Abstract
Background and Purpose
We assessed the association of prestroke comorbidities with long-term stroke outcomes among women with ischemic stroke.
Methods
Prestroke comorbid conditions in 133 women admitted with acute ischemic stroke were scored with the Charlson Index (CI). We assessed whether the CI and other specific conditions were associated with modified Rankin Score (mRS) at 90 days or more poststroke.
Results
After adjustment for initial NIHSS and age, higher CI was the sole factor independently associated with poorer 90 day mRS scores. When CI was excluded, coronary disease and diabetes were independently associated with poorer outcome.
Conclusion
The extent of comorbidities as assessed by the CI is independently associated with 90-day mRS among women with ischemic stroke, but the individual comorbidities of CHD and DM were each associated with functional outcome.
Keywords: acute stroke, outcome, stroke recovery, women and minorities, comorbidities
Women who have ischemic strokes are on average older than men, and their older age may contribute to their poorer outcomes.1 Several studies, however, show that stroke outcomes are worse in women even after adjusting for age.2–4 The additional specific conditions that predict functional outcome in women are not known.
The Charlson Index is a widely used comorbidity index that weights conditions based on their association with 1-year mortality. The score, which can be based on ICD-9 codes or data collected from medical records, is defined by the presence of 16 distinct illnesses, including stroke and hemiplegia.5 A score >2 was associated with poor outcomes among male ischemic stroke patients when the Charlson Index was modified to exclude stroke and hemiplegia.6
We previously found that the presence of prestroke coronary heart disease (CHD) was independently associated with more severe strokes.7 The objective of the present study was to determine whether general comorbidity status as measured by the Charlson Index or specific prestroke conditions such as CHD are associated with 90-day disability as reflected by the modified Rankin Index (mRS) in a cohort of women with ischemic stroke.
Patients and Methods
Consecutive women over age 18 who had an acute ischemic stroke within 24 hours before admission to Duke University Medical Center were invited to participate. Written informed consent was obtained before enrollment. The protocol was approved by the Duke University Medical Center Institutional Review Board.
The diagnosis of ischemic stroke was determined according to the World Health Organization criteria8 and confirmed with routine neuroimaging (CT or MRI scan). Women with TIA, intracerebral or subarachnoid hemorrhage, other intracranial abnormalities (subdural hematoma, brain tumor, etc.), severe dementia, or life expectancy less than 6 months were excluded.
Baseline data were collected from subjects with in-person interviews or from medical records and NIH Stroke Scale score (NIHSS) was obtained by certified study personnel at enrollment. Medical comorbidities were assessed using the modified Charlson Index, which excluded stroke and hemiplegia. Scores were assigned by the study principal investigator (C.D.B.) from medical records.5,6 The modified Rankin Score (mRS)9 was obtained from subjects 90 days or more after stroke by personal or telephone interview.
Statistical Analysis
Nonparametric tests were performed to assess associations between factors and the ordinal 90-day mRS (Table 1). Univariately significant factors (P<0.05) were entered into linear regression models applied to the ranked mRS adjusted for initial NIHSS and age. CHD is included in the Charlson Index and in the case of low Charlson scores (1, 2, or 3), CHD and Charlson Index were colinear. Therefore, a separate Charlson score was assigned that excluded CHD (Charlson without CHD). The effect of colinearity was also assessed by modeling outcomes with and without Charlson scores. The analyses were performed using SAS version 9.1.
Table 1. Demographics and Distributions of Candidate Variables for Outcomes Among Women With Ischemic Stroke (n=133).
| Variable | Frequency |
|---|---|
| Age, median (IQR) | 64 (27 to 88) |
| Race-ethnicity, n (%) | |
| Caucasian | 67 (50) |
| African American | 65 (49) |
| Hispanic | 1 (1) |
| BMI, kg/m2, median (range) | 27 (16 to 58.6) |
| Hypertension, n (%) | 97 (73) |
| Diabetes, n (%) | 46 (35) |
| Hyperlipidemia, n (%) | 68 (51) |
| Prior stroke, n (%) | 33 (25) |
| Hypertension, n (%) | 97 (73) |
| Prior TIA, n (%) | 23 (17) |
| Tobacco smoker, n (%) | 39 (30) |
| Atrial fibrillation, n (%) | 16 (12) |
| Coronary heart disease, n (%) | 28 (21) |
| Congestive heart failure, n (%) | 11 (8) |
Results
Of 142 eligible women, 9 refused and 133 (94%) were enrolled. The demographics and risk factor distributions of the cohort are shown in Table 1. The modified Charlson Index ranged from 0 to 5, and 66% of subjects scored 0 or 1. The median initial NIHSS was 4.0 (range 0 to 22). Three women (2%) died during hospitalization, 6 (4.5%) died before follow-up, 5 (3.8%) withdrew, and 17 (12.8%) were lost to follow-up, leaving 105 (78.9%) with 90-day mRS scores (median score = 1, range 0 to 4). The median time from enrollment to follow-up was 105 days (interquartile range 91 to 151 days).
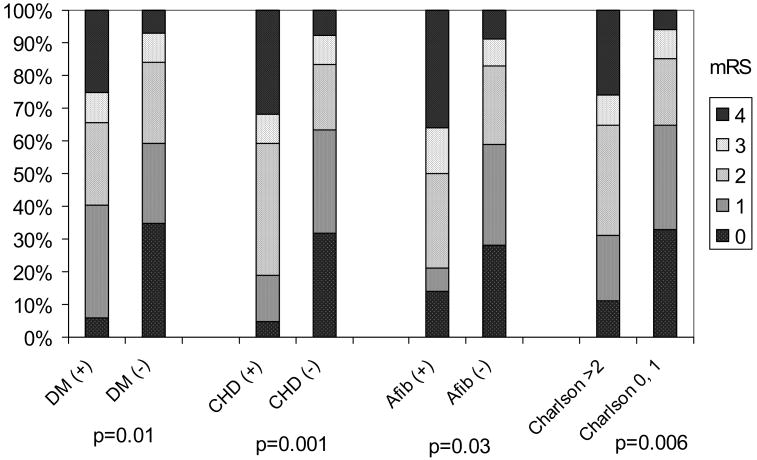
Diabetes (P=0.01), CHD (P=0.001), atrial fibrillation (P=0.03), and Charlson Index (P=0.006; Figure) were associated with higher mRS scores. Because the Charlson Index was colinear with both CHD and diabetes, separate models were generated (Table 2). The first model evaluated the modified Charlson Index (excluding stroke and hemiplegia but including CHD and DM). DM and CHD were not included as separate variables. The modified Charlson Index was the sole factor independently associated with mRS in this model (Table 2; P<0.0001). The second model excluding the Charlson Index found CHD (P=0.004) and diabetes (P<0.0001) were each independently associated with mRS (Table 2).
Figure.
Bar graph distribution of modified Rankin scores associated with specific comorbidities and the Charlson Index. Modified Rankin score categories (0 through 4) at 90 days for each condition among women with ischemic stroke. mRS indicates modified Rankin scale; DM, diabetes mellitus; CHD, coronary heart disease; Afib, atrial fibrillation.
Table 2. Multivariable Models for 90-Day Modified Rankin Scores Among Women With Ischemic Stroke (n=105).
| Model | Variables Included* | Independent Variables | Partial R2 | Model R2 | F Value | P Value |
|---|---|---|---|---|---|---|
| 1 | Charlson | Charlson | 0.126 | 0.486 | <0.0001 | |
| 2 | CHD, DM | DM | 0.092 | 0.496 | 17.10 | <0.001 |
| CHD | 0.044 | 8.89 | 0.004 | |||
| 3 | Charlson (CHD and DM removed), CHD, and DM | DM | 0.092 | 0.511 | 17.10 | <0.001 |
| CHD | 0.044 | 8.89 | 0.004 | |||
| Charlson (CHD, DM removed) | 0.016 | 3.23 | 0.076 |
All models included initial NIHSS, age, and atrial fibrillation. CHD indicates coronary heart disease; DM, diabetes mellitus.
Because DM and CHD were major contributors to the overall Charlson Index score, both conditions were eliminated from the overall Charlson Index to determine whether remaining comorbidities affected 90 day outcome. A third model included the Charlson with CHD and DM removed, and CHD and DM as separate variables. In this model, all 3 variables were independently associated with mRS scores (Table 2).
The analyses were repeated with the women who died before 90-day assigned score of 6 on the mRS. The results were similar except there was a trend reflecting a relationship between increasing age and poorer mRS scores (partial R2=0.017, P=0.06).
Discussion
In this cohort of women with ischemic stroke, overall comorbidities as reflected in the Charlson Index were associated with poor outcomes with a significant effect attributable to specific comorbidities (ie, CHD and diabetes). Age also tended to affect outcome when mortality was included.
The results are consistent with prior work in a cohort of men with stroke and provides further validation of the Charlson Index as a measure of comorbidity in stroke outcome studies.6 Similarly, a larger number of comorbidities correlated with poorer mRS scores 1 year poststroke in patients at the Mayo Clinic.10 These data suggest that functional outcome measures in stroke studies may need to be adjusted for prestroke comorbidities.
We assessed for colinearity among the Charlson Index and specific comorbidities, as well as the independent contribution of CHD and DM after removing them from the Charlson Index score. The variance in mRS (reflected in the partial R2) explained by the CHD and DM without the Charlson were similar to the Charlson alone (Table 2), suggesting that the majority of the impact of the Charlson is attributable to the presence of these 2 conditions. However, based on the trend toward an independent association of the Charlson score with outcome even after CHD and DM were removed, other comorbidities reflected in the score also contribute to outcome.
This study has several limitations. We were unable to obtain prestroke disability scores as measured by the mRS in our cohort, and 13% were lost to follow-up. Also, we studied only women presenting within 24 hours; therefore, the results may not apply to women who were unable or unwilling to seek medical attention until much later after symptom onset.
The major strength of this study is that it is one of the first longitudinal studies of detailed outcomes after stroke in women, which has been generally lacking from the major trials11,12 and cohort studies focused on women and cardiovascular disease.13
Women with prestroke comorbidities are more likely to have poor stroke outcomes. This study suggests that measurement of stroke outcomes in women may need to account for the presence of comorbid conditions, (Charlson Index), or at a minimum, CHD and diabetes because of their significant impact on stroke outcome.
Acknowledgments
Sources of Funding: This study was supported by grant number NIH K23NS041929 from the National Institute of Neurological Disorders and Stroke (NINDS) and by a National Stroke Association Research Fellowship Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the National Institutes of Health, or the National Stroke Association.
Footnotes
Disclosures: None.
References
- 1.Kelly-Hayes M, Beiser AS, Kase C, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: The framingham study. J Stroke Cerebrovasc Diseases. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 2.Glader EL, Stegmayr B, Norrving B, Terent A, Hulter-Asberg K, Wester PO, Asplund K. Sex differences in management and outcome after stroke. A swedish national perspective. Stroke. 2003;34:1970–1975. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 3.Kapral MK, Fang J, Hill MD, Silver F, Richars J, Jaigobin C, Cheung AM. Sex differences in stroke care and outcomes. Results from the registry of the canadian stroke network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 4.Di Carlo A, Lamassa M, Baldreschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in europe. Data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 5.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LB, Samsa GP, Matchar D, Horner R. Charlson index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35:1941–1945. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
- 7.Bushnell CD, Samsa GP, Goldstein LB. Hormone replacement therapy and ischemic stroke severity in women: A case control study. Neurology. 2001;56:1304–1307. doi: 10.1212/wnl.56.10.1304. [DOI] [PubMed] [Google Scholar]
- 8.Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 9.The national institute of neurological disorders; stroke rt-pa stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 10.Dombovy M, Basford J, Whisnant J, Bergstrahl E. Disability and use of rehabilitation services following stroke in rochester, minnesota, 1975–1979. Stroke. 1987;18:830–836. doi: 10.1161/01.str.18.5.830. [DOI] [PubMed] [Google Scholar]
- 11.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard B, Kooperberg C, Rossouw JE, Trevisan M, Aragaki AK, Baird A, Bray PF, Buring J, Criqui M, Herrington D, Lynch JK, Rapp SR, Torner J. Effects of conjugated equine estrogen on stroke in the women's health initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 12.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 13.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]