EMBO J 27, 3092–3103 (2008); published online 6 November 2008
An increase in pressure inside most small arteries unexpectedly results in a vasoconstriction. This phenomenon, known as the Bayliss effect, involves the stretch-induced activation of non-selective cation channels in the vascular smooth muscle cells. Recent work by Mederos y Schnitzler et al. now demonstrates that the stretch-induced channel activity originates from a fascinating interplay between a TRP cation channel and the angiotensin receptor.
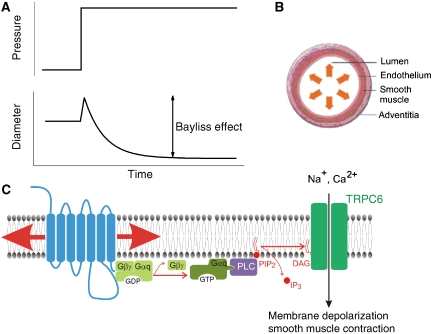
Intuitively, we all appreciate that increasing the intracellular pressure in an elastic container (e.g. a balloon or the tyre of your bicycle) leads to expansion of that container. However, many blood vessels, in particular small resistance arteries, behave oppositely: increasing intraluminal blood pressure makes them constrict instead of dilate (Figure 1A). This pressure-induced myogenic vasoconstriction is generally known as the Bayliss effect, after the English Physiologist William Maddock Bayliss, who first described it in 1902 (Bayliss, 1902). On account of the Bayliss effect, the blood flow through small arteries and arterioles in brain, kidney and several other internal organs remains relatively stable even when the perfusion pressure fluctuates, thus establishing autoregulation.
Figure 1.
The Bayliss effect. (A) Increasing pressure in certain blood vessels causes vasoconstriction, a phenomenon known as the Bayliss effect. (B) The Bayliss is mediated by the smooth muscle layer, independent of the inner layer of endothelial cells. (C) Proposed mechanism for stretch-induced activation of TRPC6 in vascular smooth muscle membranes.
Hence what is the mechanism underlying the Bayliss effect? It was already known for some time that the effect is mediated by the vascular smooth muscle, independent of the endothelial cell layer that lines the vessels (Figure 1B). The increased intraluminal pressure results in the activation of non-selective cation channels in the plasma membrane of the smooth muscle cells, which causes membrane depolarization, Ca2+ influx through voltage-gated L-type Ca2+ channels and smooth muscle contraction (Davis and Hill, 1999). However, a key unresolved question was the mechanism underlying the stretch-induced activation of the non-selective cation channels.
Several lines of evidence pointed at a possible involvement of cation channels of the TRP superfamily in the Bayliss effect. For example, downregulation of the expression of either TRPC6 or TRPM4 in the smooth muscle rat cerebral arteries using antisense oligonucleotides led to a severe loss of pressure-induced vasoconstriction (Welsh et al, 2002; Earley et al, 2004). Given that several TRP channels have been shown to function as direct sensors of a variety of stimuli, including temperature changes and a variety of endogenous and exogenous chemical stimuli (Voets et al, 2005), it appeared straightforward to propose that some TRP channels could act as mechanosensors in vascular smooth muscle cells. And indeed, it has been reported that heterologously expressed TRPC6 can be directly activated by membrane stretch (Spassova et al, 2006), although later studies have strongly questioned this proposal (Gottlieb et al, 2008). A crucial aspect of the discussion is that proving a (TRP) channel to be an intrinsic sensor of membrane stretch is far from obvious. The most important criterion to establish that a channel is directly mechanosensitive is the latency between stimulus and channel activation, which should be in the order of a few milliseconds (Christensen and Corey, 2007).
In a recent article in EMBO Journal, Mederos y Schnitzler et al (2008) report that TRPC6 does not meet the criteria for a bona fide mechanosensitive channel. Instead, they show that the TRPC6 (and the related TRPC3 and TRPC7) can be readily gated by membrane stretch in cells that co-express membrane receptors that couple to activation of Gq-type GTP-binding proteins. G-protein-coupled receptors (GPCRs) form a large and well-studied family of transmembrane proteins that sense molecules outside the cell, including neurotransmitters, hormones, odorants, tastants and more. Ligand binding causes a conformational change in the receptors, leading to the activation of trimeric G proteins. The activated Gα and Gβγ proteins can initiate various signal transduction pathways. For example, GPCRs that are coupled to Gq lead to activation of phospholipase C, which hydrolyses the membrane phospholipids phosphatidyl inositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). It was previously shown that TRPC3, TRPC6 and TRPC7 are directly activated by DAG, which clearly explained the activation of these channels upon ligand activation of Gq-coupled GPCRs (Hofmann et al, 1999).
Following up on previous results showing that the angiotensin receptor (AT2R), a GPCR that is widely expressed in the vasculature, can be activated by membrane stretch in the absence of a ligand (Zou et al, 2004; Yasuda et al, 2008), Mederos y Schnitzler et al (2008) hypothesized that a GPCR could be the element that makes TRPC6 gating in vascular smooth muscle cells mechanosensitive (Figure 1C). In their report, they provided several lines of evidence to substantiate this hypothesis. First, as mentioned above, heterologously expressed TRPC6 can be activated by manoeuvres that provoke membrane stretch (e.g. hypotonic cell swelling or hydrostatic pressure), but only when these cells co-express Gq-coupled GPCRs, such as the AT2R. Second, agents that inhibit either the function of the GPCRs or of PLC prevent stretch activation of the channel. Third, smooth muscles derived from rat aorta (A7R5 cells), which do not express stretch-activated cation channels, exhibit robust stretch-activated TRPC6-like channel activity when overexpressing AT2R. Fourth, the Bayliss effect in cerebral arteries and isolated perfused kidneys is counteracted by losartan, an inverse agonist of the AT2R. Finally, the Bayliss effect is significantly enhanced in kidneys from mice that lack the negative regulator of Gq signalling RSG2.
These findings not only provide a clear molecular picture of what happens during the Bayliss effect, they may also provide a new paradigm for mechanosensitive channel activation in other physiological settings. The finding that several Gq-coupled GPCRs can be activated by membrane stretch implies that mechanical stimuli can influence PLC activity in a large array of cells and tissues. PLC activity influences the cellular levels of PIP2, DAG and IP3, all of which can influence the activity of certain TRP channels (Nilius et al, 2007). It will be intriguing to investigate whether other mechanosensitive processes depend on similar GPCR–TRP channel cooperation.
References
- Bayliss W (1902) On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AP, Corey DP (2007) TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521 [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423 [DOI] [PubMed] [Google Scholar]
- Earley S, Waldron BJ, Brayden JE (2004) Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929 [DOI] [PubMed] [Google Scholar]
- Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E (2008) Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch 455: 1097–1103 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263 [DOI] [PubMed] [Google Scholar]
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T (2008) Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA (2007) Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217 [DOI] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL (2006) A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Talavera K, Owsianik G, Nilius B (2005) Sensing with TRP channels. Nat Chem Biol 1: 85–92 [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE (2002) Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250 [DOI] [PubMed] [Google Scholar]
- Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I (2008) Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep 9: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I (2004) Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506 [DOI] [PubMed] [Google Scholar]