and severe SDB, respectively (Figure 2A; P < 0.007 for linear trend). Although DI was comparable in normal subjects and in subjects with mild SDB, its adjusted mean was also 28.0 and 44.8% lower in patients with moderate and severe SDB, respectively (P < 0.035 for linear trend). SG and GEZI also declined with increasing severity of SDB (Figures 2C and 2D). Although a linear association between AHI and GEZI was not observed, GEZI was lower in patients with moderate and severe SDB than in normal subjects or those with mild SDB (Figure 1D).
Figure 2.
Adjusted values of insulin sensitivity (A: average SI and 95% confidence intervals), the disposition index (B: average DI and 95% confidence intervals), glucose effectiveness (C: median SG and 95% confidence intervals), and glucose effectiveness at zero insulin (D: median GEZI and 95% confidence interval). Values adjusted for age, sex, race, and percent body fat.
Analyses were undertaken to determine whether measures of nocturnal hypoxemia and sleep fragmentation were correlated with the results of the FSIVGTT. SI was most notably associated with the average degree of oxyhemoglobin desaturation (ΔSaO2) in analyses that adjusted for age, sex, race, and percent body fat (Figure 3). DI showed a similar inverse association with average ΔSaO2. For every 1% increase in the average ΔSaO2, the adjusted average DI decreased by 5.7% (P < 0.065). SG and GEZI were not associated with the degree of nocturnal hypoxemia (i.e., ΔSaO2). However, SG was independently associated with the arousal frequency even after accounting for ΔSaO2. For every five-unit increase in the respiratory event–related arousal frequency (events/h), the median adjusted value of SG decreased by 4.3 × 10−4/min. Analyses correlating sleep stage amounts (e.g., slow-wave sleep, stage 1 sleep) to metabolic parameters showed no significant associations after adjusting for percent body fat.
Figure 3.
Adjusted average values of insulin sensitivity (SI) as a function of average oxyhemoglobin desaturation (ΔSaO2) during sleep. Displayed are measured SI values (squares) and the fitted regression values (dark line with 95% confidence bands) adjusted for age, sex, race, and percent body fat.
DISCUSSION
The importance of SDB as an independent risk factor for type 2 diabetes mellitus has been a topic of significant research. The current study adds to the body of existing literature by providing strong evidence that, independent of adiposity, SDB is associated with several metabolic defects, including a decrease in insulin sensitivity, glucose effectiveness, and pancreatic β-cell function. The major implication of these findings is that SDB is characterized by multiple physiologic deficits that are known to increase the predisposition for type 2 diabetes mellitus.
Early investigations of metabolic disturbances in SDB correlated sleep-related symptoms (e.g., snoring) or polysomnographic metrics (e.g., AHI) to a wide array of measures of insulin sensitivity and glycemic status (19, 20). Although pioneering, many of these initial studies had several methodologic limitations, including small sample sizes, the use of selected clinic-based cohorts, and the lack of an appropriate control group. Thus, it is not surprising that these studies offered mixed conclusions, with some arguing for and others against an independent association. A number of published reports have addressed these concerns by including an adequate number of subjects along with objective sleep-related assessments. However, two major limitations remain. First, because of the relatively low burden, investigators have frequently relied on using a static single blood draw measure of fasting insulin levels as a proxy for insulin sensitivity. Although the fasting condition represents a steady state in which glucose is homeostatically maintained in the normal range, fasting insulin levels do not reflect pancreatic insulin secretion in response to nutrient intake. Even after incorporating fasting glucose values, as in the homeostasis model of insulin resistance index (9), it is difficult, if not impossible, to quantify the dynamic relation between insulin sensitivity and insulin secretion. Second, to account for the confounding effects of adiposity, most studies have used BMI as an index for body fat. Although BMI is relatively easy to measure, it does not discriminate lean from fat mass and thus is an imperfect measure of adiposity (7, 8). Because fat mass is a major determinant of whole-body insulin resistance (21), greater precision in measurement of body fat is needed to adequately assess the independent effects of SDB on glucose metabolism. Hence, the most unique aspects of the current study are that total body fat was quantified and that a more informative approach was used that included simultaneous assessments of insulin sensitivity and insulin secretion. Minimal model analysis of the FSIVGTT data showed that insulin sensitivity, glucose effectiveness, and the disposition index were lower in patients with SDB. It is also important to note that as insulin sensitivity decreases, the acute insulin response to glucose (or AIRg) should increase if the pancreatic β-cell can respond adequately. The fact that AIRg was comparable across groups of SDB severity indicates that the pancreatic β-cell does not compensate for the decrease in insulin sensitivity observed in patients with moderate to severe SDB. The primary assertion of the foregoing is that the SDB is associated with multiple deficits that are critical in the pathogenesis of type 2 diabetes mellitus.
The underlying mechanisms for impaired glucose metabolism in SDB are largely unknown. The genesis of metabolic dysfunction in SDB likely involves several distinct but synergistic processes, including activation of the sympathetic nervous system, increase in oxidative stress, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, and low-grade systemic inflammation. It is well established that SDB has profound effects on the sympathetic nervous system (22) and is characterized by higher levels of circulating catecholamines (23, 24). Elevations in norepinephrine and epinephrine levels can induce insulin resistance, inhibit pancreatic insulin secretion, decrease glucose effectiveness, and stimulate hepatic glucose release (25–28). Repetitive cycles of hypoxemia with reoxygenation in SDB can also initiate a cascade of biochemical reactions that increase in oxidative stress in a manner similar to that seen with ischemia-reperfusion injury. Indeed, SDB has been associated with excess production of reactive oxygen species (29–32), which can be harmful to the pancreatic β-cell given its relatively low levels of antioxidant enzymes (33). An increase in oxidative stress suppresses insulin secretion and diminishes insulin-medicated peripheral glucose uptake.
Glucose metabolism could be further disturbed in SDB through activation of the HPA axis by sleep fragmentation (34, 35) or intermittent hypoxemia (36, 37). Glucocorticoids inhibit insulin secretion and induce insulin resistance by increasing free fatty acid flux and modifying multiple aspects of the insulin-mediated glucose transport system (38, 39). Finally, low-grade systemic inflammation may be another mechanism relating SDB to metabolic dysfunction. Despite strong biologic plausibility for a causal role, the majority of interventional studies indicate that continuous positive pressure therapy does not mitigate SDB-related metabolic dysfunction. It is possible that chronic exposure to intermittent hypoxemia and sleep fragmentation lead to irreversible metabolic changes. Alternatively, the small number of subjects in most interventional studies coupled with the overwhelming effects of obesity, a strong correlate of SDB, could explain why treatment has not been found to have favorable effects.
Several caveats should be considered in interpreting our results. First, given the cross-sectional nature of our study, inferences on the cause-and-effect sequence are not possible. Second, despite the numerous insights gained by modeling the kinetics of glucose and insulin, the minimal model is not without its drawbacks (40). Because it is uses a single compartment to model glucose disposal, the minimal model oversimplifies the physiology of glucose homeostasis. Finally, visceral adiposity, which is an important contributor to metabolic risk, was not directly assessed with an abdominal CT or MRI scan. Despite these limitations, the current study has several strenghts. These include a relatively large sample size with full-montage polysomnography, exclusion of prevalent medical conditions, assessment of adiposity, and use of a dynamic test to quantify insulin sensitivity, glucose effectiveness, and pancreatic β-cell function.
In conclusion, our results demonstrate that, independent of adiposity, SDB is associated with impairments in insulin sensitivity, glucose effectiveness, and pancreatic β-cell function. These findings are especially relevant given that it is the concurrence of such defects that increases the propensity for type 2 diabetes mellitus. A critical area that must be addressed in future studies is the relative paucity of information on the specific mechanisms that could explain the metabolic alterations associated with SDB. Perhaps most urgent, however, is the need for well-controlled clinical trials to address whether positive pressure therapy can improve metabolic function and potentially avert some of the risk for type 2 diabetes mellitus and its innumerable sequelae.
Supplementary Material
Acknowledgments
The authors thank the research volunteers who participated in these studies; Kimberly Fetsch and Kelly Devine, RN, for their efforts as the research coordinators; Melissa Minotti, RPSGT, for her assistance with polysomnography; and the Johns Hopkins Bayview General Clinical Research Center sleep technicians, nurses, and nutritionists who helped implement and execute the study protocol.
Supported by grant UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), NIH Roadmap for Medical Research, and National Institutes of Heart, Lung, and Blood Institutes grants HL083640, HL07578 and AG025553.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200809-1392OC on November 14, 2008
Conflict of Interest Statement: N.M.P. has received honoraria and travel support for continuing medical education lectures or symposia sponsored by Respironics and Resmed Inc. B.A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pepperell JC, Davies RJ, Stradling JR. Systemic hypertension and obstructive sleep apnoea. Sleep Med Rev 2002;6:157–173. [DOI] [PubMed] [Google Scholar]
- 2.Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc 2008;5:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005;99:1998–2007. [DOI] [PubMed] [Google Scholar]
- 6.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 2008;5:207–217. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996;143:228–239. [DOI] [PubMed] [Google Scholar]
- 8.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord 2002;26:789–796. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 10.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: evidence for a hyperbolic function. Diabetes 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 11.Pacini G. The hyperbolic equilibrium between insulin sensitivity and secretion. Nutr Metab Cardiovasc Dis 2006;16:S22–S27. [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med 2000;108:2S–8S. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: US Government Printing Office; 1968. [DOI] [PubMed]
- 14.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 15.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677. [DOI] [PubMed] [Google Scholar]
- 16.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86. [DOI] [PubMed] [Google Scholar]
- 17.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015. [DOI] [PubMed] [Google Scholar]
- 18.Koenker R, Hallock KF. Quantile regression. J Econ Perspect 2001;15:143–156. [Google Scholar]
- 19.Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O'Donnell CP. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiolo Neurobiol 2003;136:167–178. [DOI] [PubMed] [Google Scholar]
- 20.Tasali E, Mokhlesi B, Van CE. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008;133:496–506. [DOI] [PubMed] [Google Scholar]
- 21.Paradisi G, Smith L, Burtner C, Leaming R, Garvey WT, Hook G, Johnson A, Cronin J, Steinberg HO, Baron AD. Dual energy X-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome. Diabetes Care 1999;22:1310–1317. [DOI] [PubMed] [Google Scholar]
- 22.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 2003;177:385–390. [DOI] [PubMed] [Google Scholar]
- 23.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest 1993;103:722–727. [DOI] [PubMed] [Google Scholar]
- 24.Dimsdale JE, Coy T, Ziegler MG, ncoli-Israel S, Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep 1995;18:377–381. [PubMed] [Google Scholar]
- 25.Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest 1980;65:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin IK, Weber KM, Boston RC, Alford FP, Best JD. Effects of epinephrine infusion on determinants of intravenous glucose tolerance in dogs. Am J Physiol 1988;255:E668–E673. [DOI] [PubMed] [Google Scholar]
- 27.Lembo G, Capaldo B, Rendina V, Iaccarino G, Napoli R, Guida R, Trimarco B, Sacca L. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol 1994;266:E242–E247. [DOI] [PubMed] [Google Scholar]
- 28.Avogaro A, Toffolo G, Valerio A, Cobelli C. Epinephrine exerts opposite effects on peripheral glucose disposal and glucose-stimulated insulin secretion: a stable label intravenous glucose tolerance test minimal model study. Diabetes 1996;45:1373–1378. [DOI] [PubMed] [Google Scholar]
- 29.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002;165:934–939. [DOI] [PubMed] [Google Scholar]
- 30.Christou K, Moulas AN, Pastaka C, Gourgoulianis KI. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med 2003;4:225–228. [DOI] [PubMed] [Google Scholar]
- 31.Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP, Somers VK. Oxidative stress in obstructive sleep apnoea. Eur Heart J 2005;26:2435–2439. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, Kimura H. Oxidative stress in obstructive sleep apnea. Chest 2005;127:1674–1679. [DOI] [PubMed] [Google Scholar]
- 33.Robertson RP, Harmon JS. Pancreatic islet beta-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett 2007;581:3743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry 1991;29:575–584. [DOI] [PubMed] [Google Scholar]
- 35.Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J. Nocturnal cortisol release in relation to sleep structure. Sleep 1992;15:21–27. [DOI] [PubMed] [Google Scholar]
- 36.Humpeler E, Skrabal F, Bartsch G. Influence of exposure to moderate altitude on the plasma concentraton of cortisol, aldosterone, renin, testosterone, and gonadotropins. Eur J Appl Physiol Occup Physiol 1980;45:167–176. [DOI] [PubMed] [Google Scholar]
- 37.Coste O, Beers PV, Bogdan A, Charbuy H, Touitou Y. Hypoxic alterations of cortisol circadian rhythm in man after simulation of a long duration flight. Steroids 2005;70:803–810. [DOI] [PubMed] [Google Scholar]
- 38.Dinneen S, Alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest 1993;92:2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96:513–523. [DOI] [PubMed] [Google Scholar]
- 40.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–E26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.