Abstract
Neuroendocrine malignancies of the gastroenteropancreatic axis include carcinoid and pancreatic endocrine tumors. These heterogeneous neoplasms arise from the enterochromaffin cells of the gastrointestinal tract and the islet cells of the pancreas. Histologically, most well-differentiated endocrine tumors consist of small, round, monomorphic cells, arranged in islands or trabeculae, with a distinct “salt-and-pepper” pattern of nuclear chromatin. Chromogranin and synaptophysin are useful as immunohistochemical markers of neuroendocrine differentiation. Other common features include the capacity to secrete peptide hormones and biogenic amines. A relatively indolent growth rate is characteristic of most gastrointestinal neuroendocrine tumors, with the exception of poorly differentiated tumors which are usually aggressive. Treatment strategies are designed to limit tumor progression and palliate hormonal syndromes. This article reviews the diverse biologic characteristics of gastrointestinal neuroendocrine tumors and current treatment options for metastatic disease.
Neuroendocrine malignancies of the gastroenteropancreatic axis include carcinoid and pancreatic endocrine tumors (PETs). Metastatic gastroenteropancreatic neuroendocrine tumors are typically indolent malignancies characterized by a propensity to secrete hormones and vasoactive substances, resulting in characteristic clinical syndromes. Their clinical behavior varies based on site of tumor origin and histologic differentiation, which appear to be the most important prognostic factors in the natural history of metastatic carcinoid tumors. Although most gastrointestinal neuroendocrine tumors (NETs) are characterized by a relatively slow growth rate, poorly differentiated (PD) neuroendocrine carcinomas are highly aggressive malignancies.
Survival of patients with metastatic carcinoid and pancreatic endocrine tumors appears to have improved over the years. Treatment strategies largely aim to limit tumor progression and palliate hormonal syndromes. The distinct biologic characteristics of gastrointestinal neuroendocrine tumors and current treatment options for metastatic disease are reviewed herein.
CARCINOID TUMORS
Carcinoid tumors are thought to arise from enterochromaffin cells in the intestine and bronchial tree. They were first described in 1888 by Lubarsch who identified multifocal ileal tumors in two autopsy specimens. 1 In 1907, Oberndorfer coined the term “karzinoid tumoren” to describe ileal tumors that appeared to behave more indolently than typical intestinal adenocarcinomas. 2 The term “carcinoid” (meaning “cancer like”) is somewhat of a misnomer, since even the smallest ileal neuroendocrine tumors can metastasize. Nevertheless, the terminology has persisted, despite efforts to revise the nomenclature. Approximately 70% of carcinoid tumors arise in the gastrointestinal (GI) tract and about 25% originate in the lungs.3,4 Other rare primary sites include larynx, thymus, kidneys, and ovaries.
It was not until the 1950s that the carcinoid syndrome was described and serotonin was identified as the primary secretory product associated with symptoms such as flushing and diarrhea.5,6 Serotonin is derived from the amino acid tryptophan, and is inactivated by the liver into 5-hydroxyindoleacetic acid (5-HIAA), a urinary metabolite. Consequently, the carcinoid syndrome occurs primarily in patients with metastatic tumors that secrete serotonin directly into the systemic (rather than portal) circulation. Other vasoactive substances elaborated by carcinoid tumors include biogenic amines (such as histamine, dopamine, and hydroxytryptophan), tachykinins (kallikrein, substance P), and prostaglandins.7–10
Carcinoid heart disease typically occurs in patients with high levels of circulating serotonin.11,12 Characteristic thickening and fibrosis of right-sided cardiac valves produces tricuspid regurgitation and pulmonary stenosis.13 The right heart is invariably affected due to its direct exposure to serotonin secreted by liver metastases. Left heart valves are clinically involved in only 10% of cases. The precise underlying mechanism of valvular fibroblast proliferation is uncertain.14,15
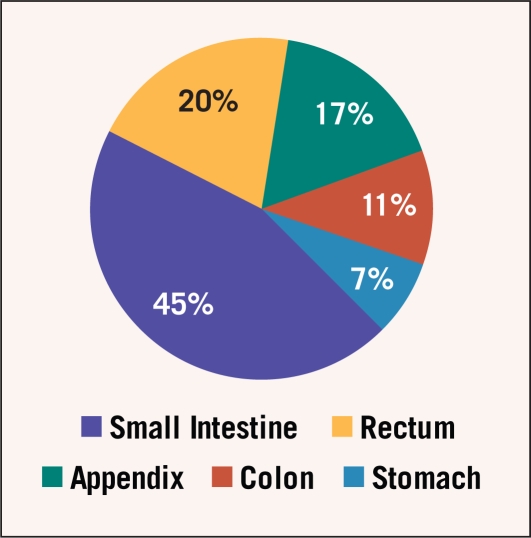
The annual clinical incidence of carcinoid tumors is estimated to be 2–4 per 100,000,3,4 although studies that incorporate autopsy data indicate a higher subclinical incidence of 8 per 100,000.16 The most common site of origin is the small intestine, followed by the rectum, appendix, colon, and stomach (Figure 1).4 Median age at diagnosis is 60, with a slight female preponderance.4
Figure 1.
Distribution of gastrointestinal carcinoid tumors by primary tumor site.4
Carcinoid Tumor Subtypes
Carcinoid tumors have distinct characteristics, depending on their site of origin. In the 1960s, Williams et al classified carcinoid tumors based on embryologic derivation, distinguishing between foregut (bronchial, stomach, duodenal), midgut (jejunal, ileal, cecal, appendiceal), and hindgut (distal colon and rectal) tumors.17 As a rule of thumb, midgut carcinoid tumors, arising primarily from the ileocecal region, produce the typical carcinoid syndrome, hindgut tumors are hormonally inactive, and foregut tumors may be associated with atypical hormonal syndromes. While this classification has some utility, it is now evident that each specific site possesses its own unique clinical characteristics.
Gastric Carcinoid Tumors
Carcinoid tumors of the stomach originate from gastric neuroendocrine cells termed “enterochromaffin-like” (ECL) cells.18 They can develop sporadically, or arise from the trophic effects of elevated serum gastrin. Three distinct types have been identified.18–20
Type I tumors occur in the setting of chronic atrophic gastritis and account for about 80% of gastric carcinoids.18,19,21–23 In this condition, serum gastrin rises in response to gastric achlorhydria. Elevated serum gastrin, in turn, causes diffuse ECL hyperplasia and development of multifocal, polypoid carcinoid tumors. These tumors are generally benign, with no reported cases of tumor-related mortality.18 The diagnosed incidence of type I gastric carcinoid tumors has been rising markedly with increasing use of upper GI endoscopy.19 Usually, these tumors can be managed conservatively with endoscopic surveillance and snare polypectomy.24 Rarely, antrectomy is recommended to eliminate the underlying gastrin stimulus.25,26
Type II gastric carcinoids likewise arise in the setting of hypergastrinemia. In these rare tumors, elevated gastrin is produced by pancreatic or duodenal gastrinomas typically in the setting of multiple endocrine neoplasia 1 (MEN1).27 As is the case in type I gastric carcinoids, tumors tend to be small, multifocal, and clinically indolent.18,27 Instances of tumor regression have been described among patients treated with somatostatin analogs.28
Sporadic gastric carcinoid tumors (type III) occur in about 15% of cases and are not associated with elevated gastrin levels.27 These tumors have a much higher malignant potential than type I or type II gastric carcinoids, and are typically managed with radical gastrectomy when discovered at an early stage.
Ileocecal Carcinoid Tumors
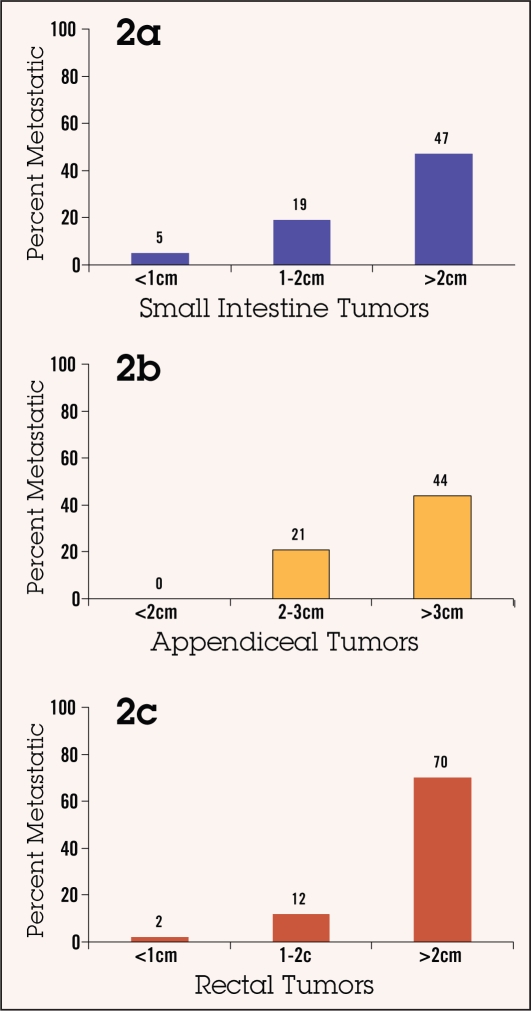
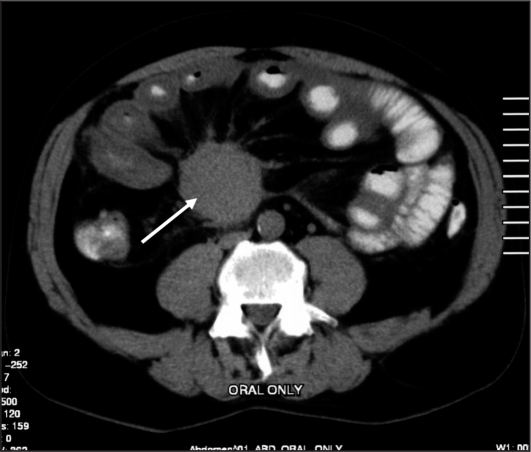
The majority of carcinoid tumors originate in the terminal ileum, where the concentration of enterochromaffin cells is highest. Up to 25% of ileal carcinoid tumors are multifocal on pathologic examination.29 Malignant potential correlates closely with tumor size, however, even tumors smaller than 1 cm can metastasize (Figure 2a).30,31 The most frequent sites of distant spread are the liver, bone, and peritoneal cavity.31,32 Lymph node metastases at the root of the mesentery are common, and may be associated with dense desmoplastic fibrosis, rendering them unresectable (Figure 3).29,31
Figure 2.
Relationship between tumor size and frequency of distant metastases in carcinoid tumors of the small intestine (2a),29 appendix (2b),40 and rectum (2c).44
Figure 3.
Metastatic carcinoid tumor to the root of the mesentery (arrow) causing typical circumferential desmoplastic fibrosis.
The carcinoid syndrome occurs primarily in patients with serotonin-secreting metastatic small intestinal carcinoid tumors. Common symptoms include flushing (a vasomotor phenomenon described as a sensation of warmth associated with erythema) and diarrhea.5,31,33,34 Bronchospastic symptoms occur less frequently. The term “carcinoid crisis” describes circulatory collapse caused by an acute release of serotonin and other vasoactive substances into the circulation.35,36 Triggers include general anesthesia37 and epinephrine.36
Appendiceal Carcinoid Tumors
Appendiceal carcinoid tumors are found in approximately 1 in 300 appendectomy specimens, nearly always incidentally.38 They typically arise from submucosal endocrine cells at the tip of the appendix.39 Median age at presentation is approximately 40 with a female predominance of 2 to 1.40 Large retrospective series confirm that metastatic disease occurs exclusively in tumors larger than 2 centimeters, regardless of local invasiveness (Figure 2b).41 Thus, simple appendectomy is sufficient for the majority of appendiceal carcinoid tumors, whereas staging studies and right hemicolectomies are generally indicated for tumors larger than 2cm.41,42
Rectal Carcinoid Tumors
Carcinoid tumors originating in the rectum are often discovered incidentally during lower endoscopy or as a result of lower GI bleeding.43 They are not associated with a hormonal syndrome.44 Malignant potential closely correlates with size.30 Tumors smaller than 1 cm rarely metastasize and can usually be resected endoscopically or transanally, whereas tumors larger than 2 cm metastasize in over 50% of cases (Figure 2c).43,45–48 The metastatic potential of intermediate size tumors appears to correlate with invasion of the muscularis propria.45
PANCREATIC ENDOCRINE TUMORS
Pancreatic endocrine tumors arise from the islet cells of the pancreas. These heterogeneous neoplasms can secrete a variety of peptide and amine hormones, in cluding insulin, gastrin, glucagon, vasoactive intestinal peptide (VIP), ACTH, serotonin, somatostatin, and parathyroid hormone. Tumors that do not produce a hormonally active product are termed “nonfunctional.” The annual incidence of pancreatic endocrine tumors is approximately 1 per 100,000.49 Up to 20% are associated with MEN1, an auto so mal dominant hereditary syndrome.
Pancreatic Endocrine Tumor Subtypes
Pancreatic endocrine tumors are classified according to the hormone they produce. Approximately 35% to 85% are considered nonfunctional.50,51 Insulinomas and gastrinomas are the most common functional subtypes, with an annual incidence of 1–4 cases per million.52 The incidence of rarer subtypes such as VIPomas and glucagonomas is estimated to be less than one per ten million.53,54
The majority of pancreatic endocrine tumors are malignant, with the exception of insulinomas, which are usually benign. Gastrinomas are most commonly associated with MEN1, where they tend to be multifocal.
Insulinomas
About 90% of insulinomas are smaller than 2 cm, and less than 10% are considered considered malignant.52,55 Patients typically present with neuroglycopenic symptoms such as dizziness, lethargy, palpitations, and diaphoresis. Diagnosis is established during a monitored fast where serum glucose is measured along with insulin in order to demonstrate hypoglycemia (glucose <45 mg/dL) associated with inappropriate insulin elevation (> 6 μU/mL).56 C-peptide can also be measured to exclude exogenous insulin administration.57
Computed tomography (CT) scans generally reveal small, round, hypervascular tumors. Other imaging techniques include magnetic resonance imaging (MRI) and ultrasonography (transabdominal, endoscopic, or intraoperative).58 Somatostatin receptor scintigraphy using octreotide tagged with radiolabeled 111Indium-pentetreotide (OctreoScan) is relatively insensitive, because up to 40% of insulinomas express insufficient somatostatin receptors. 59 In cases of occult tumor, arterial calcium stimulation with hepatic venous sampling can aid with tumor localization.60
Gastrinomas
Gastrinomas originate in the duodenum and the pancreas, typically in proximity to the pancreatic head. About 60%–80% are considered malignant and one third of patients present with distant metastases at diagnosis.61,62 The MEN1 syndrome is implicated in about 20% of cases and is associated with tumor multicentricity.63 The gastrinoma syndrome, also known as the Zollinger-Ellison syndrome,64 is caused by hypersecretion of gastrin stimulating gastric acid release into the stomach. The most common manifestations are dys pepsia, heartburn, and diarrhea.65 Peptic ulcerations can affect atypical locations such as the jejunum. Diarrhea results from the passage of excess gastric acid into the small intestine, neutralizing digestive pancreatic enzymes and causing malabsorption.
The diagnosis of gastrinoma can be established when serum gastrin levels exceed ten times the upper limit of normal (ie, > 1,000 pg/mL). It is important to note that acid blocking drugs, such as proton pump inhibitors, can elevate serum gastrin levels and lead to false-positive results.66 In cases where the diagnosis is equivocal, a secretin stimulation test can help identify gastrinomas: a serum gastrin rise of > 200 pg/mL is considered diagnostic, with a sensitivity and specificity of 83% and 100%, respectively.67 Useful imaging studies include CT scans, MRI, 111In-pentetreotide scintigraphy,68 and endoscopic ultrasonography. 69,70 Surgical duodenal transillumination can identify small duodenal gastrinomas. 71
Prior to the advent of acid blocking medications, the Zollinger-Ellison syndrome was a highly morbid condition necessitating palliative gastrectomy or vagotomy.72 Today, high-dose proton pump inhibitors effectively control symptoms in the majority of cases.73,74 Some studies support titration of acid-blocking agents to achieve an optimal gastric acid secretion rate of < 10 mEq/h.75
Rare Subtypes
VIPomas secrete vasoactive intestinal peptide.76 The resulting syndome (also known as the Verner-Morrison syndrome) is characterized by profuse watery diar rhea, often exceeding 3 liters a day.54,77,78 Due to the severity of the diarrhea, the syndrome is sometimes described as “pancreatic cholera.”79 Other complications include flushing, dehydration, hypochlorhydria, and hypokalemia.80 VIPomas are typically large at presentation (> 3 cm) and usually originate in the tail of the pancreas. The majority are malignant.54
Glucagonomas arise from the alpha cells of the pancreas.81 The clinical manifestations are protean, and may include hyperglycemia, anorexia, weight loss, venous thromboses, cheilitis, and an unusual rash called necrolytic migratory erythema (NME).53 NME characteristically manifests as painful, weeping, erythematous papules or plaques involving the face, perineum, and flexural regions.82 The underlying mechanism of NME is uncertain.
HISTOLOGIC CLASSIFICATION
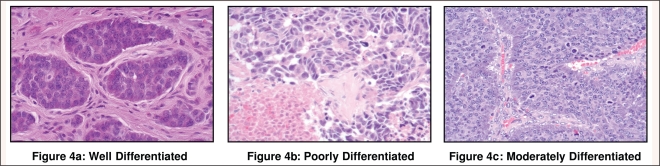
The majority of gastrointestinal neuroendocrine tumors are described histologically as well-differentiated (Figure 4a). This term identifies tumors with a relatively monomorphic population of small, round cells, a low mitotic rate of < 2 mitoses/10 high powered fields (HPF), a low Ki-67 proliferative index (< 2%), and absence of necrosis.83–88 Well-differentiated neuroendocrine tumors (WD-NETs) are clinically indolent and associated with prolonged survivals, even in the metastatic setting. Poorly differentiated (PD) neuroendocrine carcinomas (Figure 4b) are associated with cellular pleomorphism, a mitotic rate > 10 mitoses/10 HPF, a Ki-67 proliferative index >10%, and tumor necrosis. These are highly aggressive malignancies that closely resemble small-cell carcinoma of the lung, both in morphologic appearance and clinical behavior.87–89 Intermediate grade or “moderately differentiated” (Figure 4c) tumors of the gastroenteropancreatic axis are not well defined in the medical literature, but appear to have an intermediate prognosis.84,86,90
Figure 4:
Examples of well differentiated (4a), poorly differentiated (4b), and moderately differentiated (4c) gastrointestinal neuroendocrine tumors. Photographs courtesy of Nasir Aejaz, MD, Department of Pathology, H. Lee Moffitt Cancer Center and Research Institute, Tampa.
In recent years, the World Health Organi zation (WHO) developed a classification system for endocrine tumors of the gastrointestinal tract. Using the WHO (2000) nomenclature,91 the term “carcinoid” is applied to serotonin-producing WD endocrine neoplasms of the small intestine, appendix, and colon. A clinicopathologic classification has also been proposed by WHO (2004)92 for pancreatic endocrine neoplasms that distinguishes between WD endocrine tumors (benign or uncertain behavior), WD endocrine carcinomas, and PD endocrine carcinomas, based on tumor size, local invasion of adjacent organs, angioinvasion, perineural invasion, Ki-67 proliferation index, and the presence of metastases.87,91,92
GENETICS AND HEREDITARY PREDISPOSITION
Although the majority of gastroenteropancreatic tumors are sporadic, several autosomal dominant hereditary syndromes have been identified. The underlying genetic abnormalities yield insight into on-cogenic pathways of familial and sporadic tumors. Multiple endocrine neoplasia 1 (MEN1) is an autosomal dominant hereditary syndrome characterized by a predisposition to tumors of the parathyroid glands, anterior pituitary, and pancreatic islet cells.93 The underlying tumor suppressor gene mutation has been identified in the long arm of chromosome 11 (11q13).94 Its protein product “menin” has been recently cloned and appears to be a regulator of gene expression.95 Germline MEN1 genetic testing appears to have a 70%–90% sensitivity in familial MEN1 cases and a somewhat lower sensitivity in sporadic cases.96
The most frequent manifestation of MEN1 is parathyroid hyperplasia, which typically develops in the second to fourth decade.97 Pituitary adenomas form in about 15%–20% of patients. Pancreatic endocrine tumors become clinically apparent in about one third of patients, with a higher rate of subclinical disease. Gastrinomas occur most often, followed by insulinomas. Tumors are almost invariably multifocal;98 consequently, the role of curative surgical therapy is controversial.99,100 An exceptionally indolent growth pattern is characteristic of these tumors; consequently life expectancy appears to be only modestly diminished in MEN1 patients.101,102
Von-Hippel Lindau (VHL) syndrome is caused by an autosomal dominant mutation in the VHL gene located on chromosome 3p25.103 This gene is involved in the regulation of a hypoxia-inducible gene (HIF-1alpha) expression. Induction of hypoxia-associated cytokines, including erythropoietin, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), is thought to stimulate tumor growth, but the precise mechanism of tumorigenesis is unknown. A variety of tumors can develop in VHL syndrome, including renal cell carcinomas, hemangioblastomas, pheochromocytomas, pancreatic cysts, and pancreatic endo crine tumors. The latter occur in only 10% of cases,104 and tend to progress in an indolent fashion.
Tuberous sclerosis is an autosomal dominant syndrome characterized by lowgrade neoplasms and hamartomas in multiple organs, including skin, brain, and kidney. Pancreatic endocrine tumors occur in 1%–5% of cases.105 Two variants have been described: TSC1 caused by a mutation on chromosome 9q34106 encoding hamartin and TSC2 on chromosome 16p13 encoding tuberin.107 A complex of hamartin and tuberin is thought to regulate cell-cycle progression, possibly through upregulation of the mTOR cell-signaling pathway.108,109
Hereditary syndromes have not been identified in carcinoid tumors, and a family history is reported in less than 1% of patients.110 The relative risk of a carcinoid tumor diagnosis in a patient with a first degree affected relative is estimated to be 3.6,111,112 thus the absolute risk remains low and does not warrant screening.
The genetic aberrations in sporadic gastrointestinal neuroendocrine tumors are poorly understood. Oncogenes and tumor suppressor genes that are mutated in common human malignancies (p53, APC, Rb, K-ras) do not appear to be implicated in neuroendocrine tumor development.113–116 Mutations of the MEN1 gene (chromosome 11q13) occur in about 20% of sporadic, solitary pancreatic endocrine tumors,117,118 whereas chromosome 18 deletions are common in midgut carcinoid tumors.119–121 Techniques such as comparative genomic hybridization have identified gains and losses in numerous chromosomes. 120–123 These genetic abnormalities appear to increase in pancreatic endocrine tumor metastases compared to matched primary tumors.124 Nonfunctional pancreatic endocrine tumors also appear to con tain an increased frequency of chromosomal aberrations compared to functional tumors.125
Cell-signaling pathways influence tumor growth and hormonal activity. Neuroendocrine cells can express the insulin-like growth factor (IGF) as well as its receptor (IGFR).126 Cell line studies indicate that IGF-1 can act in an autocrine and paracrine fashion to inhibit apoptosis and stimulate secretion of chromogranins, possibly by activating the PI3K-AKT pathway.127 Vascular endothelial growth factor is also expressed by neuro endocrine tu mors,128,129 and elevated levels of circulating VEGF have been associated with tumor progression.130 Cyclin D1, an important component of cell cycle regulation, has been found to be overexpressed in pancreatic endocrine tumors.131
IMMUNOHISTOCHEMICAL AND SERUM MARKERS
General immunohistochemical markers of neuroendocrine differentiation include chromogranin, synaptophysin, CD56, protein gene product (PGP) 9.5, and neuronspecific enolase (NSE). Chromogranins are a family of glycoproteins associated with dense-core secretory vesicles found ubiquitously in neuronal and endocrine tissues.132 Chromogranin A (CgA) was first isolated from chromaffin cells of the adrenal medulla.133 Synaptophysin is a synaptic vesicle membrane protein also found commonly in neuronal tissues and in endocrine tumors.134 Neuron-specific enolase is a cytoplasmic enzyme detected in tumors of neuroendocrine differentiation, but lacks specificity compared to CgA and synaptophysin.135 Chromogranin positivity generally correlates with the extent of granularity on electron microscopy. WDNETs tend to exhibit diffuse and intense expression of CgA and synaptophysin, whereas PD neuroendocrine carcinomas show significantly reduced CgA expression while maintaining intense staining for synaptophysin.83
Immunostaining for specific hormones can aid in the diagnosis of neuroendocrine tumors. The various hormone-specific markers used in immunophenotyping of pancreatic endocrine tumors include in sulin, glucagon, somatostatin, gastrin, VIP, calcitonin, serotonin, ACTH, and neurotensin. This immunoreactivity, however, does not necessarily correlate with serum hormone levels or clinical syndrome. For example, a study of nonfunctional pancreatic endocrine tumors demonstrated that 87% were immunoreactive to at least one peptide hormone, such as insulin or glucagon.136
Serum and urine tumor markers include hormones and their metabolites (eg, serotonin, 5-HIAA, insulin, glucagon, gastrin) and nonspecific tumor markers such as chro mogranin, pancreatic polypeptide (PP), NSE, and substance P. Hormone levels should be assessed in accordance with the patient’s clinical syndrome. The specificity of a 24-hour 5-HIAA urine collection approaches 100% in metastatic carcinoid tumors, and sensitivity is high for detection of the carcinoid syndrome.133,137 Strict avoidance of serotonin-rich foods during urine collection is necessary to prevent false-positive test results.138
The most sensitive general serum marker of neuroendocrine tumors is chromogranin A.133 It is released into the circulation in approximately 90% of pancreatic endocrine tumors and 70%–100% in metastatic gastrointestinal carcinoid tumors.139,140 False positive tests, however, can occur with renal or hepatic impairment or with atrophic gastritis and proton pump inhibitor use (due to ECL hyperplasia). Serum levels of CgA tend to be highest in metastatic midgut carcinoid tumors and correlate with tumor burden,141,142 as well as response to treatment.143
TREATMENT OF METASTATIC DISEASE
Metastatic neuroendocrine tumors vary widely in their clinical manifestations and rate of growth. Many patients remain asymptomatic and can be managed conservatively with close observation. Others progress rapidly and develop symptoms related to hormonal secretion and/or tumor burden. Standard treatment options include somatostatin analogs, surgical cytoreduction, cytotoxic chemotherapy, interferon, and hepatic artery embolization. Novel therapies under investigation include angiogenesis inhibitors, mTOR inhibitors, and radiolabeled somatostatin analogs. The choice of treatment depends on several factors, including location of primary tumor, pattern of metastatic spread, levels of somatostatin receptor expression, and hormonal activity.
Somatostatin Analogs
The human hormone somatostatin is released by neuroendocrine cells of the gastrointestinal tract and has an inhibitory effect on bowel motility, gastrointestinal secretion, and absorption of nutrients. The actions of somatostatin are mediated through 5 receptors (SSTR 1–5).144 Several of these receptors, including SSTR sub - types 2 and 5, are important in the inhibition of gastrointestinal and pancreatic hormone secretion, whereas SSTR subtype 1 is thought to mediate cell-cycle arrest and apoptosis.145,146 The antisecretory effects of somatostatin have made it an important tool in the management of hormonally active neuroendocrine tumors, the majority of which express somatostatin receptors. However, clinical use of native human somatostatin is impeded by its short half-life of approximately 2 minutes.
The first clinically useful analog of somatostatin was octreotide, a synthetic octapeptide with a half life of 2 hours and avid binding affinity to SSTR 2. The initial clinical study of octreotide in patients with the carcinoid syndrome reported amelioration of flushing and diarrhea in 88% of patients and major reductions in urinary 5-HIAA in 72%.147 Numerous additional studies have confirmed the powerful antisecretory effects of octreotide at dose ranges of 100 μg to 500 μg administered subcutaneously 2 to 3 times daily.148,149 A long-acting depot formulation of octreotide (Sandostatin LAR®) has become available more recently, enabling monthly dosing.150 Doses of 10 mg to 60 mg administered every 4 weeks have been found to be well tolerated with no dose-related increase in adverse events. Side effects include nausea and steatorrhea. Due to the inhibitory effects of octreotide on gallbladder contractility, an increased rate of biliary stone formation is observed, but it is rarely of clinical significance. Another somatostatin analog, lanreotide, has similar somatostatinbinding properties, clinical activity and side-effect profile.151
Studies of octreotide in pancreatic endocrine tumors have also demonstrated an antisecretory effect.152 Octreotide is especially active in management of the glucagonoma and VIPoma syndromes.153,154 Its efficacy in insulinomas is limited by the relative paucity of somatostatin receptors in these tumors. Several studies have described palliation of hypoglycemia with octreotide therapy,155–157 however, others have reported exacerbation of symptoms, probably due to suppression of glucagon.158
The effects of somatostatin analogs on tumor growth are controversial. While objective response rates are observed in fewer than 5% of patients treated with octreotide,159,160 there is laboratory and clinical evidence of an inhibitory effect on cell proliferation and tumor growth.161 Studies have demonstrated growth inhibition in a variety of cell lines and xenograft models treated with somatostatin analogs.145,161–165 Proposed mechanisms in clude a direct inhibitory effect via somatostatin receptors on tumor cells vs. an indirect effect mediated by inhibition of growth factors such as IGF or VEGF.163,166,167 Clinical evidence of an antitumor effect comes from single-arm phase II studies demonstrating a relatively high rate of disease stabilization among patients with progressive metastatic tumors.168–169
Radiolabeled Somatostatin Analogs
Approximately 80% of gastroenteropancreatic neuroendocrine tumors express somatostatin receptors and can be visualized with the radiolabeled somatostatin analog 111In-pentetreotide.170,171 After somatostatin binds with its receptor, a fraction of the ligand-receptor complex internalizes. 172,173 Thus, delivering targeted radiotherapy to neuroendocrine tumor cells using the somatostatin receptor represents a logical therapeutic approach. The first clinical trials of somatostatin-labeled radiotherapy assessed the use of high, cytotoxic doses of 111In-pentetreotide.174,175 Objective responses with this compound were rare, probably due to the small particle range and short tissue penetration of Auger electrons emitted by the 111In isotope.
The next generation radiolabeled somatostatin analog was [90Y-DOTA0,Tyr3]- octreotide.176–178 The radionuclide 90Y is a β-particle emitter with a maximum tissue range of 12 mm. Objective response rates in several phase I and II trials were in the range of 10%–30%.179 Dose-limiting side effects included hematologic and renal toxicity.180
The most recent research studies involve the compound [177Lu-DOTA0,Tyr3]- octreotate, a compound with increased affinity for SSTR subtype 2.181 Whereas 90Y is a β-particle emitter, 177Lu is both a β- and γ-emitting radionuclide with a shorter range of tissue penetration (2 mm) than 90Y.182 A trial evaluating 131 patients treated with [170Lu-DOTA0,Tyr3]-octreotate at cumulative doses of 600–800 mCi demonstrated an objective response rate of 28%, with relatively mild nephrotoxicity and bone marrow suppression. Among patients who had stable disease or tumor regression, median duration of disease control was in excess of 3 years.183
Chemotherapy
Evidence is accumulating that well-differentiated carcinoid tumors are relatively resistant to cytotoxic chemotherapy. Early trials showing high response rates to streptozocin- based combinations did not employ strict radiographic criteria.184 Contemporary trials have demonstrated low response rates and short progressionfree intervals (Table 1).185–191 Thus, it appears that cytotoxic chemotherapy adds little value to the treatment of metastatic well-differentiated carcinoid tumors.
Table 1.
Trials of cytotoxic chemotherapy in metastatic carcinoid tumors.
| Regimen | Year | Response rate |
|---|---|---|
| Streptozocin + 5-FU vs. streptozocin + cyclophosphamide184 | 1979 | 33% vs. 26% |
| Streptozocin + 5-FU vs. doxorubicin 188 | 1984 | 22% vs. 21% |
| 5-FU + doxorubicin +cyclophosphamide + streptozocin189 | 1987 | 30% |
| Dacarbazine190 | 1994 | 16% |
| Doxorubicin + 5-FU vs. streptozocin + 5-FU186 | 2005 | 15% |
| Temozolomide + thalidomide191 | 2006 | 7% |
Abbreviations: 5-FU = 5-fluorouracil
In contrast, trials of cytotoxic chemotherapy in pancreatic endocrine tumors have established the activity of several drugs including streptozocin, doxorubicin, 5-fluorouracil (5-FU), and dacarbazine (Table 2). Early trials at the Mayo Clinic reported re sponse rates of 63% using streptozocin with 5-FU192 and 69% using streptozocin with doxorubicin.193 Con temporary chemotherapy trials employing stricter re sponse criteria have documented response rates of 39% using the combination of 5-FU/streptozocin/doxorubi cin194 and 33% using single-agent dacarbazine. 195 Several studies have substantiated the theory that response rates to chemotherapy differ based on primary tumor site. For example a recent trial of temozolomide combined with thalidomide demonstrated an objective response rate of 45% in pancreatic endocrine tumors vs. only 7% in metastatic carcinoid tumors.191
Table 2.
Trials of cytotoxic chemotherapy in metastatic pancreatic endocrine tumors.
| Regimen | Year | Response rate |
|---|---|---|
| Streptozocin + 5-FU vs. streptozocin192 | 1980 | 63% vs. 36% |
| Streptozocin + doxorubicin vs. streptozocin + 5-FU193 | 1992 | 69% vs. 45% |
| Dacarbazine195 | 2001 | 31% |
| Streptozocin + doxorubicin + 5-FU194 | 2006 | 39% |
| Temozolomide + thalidomide191 | 2006 | 7% |
Abbreviations: 5-FU = 5-fluorouracil
Poorly differentiated neuroendocrine tumors appear to be highly sensitive to platinum-based cytotoxic chemotherapy. This was demonstrated in a trial of cisplatin and etoposide, documenting a response rate of 67% in poorly differentiated neuroendocrine tumors vs. only 7% in well differentiated tumors.89 Unfortunately, response durations tend to be relatively short.
Interferon
Interferon-αappears to exert an antiproliferative and antisecretory effect on neuroendocrine tumors. Mechanisms include stimulation of T-cells as well as direct inhibition of tumor cell-cycle progression. 196 An early trial of human leukocyte interferon in patients with the carcinoid syndrome demonstrated an objective tumor response rate of 11%, a significant tumor marker reduction in one half, and symptomatic improvement in two thirds of patients.197 Subsequent trials with recombinant interferon-αhave confirmed objective response rates of approximately 5%–10% with disease stabilization occurring in about 50% of cases.
Several studies have examined the combination of interferon with somatostatin analogs. A trial of patients taking octreotide for progressive carcinoid syndrome reported symptomatic improvement in 49% of patients after the addition of interferon-α.159 Another study reported a 67% rate of disease stability in patients with progressive neuroendocrine tumors treated with the same combination.198 However, larger randomized trials have not confirmed that adding interferon to oc treo - tide improves outcomes compared with monotherapy.198,199
The optimal dose of interferon-αis unclear. Clinical trials have studied doses ranging from 3×106 units to 24×106 units administered daily or every other day. Side effects are often dose related and include fevers, chills, myalgias, headaches, and depression. Myelosuppression is common with higher doses. It is uncertain whether responses are dose dependent.
Angiogenesis Inhibitors
Neuroendocrine tumors are highly vascular and express both the VEGF ligand and receptor. Moreover, elevated levels of circulating VEGF (among other angiogenic cytokines) have been associated with tumor progression in neuroendocrine tumors. Thus, it is likely that VEGF-mediated angiogenesis plays an integral role in the metastatic progression of neuroendocrine tumors.
Several studies have evaluated angiogenesis inhibitors in neuroendocrine tumors. A single-arm trial of endostatin in patients with metastatic neuroendocrine tumors demonstrated no objective radio - graphic responses.200 Results were some - what more favorable in a trial of sunitinib malate, a small molecule tyrosine kinase inhibitor of VEGFR-1, -2, and -3, as well as PDGF, KIT, and Flt3. In this study, partial responses were observed in 13% of pancreatic endocrine tumors and 5% of carcinoid tumors.201
A randomized phase-II trial comparing the VEGF antibody bevacizumab to pegylated interferon-αdemonstrated prolonged progression free survival in the bevacizumab arm.202 Larger trials are planned to confirm this benefit.
Management of Liver Metastases
The liver is the predominant site of meta - static disease in gastrointestinal neuroendocrine tumors. Patients with hepatic metastases may experience symptoms resulting from tumor burden as well as from uncontrolled hormonal syndromes. Liver-directed treatment options include cytoreductive hepatic surgery, hepatic artery embolization (with or without chemotherapy), or liver transplant.
Surgery is often advocated in patients with limited liver metastases.203,204 Various ablation techniques have also been described, including cryoablation, alcohol ablation, and radiofrequency ablation (RFA).205–208 Proponents of cytoreductive surgery cite numerous retrospective studies describing palliation of symptoms and prolonged survival durations among patients undergoing cytoreductive surgery with curative or near-curative intent.209–212 Nonrandomized studies com paring surgical to nonsurgical therapies also suggest improved survivals associated with aggressive surgical management.213,214 However, patients managed surgically often present with relatively limited disease; thus comparisons with patients treated medically are inherently biased.
Hepatic artery embolization is typically performed in patients with diffuse, symptomatic and unresectable liver metastases. The rationale is that liver metastases derive their vascular supply primarily from the hepatic artery, whereas normal hepatocytes are fed primarily by the portal vein. To limit morbidity, individual hepatic artery branches are embolized selectively in two to three stages. A variety of embolic materials have been tested, including Gelfoam (Pharmacia & Upjohn Company, Kalamazoo, MI), polyvinyl alcohol (PVA) particles, and trisacryl gelatin microspheres (Embospheres; BioSphere Medical Inc, Rockland, MA) with or without the addition of antineoplastic agents, such as cisplatin, doxorubicin, streptozocin, and 5- FU (Table 3).215–224 Radioembolization using yttrium 90 microspheres has also been reported in very limited numbers of patients.225 Radiographic response rates are approximately 50%, with higher rates of symptomatic improvement and decline in tumor markers.215 In the absence of randomized comparative studies, it is unclear whether addition of chemotherapeutic agents to the embolic material (chemoembolization) improves outcomes.
Table 3.
Reviews of hepatic artery embolization in neuroendocrine tumors.
| Technique | Year | Response rate |
|---|---|---|
| Surgical occlusion or Gelfoam embolization222 | 1994 | 72% |
| Intraarterial doxorubicin followed by Gelfoam embolization219 | 1993 | 33% |
| Gelfoam embolization217 | 1998 | 52% |
| Intraarterial multiagent chemotherapy followed by Gelfoam or PVA embolization216 | 2003 | 67% |
| PVA or microsphere embolization215 | 2006 | 48% |
Abbreviations: PVA = polyvinyl alcohol.
Short-term, predictable toxicities associated with embolization include abdominal pain, nausea, fever, and transaminase elevation, all caused by ischemic hepatitis. Serious adverse effects can include hepato - renal failure, bowel or gallbladder perforation, and hepatic abscess. With prophylactic hydration and antibiotics, treatmentrelated mortality is exceptionally rare.
The benefit of liver transplantation for patients with metastatic neuroendocrine tumors is uncertain.226–230 Although data from institutional series vary, most centers document relatively high rates of postoperative morbidity and disease recurrence. In the largest meta-analysis of liver transplants, 5-year survival was 47%, with only 24% of patients free of disease recurrence. 231
DISCUSSION
The survival of patients with metastatic neuro endocrine tumors appears to have improved over the years. One of the first large epidemiologic studies examining data from 1950–1969 reported a 5-year survival rate of 19% for metastatic small bowel carcinoid tumors, 0% for metastatic tumors of the stomach, and 7% for tumors originating in the rectum.232 A more recent review of SEER (Surveillance Epidemiology and End Results) data compared survival data from the 1970s and 1980s to contemporaneous survival data.3 In this analysis, the 5-year survival rate for metastatic small bowel carcinoid tumors improved from 36% to 50%, with corresponding improvements in prognosis for metastatic tumors of gastric, rectosigmoid, and appendiceal origin. Other institutional reviews point to a substantial increase in median survival from approximately 2 years historically29,184 to over 5 years at the present time.32,141 A large retrospective study of patients with carcinoid heart disease likewise demonstrated an improvement in median survival from 1.5 years to 4.4 over the past 3 decades.233
The most important prognostic factor in the natural history of metastatic carcinoid tumors appears to be the primary tumor site, confirming the diverse biology of carcinoid tumors. Small intestine carcinoid tumors are associated with the longest survival durations, followed by metastatic rectal and stomach tumors.3,4 Other prognostic variables include gender (survival in women exceeds men), age, and extent of hepatic metastases.234
Survival of patients with pancreatic endocrine tumors has also improved over time. An analysis of SEER data indicates an increase in median survival from 13 months (1973–1980) to 39 months (1995–2000) for tumors of all stages.51 A recent retrospective institutional study of 90 patients with metastatic pancreatic endocrine tumors demonstrated a median survival of 70 months from time of diagnosis for metastases.235 Hormonally functioning tumors appear to have an improved prognosis compared to nonsecretory tumors, probably due to earlier diagnosis. Due to the scarcity of large, prospective trials, it is difficult to assess the factors contributing most strongly to improved prognosis. Random ized studies are needed to identify new biologic agents that will benefit patients with metastatic neuroendocrine tumors.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Strosberg has received honoraria from Novartis.
REFERENCES
- 1.Lubarsch O. Ueber den primären Krebs des Ileum, nebst Bemerkungen über das gleichzeitige Vorkommen von Krebs und Tuber - kolose. Virchows Arch. 1888;111:280–317. [Google Scholar]
- 2.Oberndorfer S. Karzinoide Tumoren des Dünndarms. Frankf Z Pathol. 1907;1:425–429. [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 4.Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240:117–122. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorson A, Biorck G, Bjorkman G, Walden-strom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J. 1954;47:795–817. doi: 10.1016/0002-8703(54)90152-0. [DOI] [PubMed] [Google Scholar]
- 6.Erspamer V, Asero B. Identification of enter - amine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169:800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 7.Feldman JM. Increased dopamine production in patients with carcinoid tumors. Metabolism. 1985;34:255–260. doi: 10.1016/0026-0495(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 8.Sandler M, Karim SM, Williams ED. Prostaglandins in amine-peptide-secreting tumours. Lancet. 1968;2:1053–1054. doi: 10.1016/s0140-6736(68)91528-6. [DOI] [PubMed] [Google Scholar]
- 9.Lucas KJ, Feldman JM. Flushing in the carcinoid syndrome and plasma kallikrein. Cancer. 1986;58:2290–2293. doi: 10.1002/1097-0142(19861115)58:10<2290::aid-cncr2820581022>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Skrabanek P, Cannon D, Kirrane J, Powell D. Substance P secretion by carcinoid tumours. Ir J Med Sci. 1978;147:47–49. doi: 10.1007/BF02939369. [DOI] [PubMed] [Google Scholar]
- 11.Lundin L, Norheim I, Landelius J, et al. Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation. 1988;77:264–269. doi: 10.1161/01.cir.77.2.264. [DOI] [PubMed] [Google Scholar]
- 12.Robiolio PA, Rigolin VH, Wilson JS, et al. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–795. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- 13.Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87:1188–1196. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 14.Waltenberger J, Lundin L, Oberg K, et al. Involvement of transforming growth factor-beta in the formation of fibrotic lesions in carcinoid heart disease. Am J Pathol. 1993;142:71–78. [PMC free article] [PubMed] [Google Scholar]
- 15.Beauchamp RD, Coffey RJ, Jr, Lyons RM, et al. Human carcinoid cell production of paracrine growth factors that can stimulate fibroblast and endothelial cell growth. Cancer Res. 1991;51:5253–5260. [PubMed] [Google Scholar]
- 16.Berge T, Linell F. Carcinoid tumours. Frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand [A] 1976;84:322–330. [PubMed] [Google Scholar]
- 17.Williams ED, Sandler M. The classification of carcinoid tumours. Lancet. 1963;1:238–239. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 18.Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 19.Modlin IM, Gilligan CJ, Lawton GP, et al. Gastric carcinoids. The Yale experience Arch Surg 130250–255.discussion 255–2561995 [DOI] [PubMed] [Google Scholar]
- 20.Thomas RM, Baybick JH, Elsayed AM, Sobin LH. Gastric carcinoids. An immunohistochemical and clinicopathologic study of 104 patients. Cancer. 1994;73:2053–2058. doi: 10.1002/1097-0142(19940415)73:8<2053::aid-cncr2820730807>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Moses RE, Frank BB, Leavitt M, Miller R. The syndrome of type A chronic atrophic gastritis, pernicious anemia, and multiple gastric carcinoids. J Clin Gastroenterol. 1986;8:61–65. doi: 10.1097/00004836-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Solcia E, Fiocca R, Villani L, et al. Morphology and pathogenesis of endocrine hyperplasias, precarcinoid lesions, and carcinoids arising in chronic atrophic gastritis. Scand J Gastroenterol Suppl. 1991;180:146–159. doi: 10.3109/00365529109093193. [DOI] [PubMed] [Google Scholar]
- 23.Borch K, Renvall H, Kullman E, Wilander E. Gastric carcinoid associated with the syndrome of hypergastrinemic atrophic gastritis. A prospective analysis of 11 cases. Am J Surg Pathol. 1987;11:435–444. doi: 10.1097/00000478-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ahlman H, Kolby L, Lundell L, et al. Clinical management of gastric carcinoid tumors. Digestion. 1994;55(suppl 3):77–85. doi: 10.1159/000201206. [DOI] [PubMed] [Google Scholar]
- 25.Eckhauser FE, Lloyd RV, Thompson NW, et al. Antrectomy for multicentric, argyrophil gastric carcinoids: a preliminary report. Surgery. 1988;104:1046–1053. [PubMed] [Google Scholar]
- 26.Hirschowitz BI, Griffith J, Pellegrin D, Cummings OW. Rapid regression of enterochromaffinlike cell gastric carcinoids in pernicious anemia after antrectomy. Gastroenterology. 1992;102(4 pt 1):1409–1418. [PubMed] [Google Scholar]
- 27.Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 28.Tomassetti P, Migliori M, Caletti GC, et al. Treatment of type II gastric carcinoid tumors with somatostatin analogues N Engl J Med24343551–554.2000 [DOI] [PubMed] [Google Scholar]
- 29.Moertel CG, Sauer WG, Dockerty MB, Baggenstoss AH. Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901–912. doi: 10.1002/1097-0142(196109/10)14:5<901::aid-cncr2820140502>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151–160. doi: 10.1002/jso.20179. [DOI] [PubMed] [Google Scholar]
- 31.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502–1522. doi: 10.1200/JCO.1987.5.10.1502. [DOI] [PubMed] [Google Scholar]
- 32.Pape UF, Bohmig M, Berndt U, et al. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a German referral center. Ann N Y Acad Sci. 2004;1014:222–233. doi: 10.1196/annals.1294.025. [DOI] [PubMed] [Google Scholar]
- 33.Kvols LK. Metastatic carcinoid tumors and the carcinoid syndrome. A selective review of chemotherapy and hormonal therapy. Am J Med. 1986;81:49–55. doi: 10.1016/0002-9343(86)90584-x. [DOI] [PubMed] [Google Scholar]
- 34.Kvols LK. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Ann N Y Acad Sci. 1994;733:464–470. doi: 10.1111/j.1749-6632.1994.tb17296.x. [DOI] [PubMed] [Google Scholar]
- 35.Kvols LK. Therapeutic considerations for the malignant carcinoid syndrome. Acta Oncol. 1989;28:433–438. doi: 10.3109/02841868909111218. [DOI] [PubMed] [Google Scholar]
- 36.Kahil ME, Brown H, Fred HL. The Carcinoid Crisis. Arch Intern Med. 1964;114:26–28. doi: 10.1001/archinte.1964.03860070072004. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan DJ, Brunner MD. Anesthesia for patients with carcinoid syndrome. Int Anesthesiol Clin. 1997;35:129–142. doi: 10.1097/00004311-199703540-00009. [DOI] [PubMed] [Google Scholar]
- 38.Moertel CG, Dockerty MB, Judd ES. Carcinoid tumors of the vermiform appendix. Cancer. 1968;21:270–278. doi: 10.1002/1097-0142(196802)21:2<270::aid-cncr2820210217>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Shaw PA. Carcinoid tumours of the appendix are different. J Pathol. 1990;162:189–190. doi: 10.1002/path.1711620303. [DOI] [PubMed] [Google Scholar]
- 40.Sandor A, Modlin IM. A retrospective analysis of 1570 appendiceal carcinoids. Am J Gastroenterol. 1998;93:422–428. doi: 10.1111/j.1572-0241.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- 41.Moertel CG, Weiland LH, Nagorney DM, Dockerty MB. Carcinoid tumor of the appendix: treatment and prognosis. N Engl J Med. 1987;317:1699–1701. doi: 10.1056/NEJM198712313172704. [DOI] [PubMed] [Google Scholar]
- 42.Bowman GA, Rosenthal D. Carcinoid tumors of the appendix. Am J Surg. 1983;146:700–703. doi: 10.1016/0002-9610(83)90321-5. [DOI] [PubMed] [Google Scholar]
- 43.Mani S, Modlin IM, Ballantyne G, et al. Carcinoids of the rectum. J Am Coll Surg. 1994;179:231–248. [PubMed] [Google Scholar]
- 44.Caldarola VT, Jackman RJ, Moertel CG, Dockerty MB. Carcinoid tumors of the rectum. Am J Surg. 1964;107:844–849. doi: 10.1016/0002-9610(64)90172-2. [DOI] [PubMed] [Google Scholar]
- 45.Naunheim KS, Zeitels J, Kaplan EL, et al. Rectal carcinoid tumors—treatment and prognosis. Surgery. 1983;94:670–676. [PubMed] [Google Scholar]
- 46.Fahy BN, Tang LH, Klimstra D, et al. Carcinoid of the Rectum Risk Stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14:1735–1743. doi: 10.1245/s10434-006-9311-6. [DOI] [PubMed] [Google Scholar]
- 47.Stinner B, Kisker O, Zielke A, Rothmund M. Surgical management for carcinoid tumors of small bowel, appendix, colon, and rectum. World J Surg. 1996;20:183–188. doi: 10.1007/s002689900028. [DOI] [PubMed] [Google Scholar]
- 48.Federspiel BH, Burke AP, Sobin LH, Shekitka KM. Rectal and colonic carcinoids. A clinicopathologic study of 84 cases. Cancer. 1990;65:135–140. doi: 10.1002/1097-0142(19900101)65:1<135::aid-cncr2820650127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 49.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Heitz PU, Kasper M, Polak JM, Kloppel G. Pancreatic endocrine tumors. Hum Pathol. 1982;13:263–271. doi: 10.1016/s0046-8177(82)80183-4. [DOI] [PubMed] [Google Scholar]
- 51.Halfdanarson TR, Rabe KG, Rubin J, Petersen M. Pancreatic endocrine tumors (PETs): incidence and recent trend toward improved survival. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 4584) [Google Scholar]
- 52.Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma—incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66:711–719. doi: 10.1016/s0025-6196(12)62083-7. [DOI] [PubMed] [Google Scholar]
- 53.Wermers RA, Fatourechi V, Wynne AG, et al. The glucagonoma syndrome. Clinical and pathologic features in 21 patients Medicine(Baltimore)7553–63.1996 [DOI] [PubMed] [Google Scholar]
- 54.Smith SL, Branton SA, Avino AJ, et al. Vasoactive intestinal polypeptide secreting islet cell tumors: a 15-year experience and review of the literature. Surgery. 1998;124:1050–1055. doi: 10.1067/msy.1998.92005. [DOI] [PubMed] [Google Scholar]
- 55.Scott BA, Gatenby RA. Imaging advances in the diagnosis of endocrine neoplasia. Curr Opin Oncol. 1998;10:37–42. doi: 10.1097/00001622-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Service FJ, Dale AJ, Elveback LR, Jiang NS. Insulinoma: clinical and diagnostic features of 60 consecutive cases. Mayo Clin Proc. 1976;51:417–429. [PubMed] [Google Scholar]
- 57.Service FJ, O'Brien PC, McMahon MM, Kao PC. C-peptide during the prolonged fast in insulinoma. J Clin Endocrinol Metab. 1993;76:655–659. doi: 10.1210/jcem.76.3.8445021. [DOI] [PubMed] [Google Scholar]
- 58.Rosch T, Lightdale CJ, Botet JF, et al. Localization of pancreatic endocrine tumors by endo scopic ultrasonography. N Engl J Med. 1992;326:1721–1726. doi: 10.1056/NEJM199206253262601. [DOI] [PubMed] [Google Scholar]
- 59.Modlin IM, Tang LH. Approaches to the diagnosis of gut neuroendocrine tumors: the last word (today) Gastroenterology. 1997;112:583–590. doi: 10.1053/gast.1997.v112.pm9024313. [DOI] [PubMed] [Google Scholar]
- 60.Doppman JL, Miller DL, Chang R, et al. Intraarterial calcium stimulation test for detection of insulinomas. World J Surg. 1993;17:439–443. doi: 10.1007/BF01655101. [DOI] [PubMed] [Google Scholar]
- 61.Jensen RT. Gastrointestinal endocrine tumours. Gastrinoma. Baillieres Clin Gastroenterol. 1996;10:603–643. doi: 10.1016/s0950-3528(96)90016-0. [DOI] [PubMed] [Google Scholar]
- 62.Stabile BE, Passaro E., Jr Benign and malignant gastrinoma. Am J Surg. 1985;149:144–150. doi: 10.1016/s0002-9610(85)80024-6. [DOI] [PubMed] [Google Scholar]
- 63.Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6:454–463. doi: 10.1007/s11894-004-0067-5. [DOI] [PubMed] [Google Scholar]
- 64.Zollinger RM, Ellison EH.Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas Ann Surg 142709–723.discussion, 724–7081955 [PMC free article] [PubMed] [Google Scholar]
- 65.Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients Medicine(Baltimore)79379–411.2000 [DOI] [PubMed] [Google Scholar]
- 66.Lamberts R, Creutzfeldt W, Struber HG, et al. Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis. Gastroenterology. 1993;104:1356–1370. doi: 10.1016/0016-5085(93)90344-c. [DOI] [PubMed] [Google Scholar]
- 67.Berna MJ, Hoffmann KM, Long SH, et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features Medicine(Baltimore)85331–364.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen RT, Gibril F. Somatostatin receptor scintigraphy in gastrinomas. Ital J Gastroenterol Hepatol. 1999;31(suppl 2):S179–S185. [PubMed] [Google Scholar]
- 69.Varas Lorenzo MJ, Miquel Collell JM, Maluenda Colomer MD, et al. Preoperative detection of gastrointestinal neuroendocrine tumors using endoscopic ultrasonography. Rev Esp Enferm Dig. 2006;98:828–836. doi: 10.4321/s1130-01082006001100004. [DOI] [PubMed] [Google Scholar]
- 70.Pisegna J. Zollinger-Ellison Syndrome. Curr Treat Options Gastroenterol. 1999 Jun;2(3):195–204. doi: 10.1007/s11938-999-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frucht H, Norton JA, London JF, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination. A prospective study. Gastroenterology. 1990;99:1622–1627. doi: 10.1016/0016-5085(90)90466-e. [DOI] [PubMed] [Google Scholar]
- 72.Zollinger RM, Ellison EC, Fabri PJ, et al. Primary peptic ulcerations of the jejunum associated with islet cell tumors. Twenty-five-year appraisal. Ann Surg. 1980;192:422–430. doi: 10.1097/00000658-198009000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibril F, Jensen RT. Advances in evaluation and management of gastrinoma in patients with Zollinger-Ellison syndrome. Curr Gastroenterol Rep. 2005;7:114–121. doi: 10.1007/s11894-005-0049-2. [DOI] [PubMed] [Google Scholar]
- 74.Metz DC, Strader DB, Orbuch M, et al. Use of omeprazole in Zollinger-Ellison syndrome: a prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther. 1993;7:597–610. doi: 10.1111/j.1365-2036.1993.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 75.Wolfe MM, Jensen RT. Zollinger-Ellison syndrome. Current concepts in diagnosis and management. N Engl J Med. 1987;317:1200–1209. doi: 10.1056/NEJM198711053171907. [DOI] [PubMed] [Google Scholar]
- 76.Bloom SR, Polak JM, Pearse AG. Vasoactive intestinal peptide and watery-diarrhoea syndrome. Lancet. 1973;2:14–16. doi: 10.1016/s0140-6736(73)91947-8. [DOI] [PubMed] [Google Scholar]
- 77.Krejs GJ. VIPoma syndrome. Am J Med. 1987 May;82:37–48. doi: 10.1016/0002-9343(87)90425-6. [DOI] [PubMed] [Google Scholar]
- 78.Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med. 1958;25:374–380. doi: 10.1016/0002-9343(58)90075-5. [DOI] [PubMed] [Google Scholar]
- 79.Rawnsley HM, Raffensperger EC, Cerda JJ. Cholera-like syndrome and pancreatic islet cell tumors. Med Clin North Am. 1970;54:567–575. [PubMed] [Google Scholar]
- 80.Grier JF. WDHA (watery diarrhea, hypokalemia, achlorhydria) syndrome: clinical features, diagnosis, and treatment. South Med J. 1995;88:22–24. doi: 10.1097/00007611-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 81.McGavran MH, Unger RH, Recant L, et al. A glucagon-secreting alpha-cell carcinoma of the pancreas. N Engl J Med. 1966;274:1408–1413. doi: 10.1056/NEJM196606232742503. [DOI] [PubMed] [Google Scholar]
- 82.Wilkinson DS. Necrolytic migratory erythema with carcinoma of the pancreas. Trans St Johns Hosp Dermatol Soc. 1973;59:244–250. [PubMed] [Google Scholar]
- 83.Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 85.Moyana TN, Xiang J, Senthilselvan A, Kulaga A. The spectrum of neuroendocrine differentiation among gastrointestinal carcinoids: importance of histologic grading, MIB-1, p53, and bcl-2 immunoreactivity. Arch Pathol Lab Med. 2000;124:570–576. doi: 10.5858/2000-124-0570-TSONDA. [DOI] [PubMed] [Google Scholar]
- 86.Van Eeden S, Quaedvlieg PF, Taal BG, et al. Classi fication of low-grade neuroendocrine tumors of midgut and unknown origin. Hum Pathol. 2002;33:1126–1132. doi: 10.1053/hupa.2002.129204. [DOI] [PubMed] [Google Scholar]
- 87.Capella C, Heitz PU, Hofler H, et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425:547–560. doi: 10.1007/BF00199342. [DOI] [PubMed] [Google Scholar]
- 88.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 90.Strosberg J, Coppola D, Neumann AM, Kvols L. Clinicopathologic analysis of well, moderately and poorly differentiated gastroenteropancreatic neuroendocrine tumors. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 15028) [Google Scholar]
- 91.Hamilton SR, Aaltonen LA, editors. IARC Press; Lyon: 2000. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. [Google Scholar]
- 92.DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. IARC (Lyon): 2004. World Health Organization classification of tumours, pathology and genetics of tumours of endocrine organs. [Google Scholar]
- 93.Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med. 1954;16:363–371. doi: 10.1016/0002-9343(54)90353-8. [DOI] [PubMed] [Google Scholar]
- 94.Larsson C, Skogseid B, Oberg K, et al. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- 95.Scacheri PC, Davis S, Odom DT, et al. Genome-wide analysis of menin binding provides insights into MEN1 tumorigenesis. PLoS Genet. 2006;2:e51. doi: 10.1371/journal.pgen.0020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agarwal SK, Lee Burns A, Sukhodolets KE, et al. Molecular pathology of the MEN1 gene. Ann N Y Acad Sci. 2004;1014:189–198. doi: 10.1196/annals.1294.020. [DOI] [PubMed] [Google Scholar]
- 97.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 98.Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. doi: 10.1056/NEJM199003153221103. [DOI] [PubMed] [Google Scholar]
- 99.Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease. Results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495–500. doi: 10.1046/j.1365-2796.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 100.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 101.Dean PG, van Heerden JA, Farley DR, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24:1437–1441. doi: 10.1007/s002680010237. [DOI] [PubMed] [Google Scholar]
- 102.Ebeling T, Vierimaa O, Kytola S, et al. Effect of multiple endocrine neoplasia type 1 (MEN1) gene mutations on premature mortality in familial MEN1 syndrome with founder mutations. J Clin Endocrinol Metab. 2004;89:3392–3396. doi: 10.1210/jc.2003-031513. [DOI] [PubMed] [Google Scholar]
- 103.Richards FM, Maher ER, Latif F, et al. Detailed genetic mapping of the von Hippel-Lindau disease tumour suppressor gene. J Med Genet. 1993;30:104–107. doi: 10.1136/jmg.30.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 105.Verhoef S, van Diemen-Steenvoorde R, Akkers dijk WL, et al. Malignant pancreatic tumour within the spectrum of tuberous sclerosis complex in childhood. Eur J Pediatr. 1999;158:284–287. doi: 10.1007/s004310051073. [DOI] [PubMed] [Google Scholar]
- 106.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 107.Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 108.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 109.Sandsmark DK, Pelletier C, Weber JD, Gutmann DH. Mammalian target of rapamycin: master regulator of cell growth in the nervous system. Histol Histopathol. 2007;22:895–903. doi: 10.14670/HH-22.895. [DOI] [PubMed] [Google Scholar]
- 110.Moertel CG, Dockerty MB. Familial occurrence of metastasizing carcinoid tumors. Ann Intern Med. 1973;78:389–390. doi: 10.7326/0003-4819-78-3-389. [DOI] [PubMed] [Google Scholar]
- 111.Hemminki K, Li X. Familial carcinoid tumors and subsequent cancers: a nation-wide epidemiologic study from Sweden. Int J Cancer. 2001;94:444–448. doi: 10.1002/ijc.1473. [DOI] [PubMed] [Google Scholar]
- 112.Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer. 2001;92:2204–2210. doi: 10.1002/1097-0142(20011015)92:8<2204::aid-cncr1564>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 113.Tomita T. p53 and proliferating cell nuclear antigen in endocrine tumors of pancreas and intestinal carcinoids. Pathology. 1997;29:147–153. doi: 10.1080/00313029700169774. [DOI] [PubMed] [Google Scholar]
- 114.Weckstrom P, Hedrum A, Makridis C, et al. Midgut carcinoids and solid carcinomas of the intestine: differences in endocrine markers and p53 mutations. Endocr Pathol. 1996;7:273–279. doi: 10.1007/BF02739834. [DOI] [PubMed] [Google Scholar]
- 115.Yashiro T, Fulton N, Hara H, et al. Comparison of mutations of ras oncogene in human pancreatic exocrine and endocrine tumors Surgery 114758–763.discussion 763–7541993 [PubMed] [Google Scholar]
- 116.Chung DC, Smith AP, Louis DN, et al. Analysis of the retinoblastoma tumour suppressor gene in pancreatic endocrine tumours Clin Endocrinol(Oxf)47523–528.1997 [DOI] [PubMed] [Google Scholar]
- 117.Eubanks PJ, Sawicki MP, Samara GJ, et al. Putative tumor-suppressor gene on chromosome 11 is important in sporadic endocrine tumor formation. Am J Surg. 1994;167:180–185. doi: 10.1016/0002-9610(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 118.Goebel SU, Heppner C, Burns AL, et al. Geno - type/phenotype correlation of multiple endocrine neoplasia type 1 gene mutations in sporadic gastrinomas. J Clin Endocrinol Metab. 2000;85:116–123. doi: 10.1210/jcem.85.1.6260. [DOI] [PubMed] [Google Scholar]
- 119.Tonnies H, Toliat MR, Ramel C, et al. Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut. 2001;48:536–541. doi: 10.1136/gut.48.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Terris B, Meddeb M, Marchio A, et al. Comparative genomic hybridization analysis of sporadic neuroendocrine tumors of the digestive system. Genes Chromosomes Cancer. 1998;22:50–56. doi: 10.1002/(sici)1098-2264(199805)22:1<50::aid-gcc7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 121.Kytola S, Hoog A, Nord B, et al. Comparative genomic hybridization identifies loss of 18q22- qter as an early and specific event in tumorigenesis of midgut carcinoids. Am J Pathol. 2001;158:1803–1808. doi: 10.1016/S0002-9440(10)64136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Speel EJ, Richter J, Moch H, et al. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol. 1999;155:1787–1794. doi: 10.1016/S0002-9440(10)65495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stumpf E, Aalto Y, Hoog A, et al. Chromosomal alterations in human pancreatic endocrine tumors. Genes Chromosomes Cancer. 2000;29:83–87. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1011>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 124.Zhao J, Moch H, Scheidweiler AF, et al. Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes. Cancer. 2001;32:364–372. doi: 10.1002/gcc.1201. [DOI] [PubMed] [Google Scholar]
- 125.Zikusoka MN, Kidd M, Eick G, et al. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer. 2005;104:2292–2309. doi: 10.1002/cncr.21451. [DOI] [PubMed] [Google Scholar]
- 126.Van Gompel JJ, Chen H. Insulin-like growth factor 1 signaling in human gastrointestinal carcinoid tumor cells. Surgery. 2004;136:1297–1302. doi: 10.1016/j.surg.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 127.von Wichert G, Jehle PM, Hoeflich A, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–4581. [PubMed] [Google Scholar]
- 128.Terris B, Scoazec JY, Rubbia L, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32:133–138. doi: 10.1046/j.1365-2559.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 129.La Rosa S, Uccella S, Finzi G, et al. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: correlation with microvessel density and clinicopathologic features. Hum Pathol. 2003;34:18–27. doi: 10.1053/hupa.2003.56. [DOI] [PubMed] [Google Scholar]
- 130.Pavel ME, Hassler G, Baum U, et al. Circulating levels of angiogenic cytokines can pre dict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol (Oxf) 2005;62:434–443. doi: 10.1111/j.1365-2265.2005.02238.x. [DOI] [PubMed] [Google Scholar]
- 131.Guo SS, Wu X, Shimoide AT, et al. Frequent overexpression of cyclin D1 in sporadic pancreatic endocrine tumours. J Endocrinol. 2003;179:73–79. doi: 10.1677/joe.0.1790073. [DOI] [PubMed] [Google Scholar]
- 132.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 133.Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86:858–865. doi: 10.1002/(sici)1097-0142(19990901)86:5<858::aid-cncr23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 134.Wiedenmann B, Franke WW, Kuhn C, et al. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA. 1986;83:3500–3504. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pahlman S, Esscher T, Bergvall P, Odelstad L. Purification and characterization of human neuron-specific enolase: radioimmunoassay development. Tumour Biol. 1984;5:127–139. [PubMed] [Google Scholar]
- 136.Metz DC. Diagnosis and treatment of pancreatic neuroendocrine tumors. Semin Gastrointest Dis. 1995;6:67–78. [PubMed] [Google Scholar]
- 137.Feldman JM, O'Dorisio TM. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. Am J Med. 1986;81:41–48. doi: 10.1016/0002-9343(86)90583-8. [DOI] [PubMed] [Google Scholar]
- 138.Kema IP, Schellings AM, Meiborg G, et al. Influence of a serotonin- and dopamine-rich diet on platelet serotonin content and urinary excretion of biogenic amines and their metabolites. Clin Chem. 1992;38:1730–1736. [PubMed] [Google Scholar]
- 139.Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33–38. doi: 10.1159/000051853. [DOI] [PubMed] [Google Scholar]
- 140.O'Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986;314:1145–1151. doi: 10.1056/NEJM198605013141803. [DOI] [PubMed] [Google Scholar]
- 141.Janson ET, Holmberg L, Stridsberg M, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–690. doi: 10.1023/a:1008215730767. [DOI] [PubMed] [Google Scholar]
- 142.Ferrari L, Seregni E, Bajetta E, et al. The biological characteristics of chromogranin A and its role as a circulating marker in neuroendocrine tumours. Anticancer Res. 1999;19:3415–3427. [PubMed] [Google Scholar]
- 143.Jensen EH, Kvols L, McLoughlin JM, et al. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780–785. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]
- 144.Patel YC, Greenwood MT, Panetta R, et al. The somatostatin receptor family. Life Sci. 1995;57:1249–1265. doi: 10.1016/0024-3205(95)02082-t. [DOI] [PubMed] [Google Scholar]
- 145.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ.Octreotide N Engl J Med25;334246–254.1996 [DOI] [PubMed] [Google Scholar]
- 146.Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 147.Kvols LK, Moertel CG, O’Connell MJ, et al. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 148.Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 149.Oberg K, Norheim I, Theodorsson E. Treatment of malignant midgut carcinoid tumours with a long-acting somatostatin analogue octreotide. Acta Oncol. 1991;30:503–507. doi: 10.3109/02841869109092409. [DOI] [PubMed] [Google Scholar]
- 150.Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus openlabel subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 151.Wymenga AN, Eriksson B, Salmela PI, et al. Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumors and hormone-related symptoms. J Clin Oncol. 1999;17:1111. doi: 10.1200/JCO.1999.17.4.1111. [DOI] [PubMed] [Google Scholar]
- 152.Kvols LK, Buck M, Moertel CG, et al. Treatment of metastatic islet cell carcinoma with a somatostatin analogue (SMS 201–995) Ann Intern Med. 1987;107:162–168. doi: 10.7326/0003-4819-107-2-162. [DOI] [PubMed] [Google Scholar]
- 153.O'Dorisio TM, Gaginella TS, Mekhjian HS, et al. Somatostatin and analogues in the treatment of VIPoma. Ann N Y Acad Sci. 1988;527:528–535. doi: 10.1111/j.1749-6632.1988.tb27006.x. [DOI] [PubMed] [Google Scholar]
- 154.Boden G, Ryan IG, Eisenschmid BL, et al. Treatment of inoperable glucagonoma with the long-acting somatostatin analogue SMS 201–995 N Engl J Med26;3141686–1689.1986 [DOI] [PubMed] [Google Scholar]
- 155.Izumiyama H, Gotyo N, Fukai N, et al. Glucose-responsive and octreotide-sensitive insulinoma. Intern Med. 2006;45:519–524. doi: 10.2169/internalmedicine.45.1523. [DOI] [PubMed] [Google Scholar]
- 156.Usukura M, Yoneda T, Oda N, et al. Medical treatment of benign insulinoma using octreotide LAR: a case report. Endocr J. 2007;54:95–101. doi: 10.1507/endocrj.k05-157. [DOI] [PubMed] [Google Scholar]
- 157.Vezzosi D, Bennet A, Rochaix P, et al. Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with antisst2A and anti-sst5 antibodies. Eur J Endocrinol. 2005;152:757–767. doi: 10.1530/eje.1.01901. [DOI] [PubMed] [Google Scholar]
- 158.Healy ML, Dawson SJ, Murray RM, et al. Severe hypoglycaemia after long-acting octreotide in a patient with an unrecognized malignant insulinoma. Intern Med J. 2007;37:406–409. doi: 10.1111/j.1445-5994.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- 159.Janson ET, Oberg K. Long-term management of the carcinoid syndrome. Treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol. 1993;32:225–229. doi: 10.3109/02841869309083916. [DOI] [PubMed] [Google Scholar]
- 160.Ruszniewski P, Ducreux M, Chayvialle JA, et al. Treatment of the carcinoid syndrome with the longacting somatostatin analogue lanreotide: a prospective study in 39 patients. Gut. 1996;39:279–283. doi: 10.1136/gut.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arnold R, Wied M, Behr TH. Somatostatin analogues in the treatment of endocrine tumors of the gastrointestinal tract. Expert Opin Pharmacother. 2002;3:643–656. doi: 10.1517/14656566.3.6.643. [DOI] [PubMed] [Google Scholar]
- 162.Weckbecker G, Raulf F, Stolz B, Bruns C. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol Ther. 1993;60:245–264. doi: 10.1016/0163-7258(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 163.Rohrer SP, Birzin ET, Mosley RT, et al. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282:737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- 164.Evers BM, Parekh D, Townsend CM, Jr, Thompson JC. Somatostatin and analogues in the treatment of cancer. A review. Ann Surg. 1991;213:190–198. doi: 10.1097/00000658-199103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Evers BM, Townsend CM, Jr, Upp JR, et al. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101:303–311. doi: 10.1016/0016-5085(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 166.Bousquet C, Puente E, Buscail L, et al. Antiproliferative effect of somatostatin and analogs. Chemotherapy. 2001;47(suppl 2):30–39. doi: 10.1159/000049159. [DOI] [PubMed] [Google Scholar]
- 167.Woltering EA, Watson JC, Alperin-Lea RC, et al. Somatostatin analogs: angiogenesis inhibitors with novel mechanisms of action. Invest New Drugs. 1997;15:77–86. doi: 10.1023/a:1005774713202. [DOI] [PubMed] [Google Scholar]
- 168.Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430–438. doi: 10.1136/gut.38.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ducreux M, Ruszniewski P, Chayvialle JA, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol. 2000;95:3276–3281. doi: 10.1111/j.1572-0241.2000.03210.x. [DOI] [PubMed] [Google Scholar]
- 170.Krenning EP, Bakker WH, Kooij PP, et al. Somatostatin receptor scintigraphy with indium- 111-DTPA-D-Phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J Nucl Med. 1992;33:652–658. [PubMed] [Google Scholar]
- 171.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 172.Andersson P, Forssell-Aronsson E, Johanson V, et al. Internalization of indium-111 into human neuroendocrine tumor cells after incubation with indium-111-DTPA-D-Phe1-octreotide. J Nucl Med. 1996;37:2002–2006. [PubMed] [Google Scholar]
- 173.Wangberg B, Nilsson O, Johanson VV, et al. Somatostatin receptors in the diagnosis and therapy of neuroendocrine tumor. Oncologist. 1997;2:50–58. [PubMed] [Google Scholar]
- 174.Valkema R, De Jong M, Bakker WH, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–122. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- 175.Anthony LB, Woltering EA, Espenan GD, et al. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med. 2002;32:123–132. doi: 10.1053/snuc.2002.31769. [DOI] [PubMed] [Google Scholar]
- 176.Valkema R, Pauwels S, Kvols LK, et al. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147–156. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 177.Waldherr C, Pless M, Maecke HR, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 178.Paganelli G, Zoboli S, Cremonesi M, et al. Receptor-mediated radiotherapy with 90YDOTA- D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426–434. doi: 10.1007/s002590100490. [DOI] [PubMed] [Google Scholar]
- 179.Kwekkeboom DJ, Mueller-Brand J, Paganelli G, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46(suppl 1):62S–66S. [PubMed] [Google Scholar]
- 180.Valkema R, Pauwels SA, Kvols LK, et al. Longterm follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0), Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46(suppl 1):83S–91S. [PubMed] [Google Scholar]
- 181.Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 182.Kwekkeboom DJ, Bakker WH, Kooij PP, et al. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med. 2001;28:1319–1325. doi: 10.1007/s002590100574. [DOI] [PubMed] [Google Scholar]
- 183.Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu- DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–2762. doi: 10.1200/JCO.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 184.Moertel CG, Hanley JA. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials. 1979;2:327–334. [PubMed] [Google Scholar]
- 185.Oberg K, Norheim I, Lundqvist G, Wide L. Cytotoxic treatment in patients with malignant carcinoid tumors. Response to streptozocin— alone or in combination with 5-FU. Acta Oncol. 1987;26:429–432. doi: 10.3109/02841868709113712. [DOI] [PubMed] [Google Scholar]
- 186.Sun W, Lipsitz S, Catalano P, et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23:4897–4904. doi: 10.1200/JCO.2005.03.616. [DOI] [PubMed] [Google Scholar]
- 187.Bukowski RM, Tangen C, Lee R, et al. Phase II trial of chlorozotocin and fluorouracil in islet cell carcinoma: a Southwest Oncology Group study. J Clin Oncol. 1992;10:1914–1918. doi: 10.1200/JCO.1992.10.12.1914. [DOI] [PubMed] [Google Scholar]
- 188.Engstrom PF, Lavin PT, Moertel CG, et al. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol. 1984;2:1255–1259. doi: 10.1200/JCO.1984.2.11.1255. [DOI] [PubMed] [Google Scholar]
- 189.Bukowski RM, Johnson KG, Peterson RF, et al. A phase II trial of combination chemotherapy in patients with metastatic carcinoid tumors. A Southwest Oncology Group Study. Cancer. 1987;60:2891–2895. doi: 10.1002/1097-0142(19871215)60:12<2891::aid-cncr2820601207>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 190.Bukowski RM, Tangen CM, Peterson RF, et al. Phase II trial of dimethyltriazenoimidazole carboxamide in patients with metastatic carcinoid. A Southwest Oncology Group study. Cancer. 1994;73:1505–1508. doi: 10.1002/1097-0142(19940301)73:5<1505::aid-cncr2820730530>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 191.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 192.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced isletcell carcinoma. N Engl J Med. 1980;303:1189–1194. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]
- 193.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 194.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 195.Ramanathan RK, Cnaan A, Hahn RG, et al. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol. 2001;12:1139–1143. doi: 10.1023/a:1011632713360. [DOI] [PubMed] [Google Scholar]
- 196.Detjen KM, Welzel M, Farwig K, et al. Molecular mechanism of interferon alfa-mediated growth inhibition in human neuroendocrine tumor cells. Gastroenterology. 2000;118:735–748. doi: 10.1016/s0016-5085(00)70143-0. [DOI] [PubMed] [Google Scholar]
- 197.Oberg K, Norheim I, Lind E, et al. Treatment of malignant carcinoid tumors with human leukocyte interferon: long-term results. Cancer Treat Rep. 1986;70:1297–1304. [PubMed] [Google Scholar]
- 198.Frank M, Klose KJ, Wied M, Ishaque N, Schade-Brittinger C, Arnold R. Combination therapy with octreotide and alpha-interferon: effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol. 1999;94:1381–1387. doi: 10.1111/j.1572-0241.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 199.Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3:761–771. doi: 10.1016/s1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 200.Kulke MH, Bergsland EK, Ryan DP, et al. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol. 2006;24:3555–3561. doi: 10.1200/JCO.2006.05.6762. [DOI] [PubMed] [Google Scholar]
- 201.Kulke M, Bergsland E, Ryan D. A phase 2 study to evaluate the safety and efficacy of SU11248 in patients with unresectable neuroendocrine tumors. Proc Am Soc Clin Oncol. 2003;22 (abstr 958) [Google Scholar]
- 202.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a randomized assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;23:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 203.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 204.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors Surgery 1341057–1063.discussion, 1063–10652003 [DOI] [PubMed] [Google Scholar]
- 205.Ruszniewski P, O'Toole D.Ablative therapies for liver metastases of gastroenteropancreatic endocrine tumors Neuroendocrinology 80suppl174–78.2004 [DOI] [PubMed] [Google Scholar]
- 206.Henn AR, Levine EA, McNulty W, Zagoria RJ. Percutaneous radiofrequency ablation of hepatic metastases for symptomatic relief of neuroendocrine syndromes. Am J Roentgenol. 2003;181:1005–1010. doi: 10.2214/ajr.181.4.1811005. [DOI] [PubMed] [Google Scholar]
- 207.Hellman P, Ladjevardi S, Skogseid B, et al. Radiofrequency tissue ablation using cooled tip for liver metastases of endocrine tumors. World J Surg. 2002;26:1052–1056. doi: 10.1007/s00268-002-6663-3. [DOI] [PubMed] [Google Scholar]
- 208.Gillams AR, Cassoni AM, Conway GS, Lees WR. Radiofrequency ablation of neuroendocrine liver metastases: The Middlesex experience. Abdominal Imaging. 2005;30:435–441. doi: 10.1007/s00261-004-0258-4. [DOI] [PubMed] [Google Scholar]
- 209.Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas Am J Surg 16936–42.discussion, 42–431995 [DOI] [PubMed] [Google Scholar]
- 210.McEntee GP, Nagorney DM, Kvols LK, et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108:1091–1096. [PubMed] [Google Scholar]
- 211.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am. 2003;12:231–242. doi: 10.1016/s1055-3207(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 212.Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 18788–92.discussion, 92–93,1998 [DOI] [PubMed] [Google Scholar]
- 213.Osborne DA, Zervos EE, Strosberg J, et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol. 2006;13:572–581. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 214.Touzios JG, Kiely JM, Pitt SC, et al. Neuro endocrine hepatic metastases: does aggressive management improve survival? Ann Surg 241776–783.discussion, 783–785,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Strosberg JR, Choi J, Cantor AB, Kvols LK. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13:72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 216.Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M. D. Anderson experience. Cancer J. 2003;9:261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 217.Eriksson BK, Larsson EG, Skogseid BM, et al. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83:2293–2301. [PubMed] [Google Scholar]
- 218.Mitty HA, Warner RR, Newman LH, et al. Control of carcinoid syndrome with hepatic artery embolization. Radiology. 1985;155:623–626. doi: 10.1148/radiology.155.3.4001362. [DOI] [PubMed] [Google Scholar]
- 219.Therasse E, Breittmayer F, Roche A, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189:541–547. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 220.Diaco DS, Hajarizadeh H, Mueller CR, et al. Treatment of metastatic carcinoid tumors using multimodality therapy of octreotide acetate, intra-arterial chemotherapy, and hepatic arterial chemoembolization. Am J Surg. 1995;169:523–528. doi: 10.1016/S0002-9610(99)80210-4. [DOI] [PubMed] [Google Scholar]
- 221.Ruszniewski P, Malka D. Hepatic arterial chemoembolization in the management of advanced digestive endocrine tumors. Digestion. 2000;62(suppl 1):79–83. doi: 10.1159/000051860. [DOI] [PubMed] [Google Scholar]
- 222.Moertel CG, Johnson CM, McKusick MA, et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med. 1994;120:302–309. doi: 10.7326/0003-4819-120-4-199402150-00008. [DOI] [PubMed] [Google Scholar]
- 223.Loewe C, Schindl M, Cejna M, et al. Per ma nent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. Am J Roentgenol. 2003;180:1379–1384. doi: 10.2214/ajr.180.5.1801379. [DOI] [PubMed] [Google Scholar]
- 224.Ruszniewski P, Rougier P, Roche A, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer. 1993;71:2624–2630. doi: 10.1002/1097-0142(19930415)71:8<2624::aid-cncr2820710830>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 225.Andrews JC, Walker SC, Ackermann RJ, et al. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637–1644. [PubMed] [Google Scholar]
- 226.Ahlman H, Friman S, Cahlin C, et al. Liver transplantation for treatment of metastatic neuro - endocrine tumors. Ann NY Acad Sci. 2004;1014:265–269. doi: 10.1196/annals.1294.029. [DOI] [PubMed] [Google Scholar]
- 227.Olausson M, Friman S, Cahlin C, et al. Indications and results of liver transplantation in patients with neuroendocrine tumors. World J Surg. 2002;26:998–1004. doi: 10.1007/s00268-002-6631-y. [DOI] [PubMed] [Google Scholar]
- 228.Coppa J, Pulvirenti A, Schiavo M, et al. Re section versus transplantation for liver metastases from neuroendocrine tumors. Transplant Proc. 2001;33:1537–1539. doi: 10.1016/s0041-1345(00)02586-0. [DOI] [PubMed] [Google Scholar]
- 229.Lang H, Oldhafer KJ, Weimann A, et al. Liver transplantation for metastatic neuroendocrine tumors. Ann Surg. 1997;225:347–354. doi: 10.1097/00000658-199704000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bechstein WO, Neuhaus P. Liver transplantation for hepatic metastases of neuroendocrine tumors. Ann NY Acad Sci. 1994;733:507–514. doi: 10.1111/j.1749-6632.1994.tb17301.x. [DOI] [PubMed] [Google Scholar]
- 231.Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation. 1998;66:1307–1312. doi: 10.1097/00007890-199811270-00007. [DOI] [PubMed] [Google Scholar]
- 232.Godwin JD., 2nd Carcinoid tumors. An analysis of 2,837 cases. Cancer. 1975;36:560–569. doi: 10.1002/1097-0142(197508)36:2<560::aid-cncr2820360235>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 233.Moller JE, Pellikka PA, Bernheim AM, et al. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112:3320–3327. doi: 10.1161/CIRCULATIONAHA.105.553750. [DOI] [PubMed] [Google Scholar]
- 234.McDermott EW, Guduric B, Brennan MF. Prognostic variables in patients with gastrointestinal carcinoid tumours. Br J Surg. 1994;81:1007–1009. doi: 10.1002/bjs.1800810725. [DOI] [PubMed] [Google Scholar]
- 235.Strosberg J, Kvols L. Survival analysis of 90 metastatic pancreatic endocrine carcinomas. ASCO Gastrointestinal Cancers Symposium; 2008. (abstr 149) [Google Scholar]