Abstract
Rationale: Endothelin-1 (ET-1) is increased in patients with high-altitude pulmonary edema and acute respiratory distress syndrome, and these patients have decreased alveolar fluid reabsorption (AFR).
Objectives: To determine whether ET-1 impairs AFR via activation of endothelial cells and nitric oxide (NO) generation.
Methods: Isolated perfused rat lung, transgenic rats deficient in ETB receptors, coincubation of lung human microvascular endothelial cells (HMVEC-L) with rat alveolar epithelial type II cells or A549 cells, ouabain-sensitive 86Rb+ uptake.
Measurements and Main Results: The ET-1–induced decrease in AFR was prevented by blocking the endothelin receptor ETB, but not ETA. Endothelial–epithelial cell interaction is required, as direct exposure of alveolar epithelial cells (AECs) to ET-1 did not affect Na,K-ATPase function or protein abundance at the plasma membrane, whereas coincubation of HMVEC-L and AECs with ET-1 decreased Na,K-ATPase activity and protein abundance at the plasma membrane. Exposing transgenic rats deficient in ETB receptors in the pulmonary vasculature (ET-B−/−) to ET-1 did not decrease AFR or Na,K-ATPase protein abundance at the plasma membrane of AECs. Exposing HMVEC-L to ET-1 led to increased NO, and the ET-1–induced down-regulation of Na,K-ATPase was prevented by the NO synthase inhibitor l-NAME, but not by a guanylate cyclase inhibitor.
Conclusions: We provide the first evidence that ET-1, via an endothelial–epithelial interaction, leads to decreased AFR by a mechanism involving activation of endothelial ETB receptors and NO generation leading to alveolar epithelial Na,K-ATPase down-regulation in a cGMP-independent manner.
Keywords: endothelium, lung injury, sodium-potassium-exchanging ATPase, acute respiratory distress syndrome
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although it is well known that alveolar epithelial injury decreases alveolar fluid reabsorption (AFR), there has been a paucity of data regarding the role of the endothelium in affecting the alveolar epithelial function and thus the regulation of AFR.
What This Study Adds to the Field
In this study, we show that there is cross-talk between the pulmonary endothelium and alveolar epithelium, specifically that endothelin-1 (ET-1) activates the endothelial ETB receptor that generates nitric oxide and thus causes alveolar epithelial dysfunction.
Alveolar fluid reabsorption (AFR) is regulated by active Na+ transport, whereby Na+ enters alveolar epithelial cells (AECs) via the apical Na+ channels and exits via the basolateral Na,K-ATPase (1, 2). During acute lung injury epithelial and endothelial dysfunction occurs (2–4), leading to edema formation and impairment of the mechanisms responsible for AFR (2–4). In models of lung injury a decrease in the number of Na,K-ATPase molecules at the plasma membrane, via endocytosis and subsequent protein degradation, results in inhibition of Na+ transport and thus AFR (5–8). Therefore, regulation of the Na,K-ATPase represents an important mechanism by which to modulate alveolar epithelial function (6, 9).
Endothelin-1 (ET-1), a potent vasoactive peptide released during injurious stimuli (10, 11), has been reported to increase pulmonary microvascular pressure and lung edema formation via ETA and ETB receptors (12–14). ET-1 is increased in serum and bronchoalveolar lavage of patients with acute lung injury, suggesting lung endothelial–epithelial dysfunction (13, 15–17). The endothelium as a source of oxidative injury releases nitric oxide (NO) via endothelial ETB receptor activation (11, 18, 19). Several reports have indicated that NO down-regulates active sodium transport in AECs via a cGMP-mediated inhibition of epithelial cation channels (20–22). Also, it has been reported that NO decreases sodium absorption across AEC monolayers by inhibiting both amiloride Na+ channels and Na,K-ATPase through a cGMP-independent mechanism (21). Accordingly, we hypothesized that ET-1 decreases active sodium transport in AECs and consequently reduces AFR.
In the present study, we provide the first evidence that endothelial–epithelial interaction is necessary for ET-1 to impair alveolar epithelial function by down-regulating Na,K-ATPase in AECs via the activation of endothelial ETB receptors and NO generation in a cGMP-independent pathway leading to alveolar epithelial dysfunction.
Some of the results of these studies have been previously reported in the form of an abstract (23, 24).
METHODS
Materials
Na,K-ATPase α1 subunit monoclonal antibody (clone 464.6) was purchased from Upstate Biotechnology (Lake Placid, NY). EZ-Link NHS-SS-biotin and streptavidin beads were purchased from Pierce Biochemical (Rockford, IL). Endothelin B receptor antibody was from Novus Biologicals (Littleton, CO). Endothelin-1 antibody was from Alexis Biochemicals as marketed by Axxora (San Diego, CA). Spermine NONOate was from Cayman Chemical (Ann Arbor, MI). Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME), amiloride hydrochloride, cyclic guanosine monophosphate analog 8-BrcGMP (8-bromoguanosine-3′,5′-cyclomonophosphate), and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were from Sigma-Aldrich (St. Louis, MO). 4,5-Diaminofluorescein diacetate (DAF-2 DA) was from Calbiochem (San Diego, CA). All other reagents were commercial products of the highest grade available.
Animals
One hundred and twenty-five pathogen-free male Sprague-Dawley rats weighing 250–310 g (Harlan Sprague-Dawley, Inc., Indianapolis, IN) were studied. In addition, two groups of rats, male and female, were studied: wild-type control [dopamine β-hydroxylase [DBH] transgene−/−, ETB receptor+/+] (12 rats) and transgenic (sl/sl) [DBH transgene+/+, ETB receptor (sl/sl)] (10 rats). These animals were provided by M. Yanagisawa (Southwestern Medical School, University of Texas Southwestern Medical Center, Dallas, Texas). The genotype of each animal was confirmed by polymerase chain reaction of genomic DNA, performed according to standard techniques as previously described (25). All animals were provided food and water ad libitum and maintained on a 12:12 hour light/dark cycle. This study was conducted in accordance with both local institutional guidelines and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Alveolar Epithelial Type II Cells and Human Endothelial Lung Microvascular Cells
Rat alveolar type II (ATII) cells were isolated from the lungs of Sprague-Dawley rats weighing 200–225 g, as previously described (26). ATII and human alveolar epithelial cells (A549 cells [CCL-185; American Type Culture Collection, Manassas, VA]) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Human endothelial lung microvascular cells (HMVEC-L) were purchased from Lonza (Walkersville, MD). HMVEC-L were plated, grown, and maintained as specified by the supplier, using endothelial cell medium (EGM-2 BulletKit; Lonza, Walkersville, MD) containing the following supplements: human epidermal growth factor, hydrocortisone, GA-1000 (gentamicin, amphotericin-B), fetal bovine serum, vascular endothelial growth factor, human fibroblast growth factor (basic), long R3 insulin-like growth factor-I, ascorbic acid, and heparin. All cells were incubated in a humidified atmosphere of 5% CO2−95% air at 37°C. ATII cells isolated from pathogen-free male Sprague-Dawley rats were coincubated with HMVEC-L and endothelial cell medium for various time intervals, 30 minutes and 6 hours. A549 cells were coincubated with HMVEC-L and endothelial cell medium for 30 minutes. All AECs coincubated with HMVEC-L were then switched to DMEM without serum 30 minutes before treatment.
Isolated Lung Experiments
Briefly, rats were anesthetized with pentobarbital (50 mg/kg body weight), tracheotomized, and mechanically ventilated at a rate of 35 rpm, tidal volume of 5 ml/kg, positive end-expiratory pressure of 0 cm H2O, and 100% oxygen for 5 minutes. The chest was opened via a median sternotomy, after which 400 U of heparin sodium was injected into the right ventricle. After exsanguination, the heart and lungs were removed en bloc. The pulmonary artery and left atrium were catheterized and perfused continuously with a solution of 3% bovine serum albumin in buffered physiological salt solution (135.5 mM Na+, 119.1 mM Cl−, 25 mM HCO3−, 4.1 mM K+, 2.8 mM Mg+, 2.5 mM Ca2+, 0.8 mM SO42–, 8.3 mM glucose). Trace amounts of fluorescein isothiocyanate (FITC)-conjugated albumin was also added to the perfusate. The recirculating volume of the constant pressure perfusion system was 90 ml; arterial and venous pressures were set at 12 and 0 cm H2O, respectively. The vascular pressures were recorded every 10 seconds with a multichannel recorder (Cyber Sense Inc., Nicholasville, KY). The lungs were immersed in a “pleural” bath (100 ml) filled with the same bovine serum albumin solution. The entire system was maintained at 37°C in a water bath. Perfusate pH was maintained at 7.40 by bubbling with a gas mixture of 95% O2−5% CO2. The lungs were then instilled via the tracheal cannula in two sequential phases with a total volume of 5 ml of the bovine serum albumin solution containing Evans blue dye-conjugated albumin (0.1 mg/ml), 22Na+ (0.02 μCi/ml), and [3H]mannitol (0.12 μCi/ml). Samples were taken from the instillate, perfusate, and bath solutions after an equilibration time of 10 minutes from the instillation and again 60 minutes later. To ensure homogeneous sampling of the instillate, a volume of 2 ml was aspirated and reintroduced into the airspaces three times before removing each sample. All samples were centrifuged at 3,000 × g for 10 minutes. Absorbance analysis of the supernatant or Evans blue dye–albumin was performed at 620 nm in a Hitachi model U2000 spectrometer (Hitachi, San Jose, CA). Analysis of FITC–albumin (excitation, 487 nm; emission, 520 nm) was performed in a model LS-3B fluorometer (PerkinElmer, Oakbrook, IL) (27). Additional detail on the method for performing these measurements is provided in the online supplement.
Cell Surface Labeling
Cells were labeled for 20 minutes with EZ-Link Sulfo-NHS-SS-biotin (1 mg/ml) and pulled down (captured and purified) with streptavidin as previously described (6, 7). Proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted with an antibody specific for Na,K-ATPase α1 subunit.
Determination of Na,K-ATPase Activity
Na,K-ATPase activity was determined by ouabain-sensitive 86Rb+ uptake (Amersham Biosciences, Arlington Heights, IL) as previously described (6, 7, 29, 30). Protein was quantified in aliquots by the Bradford method (31).
Determination of NO from Endothelial and Alveolar Epithelial Cells through DAF-2 DA Imaging
Epithelial and endothelial cells were cultured on 35-mm glass coverslips and were washed twice with phosphate-buffered saline before a 15- or 30-minute incubation in 1 or 12 μM DAF-2 DA at 37°C in serum-free and phenol and red-free medium (Cellgro; Mediatech, Herndon, VA). Additional detail on the method for making these measurements is provided in the online supplement.
Reverse Transcription-Polymerase Chain Reaction
Additional detail on the method for making these measurements is provided in the online supplement.
Statistical Analysis
Data are reported as means ± SEM. Statistical analysis was done by one-way analysis of variance and Tukey correction. When two groups were compared analysis was done by Student t test. Results were considered significant when P < 0.05.
RESULTS
ET-1 Decreases Alveolar Fluid Reabsorption in Isolated Rat Perfused Lungs
ET-1 decreased AFR by about 40% when perfused for 60 minutes in isolated rat lungs, without affecting the passive fluxes of small or large solutes or FITC flux, indicating that there were no significant changes in epithelial barrier permeability (Figures 1A, 1B, and 1C). Because epithelial Na+ channels and Na,K-ATPase contribute to AFR, we set out to determine whether ET-1 had an effect on either epithelial Na+ channels or Na,K-ATPase in this ex vivo model. As shown in Figure 2A, amiloride or ET-1 decreased AFR by about 50% and the combination of both caused complete inhibition of AFR, suggesting that ET-1 does not act on the amiloride-sensitive Na+ channels. Conversely, the addition of ET-1 and ouabain alone or in combination resulted in a 50% decrease in AFR without evidence of an additive effect (Figure 2B).
Figure 1.
Endothelin-1 (ET-1) decreases alveolar fluid reabsorption (AFR) in isolated perfused rat lungs with no changes in permeability. (A) Isolated rat lungs were perfused for 1 hour with vehicle (control, CT) or ET-1 (10−11−10−6 M) and AFR was measured as described in Methods (n = 6). (B) Passive movement of 22Na+ (solid columns) and [3H]mannitol (open columns) was measured as described in Methods (n = 6). Differences among groups were not statistically significant. (C) Fluorescein isothiocyanate (FITC) flux was measured as described in Methods (n = 6). Differences among groups were not statistically significant. Graphs represent means ± SEM. **P < 0.01.
Figure 2.
Endothelin-1 (ET-1) decreases alveolar fluid reabsorption (AFR) in isolated perfused rat lungs via a decrease in Na,K-ATPase activity. (A) Isolated rat lungs were perfused for 1 hour with vehicle (control, CT) or 10−7 M ET-1 in the presence or absence of 10−4 M amiloride instilled into the airspace. AFR was measured as described in Methods (n = 5). (B) Isolated rat lungs were perfused for 1 hour with vehicle (CT) or 10−7 M ET-1 in the presence or absence of 5 × 10−4 M ouabain (Ouab). AFR was measured as described in Methods (n = 6). Graphs represent means ± SEM. ***P < 0.001.
Endothelial Cells Are Required for the ET-1–mediated Down-regulation of Alveolar Epithelial Na,K-ATPase and Decreased Alveolar Fluid Reabsorption
To determine whether expression of ET receptors was present in alveolar epithelial type I (ATI), ATII, and ATII Day 7 cells, we designed oligonucleotide primers for these receptors and prepro-endothelin (Table 1). As shown in Figure E1A in the online supplement, there was a strong signal for ETB receptor and prepro-endothelin mRNA in all three cell types. Furthermore, Western blot analysis showed the presence of ETB receptor in total cell lysate and plasma membrane of isolated ATII cells (Figure E1B). In addition, immunohistochemistry experiments were performed in rat lungs, where the ATII cell marker LB 180 and the ETB receptor signal colocalized (Figure E1C).
TABLE 1.
OLIGONUCLEOTIDE PRIMERS FOR AMPLIFICATION BY POLYMERASE CHAIN REACTION
| Primer Sequences (5′→3′) | GenBank ID | Expected Size (bp) | |
|---|---|---|---|
| ETA | ETA-5′: CGTCTTCTGCTTGGTTGTCA | NM_012550 | 238 |
| ETA-3′: GCAACAGAGGCATGACTGAA | |||
| ETB | ETB-5′: TGCACACCTTTCCGCAAGCACG | AF074963 | 919 |
| ETB-3′: AGCTGGTGCCCTTCATACAGAAGGC | |||
| PPT | ET-1-5′: GAAGTGTATCTATCAGCAGC | M64711 | 334 |
| ET-1-3′: GGAACACCTCAACCTCTCTTGG | |||
| GAPDH | GAPDH-5′: ACCACAGTCCATGCCATCAC | BC064681 | 452 |
| GAPDH-3′: TCCACCACCCTGTTGCTGTA |
Definition of abbreviations: GAPDH = glyceraldehyde-3-phosphate dehydrogenase; ETA and ETB = endothelin receptors A and B, respectively; PPT = prepro-endothelin.
To determine whether ET-1 had a direct effect on Na,K-ATPase activity and protein membrane abundance in AECs, rat ATII cells were incubated with ET-1 for 15, 30, and 60 minutes. ET-1 did not decrease Na,K-ATPase activity (Figure 3A) or Na,K-ATPase α1 protein abundance at the plasma membrane (Figure 3B). To determine whether activation of endothelial cells by ET-1 is required for regulation of Na,K-ATPase function in the alveolar epithelium we coincubated HMVEC-L with polarized ATII cells for 30 minutes and 6 hours, followed by treatment with ET-1 (10−7 M) for 30 minutes. This resulted in a decrease in Na,K-ATPase activity and α1 subunit protein abundance at the plasma membrane in ATII cells (Figures 4A and 4B). In addition, we observed that the results obtained for rat ATII cells were reproduced when human A549 cells were coincubated with HMVEC-L and then treated with ET-1 (10−7 M) for 30 minutes, as Na,K-ATPase activity decreased approximately 50% (Figure 4C). Furthermore, as depicted in Figure 4D, ET-1 decreased AFR only when added to the vascular space.
Figure 3.
Direct incubation of endothelin-1 (ET-1) on rat alveolar type II (ATII) cells does not decrease Na,K-ATPase activity or protein abundance at the plasma membrane. (A) ATII cells were incubated for 30 minutes with vehicle (control, CT) or with 10−7 M ET-1. Na,K-ATPase activity was determined in ATII cells by ouabain-sensitive 86Rb+ uptake (n = 6). Differences among groups were not statistically significant. (B) ATII cells were incubated with vehicle (CT) or with 10−7 M ET-1 for various time intervals. Na,K-ATPase α1 subunit at the plasma membrane was determined by biotin–streptavidin pulldown and Western blot. A representative Western blot of Na,K-ATPase α1 subunit is shown (n = 3). Differences among groups were not statistically significant. Graphs represent means ± SEM.
Figure 4.
Lung human microvascular endothelial cells (HMVEC-L) are required for the endothelin-1 (ET-1) decrease in Na,K-ATPase activity and fluid reabsorption in alveolar epithelial cells (AECs). (A) HMVEC-L were coincubated with alveolar type II (ATII) cells for 30 minutes and 6 hours, followed by treatment for 30 minutes with vehicle (control, CT) or with 10−7 M ET-1. Na,K-ATPase activity was determined by ouabain-sensitive 86Rb+ uptake in ATII cells (n = 4). (B) HMVEC-L were coincubated with ATII cells for 30 minutes, followed by treatment for 30 minutes with vehicle (CT) or with 10−7 M ET-1. Na,K-ATPase α1 subunit at the plasma membrane was determined by biotin–streptavidin pulldown and Western blot. A representative Western blot of Na,K-ATPase α1 subunit is shown (n = 3). (C) A549 cells were treated for 30 minutes with vehicle (CT) or with 10−7 M ET-1 and coincubated with HMVEC-L for 30 minutes followed by treatment with vehicle (CT) or with 10−7 M ET-1 for 30 minutes. Na,K-ATPase activity was determined by ouabain-sensitive 86Rb+ uptake in A549 cells (n = 3). (D) ET-1 (10−7 M) was perfused for 1 hour or instilled into the airspace of isolated rat lungs and AFR was measured as described in Methods (n = 4). Graphs represent means ± SEM. **P < 0.01; ***P < 0.001.
ET-1 Decreases Alveolar Fluid Reabsorption via Activation of Endothelial ETB Receptor
To determine the type of ET receptor involved in the ET-1–induced decrease in AFR, we conducted experiments with a specific endothelin receptor agonist and antagonists. As shown in Figure 5A, isolated rat lungs perfused with the nonselective ET receptor antagonist PD 142893 prevented the ET-1–induced decrease in AFR. Conversely, perfusion with the selective ETA antagonist BQ-123 did not prevent the ET-1–mediated decrease in AFR. When the specific ETB receptor agonist IRL-1620 was perfused through the pulmonary circulation, in the presence and absence of the ETA antagonist BQ-123, there was, in both cases, a significant decrease in AFR, comparable to that seen with ET-1, with no effect on the passive fluxes of small or large solutes (Figure 5B). To further determine the role of the ETB receptor, we studied whether ET-1 decreased AFR in a transgenic rat model that does not express ETB receptors in the lung endothelium and has minimally functional ETB receptors in the lung parenchyma (32). ET-1 decreased AFR in wild-type rats, but not in transgenic rats, without affecting the passive fluxes of small or large solutes (Figures 6A and 6B). Moreover, the decrease in Na,K-ATPase α1 subunit protein abundance at the basolateral membranes observed in wild-type rats was not observed in transgenic rats (Figure 6C).
Figure 5.
Endothelin-1 (ET-1) decreases alveolar fluid reabsorption via activation of the endothelial ETB receptor. (A) Isolated rat lungs were perfused for 1 hour with vehicle (CT), 10−7 M ET-1, 10−6 M PD 142893 (PD), PD + ET-1, 10−6 M BQ-123 (BQ), BQ + ET-1, 10−6 M IRL-1620, or BQ + IRL-1620 and alveolar fluid reabsorption (AFR) was measured as described in Methods (n = 5). (B) Passive movement of 22Na+ (solid columns) and [3H]mannitol (open columns) was measured as previously described (5) (n = 6). Differences among groups were not statistically significant. Graphs represent means ± SEM. **P < 0.01.
Figure 6.
Endothelin-1 (ET-1) decreases alveolar fluid reabsorption and Na,K-ATPase α1 subunit abundance at the basolateral membrane in rat lungs, via activation of endothelial ETB receptor. (A) Isolated rat lungs of wild-type (WT) and ETB receptor transgenic rats were perfused for 1 hour with vehicle (control, CT) or 10−7 M ET-1 and alveolar fluid reabsorption (AFR) was measured as described in Methods (n = 8). (B) Passive movement of 22Na+ (solid columns) and [3H]mannitol (open columns) was measured as previously described (5) (n = 6). Differences among groups were not statistically significant. (C) Basolateral membranes were purified from the peripheral lung tissue of rat lungs (28) treated as in (A), and Na,K-ATPase protein abundance was assessed by Western blot. A representative Western blot of Na,K-ATPase α1 subunit is shown (n = 3). Graphs represent means ± SEM. **P < 0.01; ***P < 0.001.
ET-1 Decreases Alveolar Fluid Reabsorption via Endothelial Cell NO Generation
It has been described previously that ETB activation generates NO in endothelial cells (11, 18, 19). Thus, we investigated whether the ET-1–induced NO production was responsible for the ET-1–induced decrease in AFR. In our system, HMVEC-L respond to ET-1 by increasing NO production, which was blocked by preincubation with the nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), 100 μM for 30 minutes (Figure 7A). This dose was based on previous reports that showed nitric oxide synthase inhibition as well as restoration of AFR (22, 33). As shown in Figure 7B, when ATII cells were treated with ET-1 there was no increase in NO production. As a positive control for the NO measurement, ATII cells were incubated in the presence of the NO donor spermine NONOate (1 mM). Furthermore, when ATII and A549 cells were treated with spermine NONOate at different concentrations (10–250 μM for 30 min), they had a decrease in Na,K-ATPase activity (Figures 8A and 8B). In addition, pretreatment of HMVEC-L with l-NAME prevented the ET-1–mediated decrease in Na,K-ATPase activity and protein abundance in ATII cells (Figures 8C and 8D). Moreover, as shown in Figure 8E, pretreatment of rat lungs with l-NAME, 100 μM for 30 minutes, prevented the ET-1–induced decrease in AFR.
Figure 7.
Endothelin-1 (ET-1) increases nitric oxide (NO) generation in human microvascular endothelial cells (HMVEC-L) but not in alveolar type II (ATII) cells. (A) NO was determined by immunofluorescence as described in Methods. HMVEC-L were pretreated with vehicle (Basal) or with 100 μM l-NAME for 30 minutes followed by 15 minutes of equilibration to assess baseline rates of fluorescence changes, followed by treatment with ET-1 (10−7 M) for 5 minutes (n = 3). (B) NO was determined by immunofluorescence as described in Methods. After 15 minutes of equilibration to assess baseline rates of fluorescence changes, ET-1 (10−7 M) or spermine NONOate (1 mM) was added for 5 minutes (n = 3). Graphs represent means ± SEM. **P < 0.01; ***P < 0.001.
Figure 8.
Endothelin-1 (ET-1) decreases alveolar fluid reabsorption via nitric oxide generation from endothelial cells. (A) alveolar type II (ATII) cells were incubated for 30 minutes with vehicle (control, CT) or with 50, 100, and 250 μM spermine NONOate. Na,K-ATPase activity was determined in ATII cells by ouabain-sensitive 86Rb+ uptake (n = 3). (B) A549 cells were incubated for 30 minutes with vehicle (CT) or with 10 μM spermine NONOate. Na,K-ATPase activity was determined in A549 cells by ouabain-sensitive 86Rb+ uptake (n = 3). (C) Human microvascular endothelial cells (HMVEC-L) were treated for 30 minutes with vehicle (CT) or with 10−7 M ET-1, in the presence and absence of 100 μM l-NAME, and coincubated with ATII cells. Na,K-ATPase activity was determined in ATII cells by ouabain-sensitive 86Rb+ uptake (n = 3). (D) HMVEC-L were treated for 30 minutes with vehicle (CT) or 10−7 M ET-1 in the presence and absence of 100 μM l-NAME and coincubated with ATII cells. Na,K-ATPase α1 subunit at the plasma membrane was determined by biotin–streptavidin pulldown and Western blot. A representative Western blot of Na,K-ATPase α1 subunit is shown (n = 4). (E) Isolated rat lungs were perfused for 1 hour with vehicle (CT) or with 10−7 M ET-1 in the presence and absence of 100 μM l-NAME, and alveolar fluid reabsorption was measured as described in Methods (n = 5). Graphs represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
ET-1 Decreases Alveolar Fluid Reabsorption via a cGMP-independent Pathway
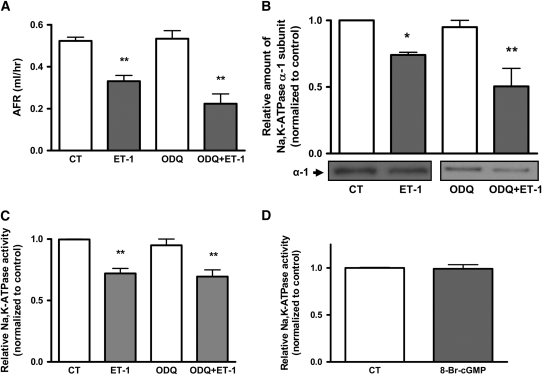
Classically, it has been described that NO acts via activation of soluble guanylate cyclase (22, 34, 35). To determine whether the decrease in AFR is via a cGMP-dependent pathway, we pretreated isolated rat lungs with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), a highly selective inhibitor of soluble guanylate cyclase. Figure 9A shows that when ODQ, 15 μM, was instilled into the airspace, it did not block the effect of the ET-1–induced decrease in AFR. Moreover, pretreatment of ATII cells with ODQ, 15 μM for 30 minutes, did not prevent the ET-1–induced decrease in Na,K-ATPase activity and protein abundance at the plasma membrane (Figures 9B and 9C) in the coculture system. In addition, the cGMP agonist 8-BrcGMP (100 μM), when added exogenously to ATII cells for 30 minutes, did not decrease Na,K-ATPase activity (Figure 9D).
Figure 9.
Endothelin-1 (ET-1) decreases alveolar fluid reabsorption (AFR) via a cGMP-independent pathway. (A) Isolated rat lungs were perfused for 1 hour with vehicle (control, CT) or 10−7 M ET-1 in the presence and absence of 15 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) instilled into the airspace. AFR was measured as described in Methods (n = 4). (B) Human microvascular endothelial cells (HMVEC-L) were treated for 30 minutes with vehicle (CT) or 10−7 M ET-1 in the presence or absence of 15 μM ODQ and coincubated with alveolar type II (ATII) cells. Na,K-ATPase α1 subunit at the plasma membrane was determined by biotin–streptavidin pulldown and Western blot. A representative Western blot of Na,K-ATPase α1 subunit is shown (n = 3). (C) HMVEC-L were treated as in (B) and coincubated with ATII cells. Na,K-ATPase activity was determined in ATII cells by ouabain-sensitive 86Rb+ uptake (n = 3). (D) ATII cells were incubated for 30 minutes with vehicle (CT) or 100 μM 8-BrcGMP. Na,K-ATPase activity was determined by ouabain-sensitive 86Rb+ uptake (n = 3). Graphs represent means ± SEM. Differences among groups were not statistically significant. *P < 0.05; **P < 0.01.
DISCUSSION
It is widely accepted that during lung injury AFR is decreased as the result of epithelial injury leading to the down-regulation of alveolar epithelial Na,K-ATPase (36, 37). However, the interactions of endothelial and epithelial cells in the regulation of alveolar epithelium function have not been elucidated.
ET-1 increases pulmonary microvascular pressure, causing edema formation, and several studies have suggested that ET-1 receptor inhibition has a protective effect in models of acute lung injury (12, 38–40). ET-1 is a potent vasoactive peptide released by endothelial and epithelial cells during stimuli such as shear stress, hypoxia, and inflammation (11, 18, 19). Levels of ET-1 are increased in patients with acute respiratory distress syndrome (ARDS) and high-altitude pulmonary edema (13, 16, 17, 41–43). We demonstrated that ET-1 decreased AFR by 50% in isolated perfused rat lungs without having any significant effect on the epithelial barrier (see Figure 1). In addition, the ET-1–induced decrease in AFR was further reduced when amiloride was present in the airspace. Conversely, when ouabain was perfused via the pulmonary circulation there was no further reduction in AFR, suggesting that the ET-1–induced decrease in AFR occurs via an effect on Na,K-ATPase (see Figure 2).
ET-1 has multiple effects depending on which receptor is activated. Jain and colleagues reported the presence of these receptors in ATII cells (14). Although ETB receptors are expressed in ATII cells (see Figure E1), ET-1–induced down-regulation of sodium transport in AECs requires the activation of endothelial cells as evidenced by the lack of response of ATII cells when they were directly incubated with ET-1 (see Figure 3). Conversely, when ATII cells where coincubated with HMVEC-L in the presence of ET-1, Na,K-ATPase activity and protein abundance at the plasma membrane were decreased. Furthermore, this effect remained even after coincubating HMVEC-L and ATII cells for a longer time period (see Figures 4A and 4B). In addition, the ET-1–induced decrease in Na,K-ATPase activity occurred in A549 cells coincubated with HMVEC-L, demonstrating that the ET-1–induced Na,K-ATPase down-regulation is independent of the cell species, as rat (ATII) and human (A549) alveolar epithelial cells responded similarly (see Figure 4C). Consistent with these in vitro findings, ET-1 decreased AFR only when added to the vascular space, suggesting that ET-1 decreases AFR by down-regulating the Na,K-ATPase function in AECs via its effects on endothelial cells. Consequently, we hypothesized that endothelial ETB receptor is involved in the ET-1–induced decrease in AFR. ETB receptor in smooth muscle has a vasoconstrictor effect, whereas in endothelial cells it causes the release of NO, leading to vasodilation (11, 18, 19). To determine the type of ET receptor involved in the ET-1–induced decrease in AFR, we performed studies using a specific endothelin receptor agonist and antagonists, showing that the ETB receptor is responsible for the ET-1–induced decrease in AFR (see Figure 5). To further elucidate the role of the ETB receptor in the ET-1–induced decrease in AFR we used an ETB transgenic rat model (25, 32). The spotting lethal rat strain has a nonfunctional ETB receptor leading to intestinal agangliosis, intestinal obstruction, and death shortly after birth (44). Gariepy and colleagues rescued the spotting lethal rat by transgenic expression of the ETB gene, using the DBH promoter. Rats carrying the transgene were crossed with spotting lethal rats, which resulted in animals that express ETB only under the transcriptional control of the DBH promoter. These animals lack expression of the ETB receptor except in adrenergic tissue (25). Ivy and colleagues characterized ETB receptor expression in these animal lungs, finding no mRNA signal in the pulmonary arteries and virtually nonfunctional ETB receptors in the lung parenchyma (32). Lungs isolated from these transgenic rats did not have an ET-1–induced decrease in AFR or a down-regulation of Na,K-ATPase protein abundance at the basolateral membrane (see Figure 6), further demonstrating that ETB receptors are required for the ET-1–induced decrease in AFR.
As it has been previously described, NO decreases amiloride-sensitive sodium transport in AECs and inhibits AFR (20–22, 45–47). Guo and colleagues showed inhibition of Na,K-ATPase in ATII cells after exposure to propylamine propylamine (PAPA) NONOate (21). Kaestle and colleagues showed that acute elevation of hydrostatic pressures in isolated perfused rat lungs caused an increase in endothelial NO levels leading to a decrease in AFR (22). Conversely, mechanisms associated with a decrease in AFR in noncardiogenic pulmonary edema such as ARDS or high-altitude pulmonary edema cannot be explained by an increase in hydrostatic pressure causing an increase in NO release. In addition, patients with congestive heart failure have increased levels of ET-1 (48–51) and ET-1 is released from endothelial cells when exposed to stretch or shear stress (11, 52). Here, we provide evidence that ET-1, which is increased in cardiogenic and noncardiogenic pulmonary edema, increases endothelial NO levels, which are responsible for the decrease in AFR (see Figures 7 and 8). Although our results are consistent with previous reports that NO decreases active sodium transport (20–22, 45), the ET-1–mediated effect appears not to inhibit the amiloride-sensitive sodium channels but the Na,K-ATPase. We speculate that the difference in the effects could be explained by the endothelial source of NO, which probably interacts with the basolateral AEC domains where Na,K-ATPase is located, as opposed to the epithelial apical amiloride-sensitive sodium channels (1, 2, 4, 9). Also, the ET-1–mediated decrease in active sodium transport occurs via a cGMP-independent pathway, as opposed to most reports, which have shown it to be a cGMP-dependent pathway (20–22, 45). Our results are in agreement with the initial description by Goodman and colleagues, in which the exogenous addition of 8-Br-cGMP did not alter dome formation in AECs (53), and with Guo and colleagues, who reported that when ATII cells were exposed to various concentrations of NO donors there was no increase in cGMP levels and 8-BrcGMP added to the bathing solution of ATII cells did not alter their short circuit currents (21). Several mechanisms have been proposed to explain the decrease in epithelial sodium transport via a cGMP-independent mechanism, including reactive nitrogen species promoting the endocytosis of Na,K-ATPase, posttranslational modifications of Na,K-ATPase by oxidizing and/or nitrosylating key thiol residues, or modifying structural proteins such as actin (21, 54).
In summary we provide the first evidence that ET-1 decreases the ability of the lungs to clear alveolar fluid. Furthermore, the endothelial activation by ETB receptors and NO generation leads to Na,K-ATPase down-regulation and decreased AFR via a cGMP-independent mechanism in the alveolar epithelium (Figure 10). These findings have potential clinical implications, as patients with ARDS have higher levels of ET-1 (17, 41, 42), which could be pharmacologically modulated with endothelin receptor antagonists.
Figure 10.
Endothelin-1 binds to the ETB receptor in endothelial cells, releasing nitric oxide, which leads to the down-regulation of Na,K-ATPase function in alveolar type II (ATII) cells.
Supplementary Material
Supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants R01 HL048129-14 and K01HL080966-01.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200804-540OC on October 23, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600. [DOI] [PubMed] [Google Scholar]
- 2.Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med 2007;33:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidot DM, Folkesson HG, Jain L, Sznajder JI, Pittet JF, Matthay MA. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol 2006;291:L301–L306. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2005;2:206–213. [DOI] [PubMed] [Google Scholar]
- 5.Briva A, Vadasz I, Lecuona E, Welch LC, Chen J, Dada LA, Trejo HE, Dumasius V, Azzam ZS, Myrianthefs PM, et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE 2007;2:e1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res 2006;98:1314–1322. [DOI] [PubMed] [Google Scholar]
- 7.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-ζ. J Clin Invest 2003;111:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadasz I, Morty RE, Olschewski A, Konigshoff M, Kohstall MG, Ghofrani HA, Grimminger F, Seeger W. Thrombin impairs alveolar fluid clearance by promoting endocytosis of Na+,K+-ATPase. Am J Respir Cell Mol Biol 2005;33:343–354. [DOI] [PubMed] [Google Scholar]
- 9.Dada LA, Sznajder JI. Mechanisms of pulmonary edema clearance during acute hypoxemic respiratory failure: role of the Na,K-ATPase. Crit Care Med 2003;31:S248–S252. [DOI] [PubMed] [Google Scholar]
- 10.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol 2007;293:L52–L59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marasciulo FL, Montagnani M, Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem 2006;13:1655–1665. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter TC, Stenmark KR. Endothelin receptor blockade decreases lung water in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 2000;279:L547–L554. [DOI] [PubMed] [Google Scholar]
- 13.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res 2001;2:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain R, Shaul PW, Borok Z, Willis BC. Endothelin-1 induces alveolar epithelial–mesenchymal transition through endothelin type A receptor–mediated production of TGF-β1. Am J Respir Cell Mol Biol 2007;37:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink MP. Role of reactive oxygen and nitrogen species in acute respiratory distress syndrome. Curr Opin Crit Care 2002;8:6–11. [DOI] [PubMed] [Google Scholar]
- 16.Modesti PA, Vanni S, Morabito M, Modesti A, Marchetta M, Gamberi T, Sofi F, Savia G, Mancia G, Gensini GF, et al. Role of endothelin-1 in exposure to high altitude: Acute Mountain Sickness and Endothelin-1 (ACME-1) Study. Circulation 2006;114:1410–1416. [DOI] [PubMed] [Google Scholar]
- 17.Nakano Y, Tasaka S, Saito F, Yamada W, Shiraishi Y, Ogawa Y, Koh H, Hasegawa N, Fujishima S, Hashimoto S, et al. Endothelin-1 level in epithelial lining fluid of patients with acute respiratory distress syndrome. Respirology 2007;12:740–743. [DOI] [PubMed] [Google Scholar]
- 18.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 2004;61:227–237. [DOI] [PubMed] [Google Scholar]
- 19.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000;102:2434–2440. [DOI] [PubMed] [Google Scholar]
- 20.Jain L, Chen XJ, Brown LA, Eaton DC. Nitric oxide inhibits lung sodium transport through a cGMP-mediated inhibition of epithelial cation channels. Am J Physiol 1998;274:L475–L484. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, DuVall MD, Crow JP, Matalon S. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol 1998;274:L369–L377. [DOI] [PubMed] [Google Scholar]
- 22.Kaestle SM, Reich CA, Yin N, Habazettl H, Weimann J, Kuebler WM. Nitric oxide–dependent inhibition of alveolar fluid clearance in hydrostatic lung edema. Am J Physiol Lung Cell Mol Physiol. 2007. [DOI] [PubMed]
- 23.Comellas A, Litvan J, Briva A, Lecuona E, Chen J, Sznajder JI. Endothelin decreases lung edema clearance and Na,K-ATPase activity in alveolar epithelial cells via ET-B receptor and nitric oxide generation [abstract]. FASEB J 2006;20:A1071. [Google Scholar]
- 24.Comellas AP, Litvan J, Lecuona E, Azzam Z, Butti M, Yanagisawa M, Sznajder JI. Endothelin decreases lung edema clearance in alveolar epithelial cells via ET-B receptor and NO generation [abstract]. Am J Respir Crit Care Med 2007;175:A841. [Google Scholar]
- 25.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest 1998;102:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridge KM, Rutschman DH, Factor P, Katz AI, Bertorello AM, Sznajder JL. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. Am J Physiol 1997;273:L246–L255. [DOI] [PubMed] [Google Scholar]
- 27.Rutschman DH, Olivera W, Sznajder JI. Active transport and passive liquid movement in isolated perfused rat lungs. J Appl Physiol 1993;75:1574–1580. [DOI] [PubMed] [Google Scholar]
- 28.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. β-Adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem 2006;281:19892–19898. [DOI] [PubMed] [Google Scholar]
- 29.Dada LA, Welch LC, Zhou G, Ben-Saadon R, Ciechanover A, Sznajder JI. Phosphorylation and ubiquitination are necessary for Na,K-ATPase endocytosis during hypoxia. Cell Signal 2007;19:1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comellas AP, Pesce LM, Azzam Z, Saldias FJ, Sznajder JI. Scorpion venom decreases lung liquid clearance in rats. Am J Respir Crit Care Med 2003;167:1064–1067. [DOI] [PubMed] [Google Scholar]
- 31.Ridge KM, Dada L, Lecuona E, Bertorello AM, Katz AI, Mochly-Rosen D, Sznajder JI. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-ɛ and -δ. Mol Biol Cell 2002;13:1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivy D, McMurtry IF, Yanagisawa M, Gariepy CE, Le Cras TD, Gebb SA, Morris KG, Wiseman RC, Abman SH. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 2001;280:L1040–L1048. [DOI] [PubMed] [Google Scholar]
- 33.Fouty B, Komalavilas P, Muramatsu M, Cohen A, McMurtry IF, Lincoln TM, Rodman DM. Protein kinase G is not essential to NO-cGMP modulation of basal tone in rat pulmonary circulation. Am J Physiol 1998;274:H672–H678. [DOI] [PubMed] [Google Scholar]
- 34.McKee M, Scavone C, Nathanson JA. Nitric oxide, cGMP, and hormone regulation of active sodium transport. Proc Natl Acad Sci USA 1994;91:12056–12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 1991;88:4651–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saldias FJ, Lecuona E, Comellas AP, Ridge KM, Sznajder JI. Dopamine restores lung ability to clear edema in rats exposed to hyperoxia. Am J Respir Crit Care Med 1999;159:626–633. [DOI] [PubMed] [Google Scholar]
- 37.Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med 1999;159:603–609. [DOI] [PubMed] [Google Scholar]
- 38.Cox RA, Enkhabaatar P, Burke AS, Katahira J, Shimoda K, Chandra A, Traber LD, Herndon DN, Hawkins HK, Traber DL. Effects of a dual endothelin-1 receptor antagonist on airway obstruction and acute lung injury in sheep following smoke inhalation and burn injury. Clin Sci (Lond) 2005;108:265–272. [DOI] [PubMed] [Google Scholar]
- 39.Fujii Y, Magder S, Cernacek P, Goldberg P, Guo Y, Hussain SN. Endothelin receptor blockade attenuates lipopolysaccharide-induced pulmonary nitric oxide production. Am J Respir Crit Care Med 2000;161:982–989. [DOI] [PubMed] [Google Scholar]
- 40.Hubloue I, Biarent D, Abdel Kafi S, Bejjani G, Melot C, Naeije R, Leeman M. Endothelin receptor blockade in canine oleic acid–induced lung injury. Intensive Care Med 2003;29:1003–1006. [DOI] [PubMed] [Google Scholar]
- 41.Druml W, Steltzer H, Waldhausl W, Lenz K, Hammerle A, Vierhapper H, Gasic S, Wagner OF. Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis 1993;148:1169–1173. [DOI] [PubMed] [Google Scholar]
- 42.Langleben D, DeMarchie M, Laporta D, Spanier AH, Schlesinger RD, Stewart DJ. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis 1993;148:1646–1650. [DOI] [PubMed] [Google Scholar]
- 43.Sartori C, Vollenweider L, Loffler BM, Delabays A, Nicod P, Bartsch P, Scherrer U. Exaggerated endothelin release in high-altitude pulmonary edema. Circulation 1999;99:2665–2668. [DOI] [PubMed] [Google Scholar]
- 44.Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci USA 1996;93:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardiman KM, McNicholas-Bevensee CM, Fortenberry J, Myles CT, Malik B, Eaton DC, Matalon S. Regulation of amiloride-sensitive Na+ transport by basal nitric oxide. Am J Respir Cell Mol Biol 2004;30:720–728. [DOI] [PubMed] [Google Scholar]
- 46.Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 2006;173:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen VG, Baird MS, Chen L, Matalon S. DETANONOate, a nitric oxide donor, decreases amiloride-sensitive alveolar fluid clearance in rabbits. Am J Respir Crit Care Med 2000;161:1154–1160. [DOI] [PubMed] [Google Scholar]
- 48.Yip HK, Wu CJ, Chang HW, Yang CH, Yu TH, Chen YH, Hang CL. Prognostic value of circulating levels of endothelin-1 in patients after acute myocardial infarction undergoing primary coronary angioplasty. Chest 2005;127:1491–1497. [DOI] [PubMed] [Google Scholar]
- 49.Lepailleur-Enouf D, Egidy G, Philippe M, Louedec L, Henry J, Mulder P, Michel J. Pulmonary endothelinergic system in experimental congestive heart failure. Cardiovasc Res 2001;49:330–339. [DOI] [PubMed] [Google Scholar]
- 50.Sirvio ML, Helin K, Stewen P, Tikkanen I, Fyhrquist F. Endothelin-1 in heart and pulmonary tissue in experimental heart failure. Blood Press 1997;6:250–255. [DOI] [PubMed] [Google Scholar]
- 51.Tsutamoto T, Hisanaga T, Fukai D, Wada A, Maeda Y, Maeda K, Kinoshita M. Prognostic value of plasma soluble intercellular adhesion molecule-1 and endothelin-1 concentration in patients with chronic congestive heart failure. Am J Cardiol 1995;76:803–808. [DOI] [PubMed] [Google Scholar]
- 52.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, Maemura K, Kurihara H, Aikawa R, et al. Endothelin-1 is involved in mechanical stress–induced cardiomyocyte hypertrophy. J Biol Chem 1996;271:3221–3228. [DOI] [PubMed] [Google Scholar]
- 53.Goodman BE, Brown SE, Crandall ED. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol 1984;57:703–710. [DOI] [PubMed] [Google Scholar]
- 54.Song W, Matalon S. Modulation of alveolar fluid clearance by reactive oxygen–nitrogen intermediates. Am J Physiol Lung Cell Mol Physiol 2007;293:L855–L858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.