Abstract
Background
Studies of bone marrow transplantation from wild-type mice or rats to GalT knockout mice have demonstrated that induction of mixed chimerism tolerizes not only T cells, but also natural antibody-producing B cells, even across xenogeneic barriers. Given that rodent cells express lower levels of the αGal epitope than the more clinically relevant porcine species, the consequences of exposure to cells expressing high levels of αGal on the ability to induce B cell tolerance are unknown.
Methods
The effects on chimerism and anti-αGal B cell tolerance of an i.p. injection of 109 porcine RBC were evaluated in GalT knockout mice receiving wild-type allogeneic BMT after non-myeloablative conditioning with T cell depleting mAbs, thymic irradiation and low dose total body irradiation.
Results
Achievement of mixed chimerism and tolerance of anti-αGal producing B cells was not affected by exposure to high density αGal at the time of BMT. The absence of induced anti-αGal or anti-pig antibody responses in conditioned control mice suggested that the B cell xeno-response to pig is T cell-dependent.
Conclusion
High αGal density on pig cells might not preclude the ability to achieve tolerance of pre-existing αGal-reactive human B cells via induction of mixed chimerism. This strategy has the potential to induce B cell tolerance to non-αGal epitopes, against which Nabs have been found in the sera of healthy humans.
Keywords: antigen density, tolerance, bone marrow transplantation, natural Ab, αGal
Introduction
Humans lack a functional α1,3-galactosyltransferase (GalT) and therefore produce abundant natural antibodies (Nabs) directed against the α Gal epitope (1). In contrast, cells from pigs, which are considered to be the most promising potential source animals for human xenotransplantation, express very high levels of αGal on both proteins and lipids (2). The abundant anti-αGal natural antibodies (Nabs) found in humans and other old world primates was the major impediment to the success of xenotransplantation from pigs before the development of GalT KO pigs, which permitted a record 83-day survival of a life-supporting pig-to-baboon kidney xenograft (3,4). The development of GalT KO pigs has revealed that natural anti non-αGal antibodies exist in the sera of healthy individuals humans and other old world primates, and that these antibodies can lead to the activation of porcine endothelial cells and promote graft destruction (5). Studies in mice have revealed that mixed chimerism is an effective means of inducing tolerance to non-Gal xenoantigens in addition to anti-αGal (6,7).
GalT KO mice, which lack a functional α1,3-galactosyltransferase enzyme, also produce anti-αGal Nabs. We have previously shown that establishment of mixed chimerism from Gal+ allogeneic or xenogeneic donors in GalT KO mice induces tolerance of pre-existing anti-αGal antibody-producing B1-b B cells (8). In addition, pre-sensitized GalT KO mice, in which increased levels of IgM and class-switched IgG anti-αGal antibodies are present, can still achieve mixed chimerism and be rendered tolerant to αGal with a high dose of Gal+ BMT (9). B cell tolerance has been shown to dependent on different mechanisms when antigen density and location varies (10). Given that mouse cells express lower levels of the αGal epitope than the more relevant porcine species (11) (2), it remained possible that our strategy of mixed chimerism to induce tolerance of pre-existing anti-αGal antibody-producing B cells might be less effective when donor cells express higher levels of αGal Ag. We have therefore addressed the ability to achieve tolerance via mixed chimerism when αGal is present at high levels on a cell population given at the time of chimerism induction. We examined the effects of co-transplantation of porcine red blood cells (RBC) on the ability to achieve mixed chimerism from GalT+ allogeneic donors in GalT KO mice. Our studies show that our non-myeloablative conditioning regimen markedly reduces the anti-αGal and anti-pig antibody responses and that the presence of αGal antigens at high levels does not impede the ability to achieve mixed chimerism or B cell tolerance using αGal+ BMT donors.
Materials and Methods
Animals
Mice
GalT KO mice (C57BL/6 (H-2b) background), from a line originally provided by Dr. John Lowe (University of Michigan), were bred in our facility. Age-matched BMT recipients were 8–14 weeks old. B10.A (H-2a) mice were purchased from Frederick Cancer Research Center (Frederick, MD) or from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained in a specific pathogen-free microisolator environment.
Porcine cells
Blood from SLAdd wild-type (WT) or SLAdd GalT KO (GalT KO) pigs was collected in heparin tubes, then transferred in a 15ml conical and centrifuged for 20 min at 2500rpm. Sera and PBMC were removed and red blood cells (RBC) were conserved at 2×109 per ml in Alsever’s Solution (Sigma-Aldrich, Saint-Louis, MI) at 4°C.
Immunization
109 WT pig RBC were injected i.p. at indicated time points as a source of cells expressing αGal at high density. Recipients of 109 GalT KO pig RBC i.p. were used as a control group.
To enhance anti-αGal Ab production, all recipient mice were immunized with αGal-bearing xenogenic rabbit RBC (Cocalio Biologicals, Reamstown, PA) at 16 to 18 weeks post-BMT, 8 days prior to sacrifice (17 to 19 weeks post-BMT).
Non-myeloablative conditioning regimen and BMT
Mixed allogeneic chimerism was induced in GalT KO mice using a non-myeloablative conditioning regimen as previously described (12). Briefly, recipient mice were CD4 and CD8 depleted by i.p. injection of GK1.5 (1.8mg/mouse) (13) and 2.43 (1.4mg/mouse) (14) on Day -5. On Day 0, mice received 3Gy total body irradiation (TBI) and 7Gy selective thymic irradiation (TI) prior to the i.v. injection of 30×106 allogeneic B10.A BM cells on Day 0. MAbs for in vivo use were produced at the National Cell Culture Center, Minneapolis, Minnesota.
FCM analysis
Xenoantibody and anti-αGal antibody detection
WT or GalT KO pig RBC were washed in PBS, plated at 12×104 per well in a 96- well-V-bottom plate and incubated for 30min at 4°C with 50µl of serially diluted sera. Cells were washed, then incubated with biotinylated anti-IgM (12.5ug/ml) followed by another wash and incubation with Allophycocyanin (APC)-conjugated (3.125µg/ml) or phycoerythrin (PE)-conjugated streptavidin (3.91µg/ml) (Becton Dickinson (BD)/PharMingen, San Diego, CA). Samples were analyzed by flow cytometry (FCM) on a FACScan cytometer (BD Immunocytometry Systems, Mountain View, California, USA). Median Channel Fluorescence (MCF) is presented for the 1:60 serum dilution, which was on the sensitive portion of the dilution curve for positive sera.
Chimerism
Mixed chimerism analysis was performed every two weeks by FCM on white blood cells (WBC). Donor-derived cells were identified among gated live cells (propidium iodide negative) using fluorescein isothiocyanate (FITC)-conjugated anti-H-2Dd mAb 34-2-12. Cells were counterstained with PE-conjugated anti-CD4 (BD/Pharmingen), or Mac-1 (Caltag, San Francisco, CA) and with APC-conjugated anti-CD8 or anti-B220 mAbs (BD/Pharmingen), respectively. Negative control mAbs included HOPC1-FITC (prepared in our laboratory) and rat anti-mouse IgG2a-PE or -APC. A mouse was considered chimeric when it demonstrated ≥ 5% of donor cells in all lineages tested.
αGal+ pig RBC labeling
WT and GalT KO pig RBC were stained with biotinylated Griffonia simplicifolia IB4 lectin (which specifically recognizes αGal) and revealed with PE-conjugated streptavidin (BD/Pharmingen).
Enzyme-linked immunispot (ELISpot) for detecting anti-αGal Ab-producing cells
Elispot assay was performed as previously described (15). A nitrocellulose membrane 96-well plate (Millipore, Bedford, MA) was coated with 10µg/ml of Gal- BSA (Alberta Research Council, Alberta, Canada) or BSA (IgG free, Jackson Immunoresearch PA, USA). Nonspecific binding sites were blocked with 0.4% BSA in IMDM. Two-fold serial dilutions of the initial cell suspension (containing 1–7×106 cells per well) in IMDM supplemented with 0.4% BSA, 5 mg/ml insulin, 5 mg/ml transferrin, 5 ng/ml sodium selenite (all from Sigma, St. Louis, MO), 50 mM 2-mercapto-ethanol and 1 µg/ml gentamycin were added to wells in triplicate. After a 24-h culture at 37°C, bound Abs were detected using HRP-conjugated goat anti-mouse IgM Abs (Southern Biotech) (1µg/ml), followed by color development with 3-amino-9-ethyl carbazole (Sigma). Plates were then analyzed using an automated ELISpot reader (C.T.L., Cleveland, OH). Specific anti-αGal IgM production was calculated by subtracting the number of spots against BSA from the number of spots against Gal-BSA.
ELISA assay for detecting anti-αGal Ab
ELISA plates were coated with 10µg/ml of Gal-BSA or BSA in a BupH™ CarbonateBicarbonate buffer (Pierce, IL, USA) and blocked with SuperBlock® blocking buffer (Pierce, IL, USA). Duplicates of serially diluted serum samples were incubated and bound Abs were detected using horseradish peroxidase-conjugated goat anti-mouse IgM-specific Ab (Southern Biotech, Birmingham, AL). Color development was achieved using 0.2mg/ml o-phenylenediamine dihydrochloride (OPD, Sigma) in substrate buffer. The OPD reaction was stopped using 3M H2SO4 and absorbance at 490nm was measured. The serum dilution corresponding to the OD value of two times the background was set as the titer. In all experiments, binding of serum antibodies to BSA was examined in parallel and subtracted from binding of serum antibodies to Gal-BSA. Sera were tested twice and one representative result is shown.
Results
WT but not GalT KO pig RBC induce anti-αGal Ab production
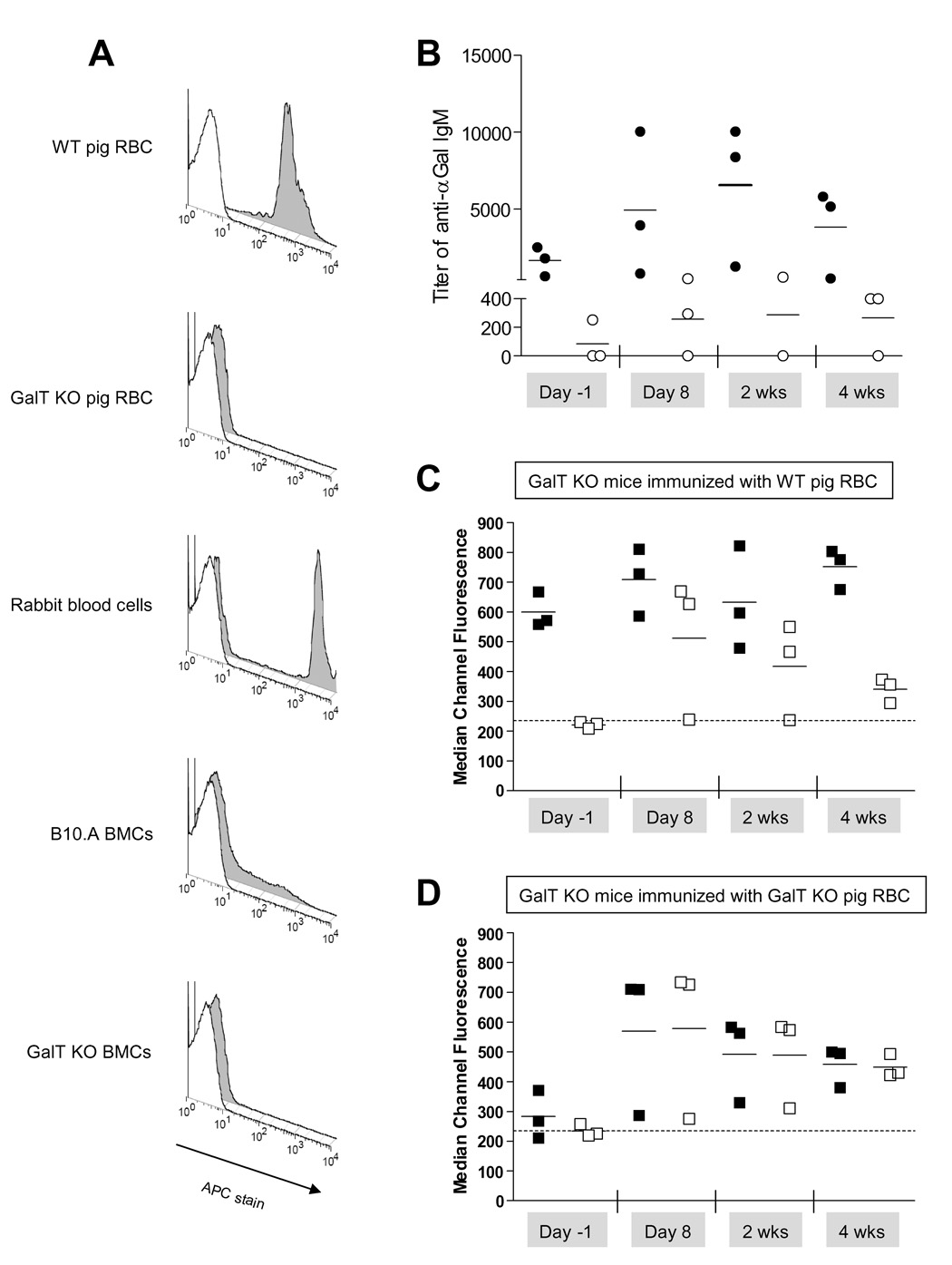
The density of αGal antigen expression varies between species (2,11). The IB4 lectin was used for FCM staining to measure αGal expression because it specifically binds the αGal antigen. As shown in Figure 1A, WT pig RBC express markedly higher levels of αGal than B10.A BMC, while GalT KO pig RBC and bone marrow cells from GalT KO mice do not express measurable αGal. As expected, the titer of anti-αGal IgM in sera of GalT KO mice only increased when they were immunized with WT pig RBC and not when they were immunized with GalT KO pig RBC (Figure 1B). The slight increase observed at 4 weeks post-immunization in mice immunized with GalT KO pig RBC is likely due to their increased age by this time, as anti-αGal natural antibodies have been shown to increase with age (16). Before immunization, some GalT KO mice contained measurable anti-αGal IgM in their sera, as IgM binding was detected to WT but not GalT KO pig RBC (Figures 1 C–D). Non-αGal anti-pig Abs were not detected in the sera of naïe GalT KO mice (Figures 1 C–D), but immunization with GalT KO or WT pig RBC resulted in the development of anti-pig xeno-Ab, as detected by IgM binding to WT and GalT KO pig RBC. The levels of xeno-Abs peaked at 8 days post-immunization and declined but were still elevated by 4 weeks following immunization (Figures 1 C–D) regardless of the type of pig cells used for immunization. I.p. immunization with WT pig RBC induced not only a xenogenic Ab response but also an anti-αGal response (Figure 1 B). Thus, a single i.p. injection of 109 WT or GalT KO pig RBC induces a xenogeneic immune response and a specific anti-αGal response when GalT KO mice are immunized with WT pig RBC.
Figure 1. Immunization of naïe GalT KO mice by i.p. injection of GalT KO or WT pig RBC.
(A) FCM analysis of αGal expression on different cell types. Open histogram represents the isotype control, grey histogram represents IB4 stain. (B) Titer of anti-αGal IgM in the serum was calculated as described in Methods section (ELISA) and is shown before and after the immunization of GalT KO mice with either WT (●) or GalT KO (○) pig RBC. (C–D) Analysis of anti-xeno and anti-αGal IgM in the sera is shown over time in regard to the i.p. immunization. Anti-pig IgM and anti-αGal IgM binding on pig RBC were analyzed by FCM. Each symbol represents an individual animal. (C) WT (■) or GalT KO (□) pig RBC were incubated with sera collected from GalT KO mice immunized with WT pig RBC and counterstained with anti-IgM. Dotted line represents the MCF obtained when WT pig RBC were incubated with serum from a naive WT mouse. (D) WT (■) or GalT KO (□) pig RBC were incubated with sera from GalT KO mice immunized with GalT KO pig RBC and counterstained with anti-IgM. Dotted line represents the MCF obtained when WT pig RBC were incubated with serum from a naive WT mouse. One representative experiment of 2 is shown (n=23 animals/group/experiment).
Conditioned control mice are refractory to immunization by WT or GalT KO pig RBC
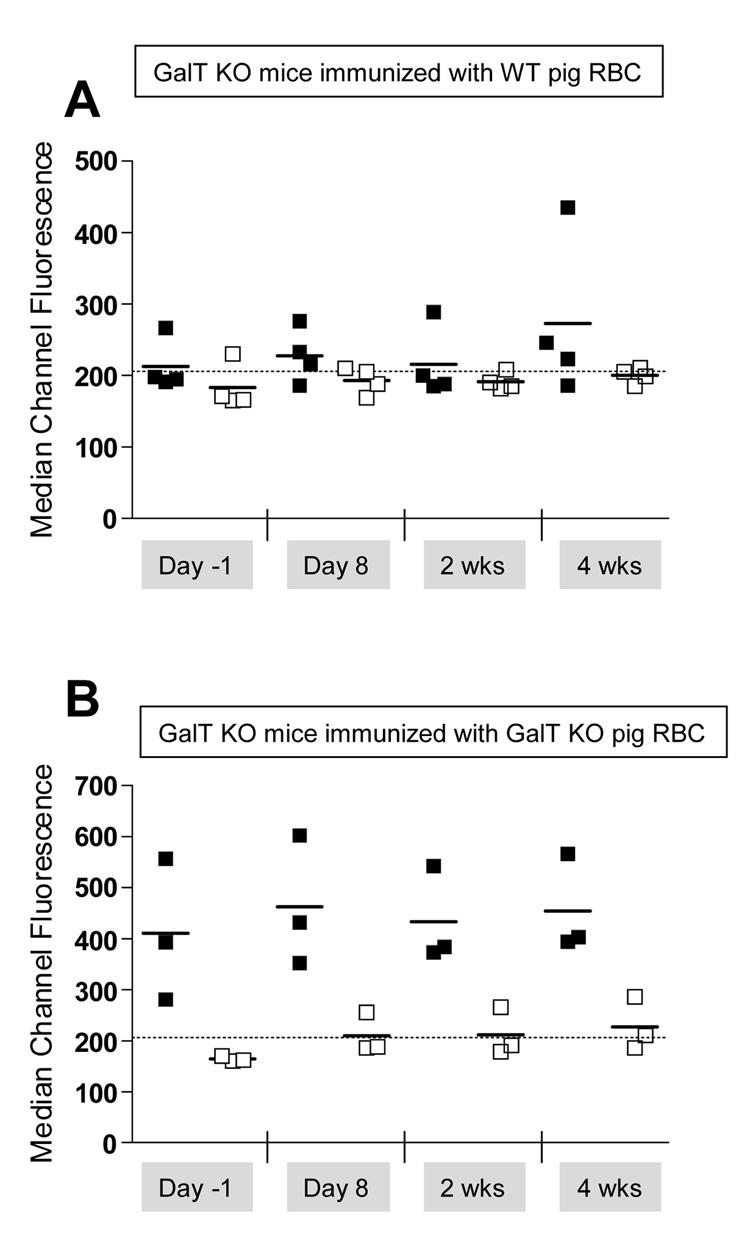
Induction of anti-αGal Ab production by i.p. injection of xenogenic GalT+ cells has been shown to be partly T cell dependent (2). Thus, we evaluated the consequences of a T cell-depleting non-myeloablative conditioning regimen (anti-CD4 and anti-CD8 injection on Day-5, 3Gy TBI, and 7Gy TI on Day 0) on the ability to induce xenogenic and anti-αGal immune responses in GalT KO recipient mice after immunization with either WT or GalT KO pig RBC. Development of anti-αGal and xeno-Abs was followed in the sera. As shown in Figures 2 A–B, immunization of conditioned GalT KO mice with either WT or GalT KO pig RBC did not induce the production of anti-pig IgM. Moreover, no increase in anti-αGal IgM was detected in the sera of GalT KO mice immunized with WT pig RBC (Figure 2A). These results suggest that the conditioning regimen used is sufficient to abrogate the production of anti-αGal and other anti-pig Abs, despite the strong immunogenicity of the pig RBC given i.p
Figure 2. Non-myeloablative conditioning regimen impairs the immunization of naïe GalT KO mice by WT or GalT KO pig RBC.
GalT KO mice underwent conditioning as in the Methods section followed by i.p. injection with either WT (A) or GalT KO (B) pig RBC on Day 0. (A) WT (■) or GalT KO (□) pig RBC were incubated with sera collected from GalT KO mice immunized with WT pig RBC, counterstained with anti-IgM and analyzed by FCM. (B) WT (■) or GalT KO (□) pig RBC were incubated with sera collected from GalT KO mice immunized with GALT KO pig RBC, counterstained with anti-IgM and analyzed by FCM. Dotted line represents the MCF obtained when WT pig RBC were incubated with serum from a naive WT mouse.
Presence of cells expressing αGal at high antigen density does not impair the development of chimerism or the induction of tolerance by αGal+ bone marrow cells in GalT KO mice
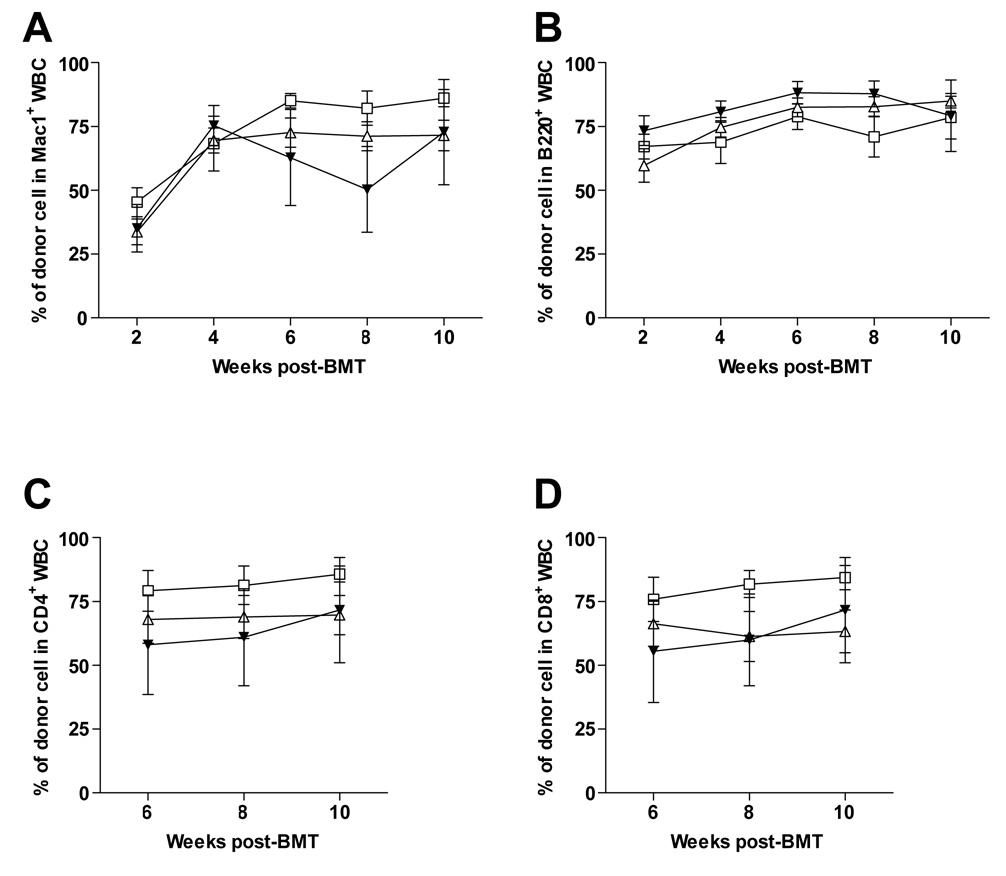
Pig cells have a higher density of expression of the αGal epitope than mouse cells (Figure 1A and (2,11)). Thus we examined whether or not the presence of cells expressing the αGal epitope at high density would affect tolerance induction of pre-existing anti-Gal-bearing B cells in GalT KO recipients. Therefore, GalT KO mice were conditioned as in Methods and received 30×106 GalT+ B10.A BMCs i.v. followed by WT or GalT KO pig RBC injected i.p. on the same day. Development of chimerism was followed in the blood by FCM. The ability to achieve multilineage peripheral blood chimerism was not affected by the strongly αGal+ porcine RBCs given on the day of the BMT (Figure 3A–D). Likewise, similar chimerism levels were detected long-term within the spleen and the PerC in GalT KO mice regardless of whether or not they received WT or GalT KO pig RBC on Day 0 (data not shown). Two of 3 mice not receiving pig RBC achieved mixed chimerism and 7 of 8 animals receiving wild-type pig RBC achieved durable chimerism, while 5 of 8 animals receiving GalT KO pig RBC demonstrated this outcome (Figures 3 A–D).
Figure 3. Exposure to high levels of αGal does not impair establishment of mixed chimerism in GalT KO recipient mice.
GalT KO mice underwent conditioning as described in Methods section, followed by i.v. injection of allogeneic GalT+ B10.A BMT (□) and i.p. injection of either WT (Δ) or GalT KO (▼) pig RBC on Day 0. Percentages of donor WBC are shown for the Mac-1+ (A), B220+ (B), CD4+ (C), and CD8+ (D) lineages at the indicated time points Mean (±SEM) of two experiments are shown with 8 to 13 animals per group
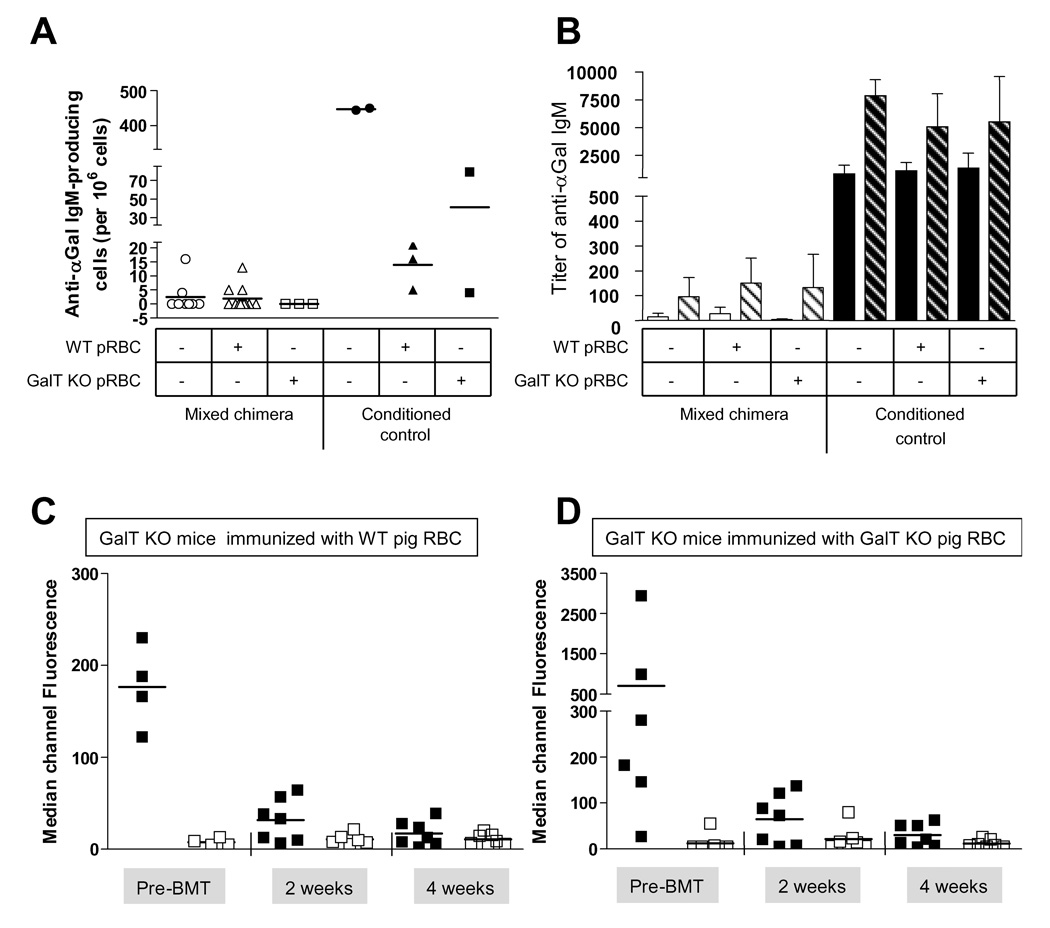
To determine whether or not anti-αGal-producing B cell tolerance was affected by exposure to a high density of αGal-antigen, we attempted to enhance anti-αGal Ab production by immunizing the mice with rabbit RBC (RRBC), which express αGal at even higher levels than WT pig RBC (Figure 1A) 16 or 20 weeks after BMT. Eight days after immunization, splenocytes were tested in vitro for the ability to produce anti-αGal IgM in an ELISPOT assay. Splenocytes from chimeric mice did not produce anti-αGal IgM, whereas those from conditioned control mice did (Figure 4A). For reasons that are unclear, Figure 4A also shows that conditioned control mice that had been previously i.p. injected with pig RBC had a lower frequency of anti-αGal IgM-producing B cells than conditioned control mice that had not received pig RBC. Sera were analyzed by Elisa and the titer of specific anti-αGal IgM was compared to the titer obtained prior to immunization with RRBC. Conditioned control mice showed a high titer of anti-αGal IgM before RRBC immunization and this titer increased after RRBC immunization, regardless of whether or not they were immunized with wild type or KO pig RBC. Chimeric mice were tolerant, showing no production of anti-αGal IgM regardless of whether or not they received WT or GalT KO pig RBC on the day of BMT (Figure 4B). Mixed chimeras and conditioned control mice failed to demonstrate strong anti-pig Ab responses when immunized with WT or GalT KO pig RBC at the time of BMT (Figures 4 C–D), consistent with the interpretation that conditioning overcomes the anti-pig xenoantibody response in addition to the anti-αGal response. These results suggest a requirement for host T cells in inducing anti-pig and anti-αGal IgM antibody responses following immunization with pig RBC.
Figure 4. Exposure to high levels of αGal does not impair αGal-bearing-B cell tolerance after establishment of mixed chimerism in GalT KO recipient mice.
(A) Chimeric mice (empty symbols) and conditioned control mice (filled symbols) were analyzed 17 to 19 weeks post-BMT, 8 days after i.p. injection with rabbit RBC. B cell tolerance was assessed in the spleen by Elispot assay revealing specific αGal IgM production. (B) Serum was collected from chimeric mice (white bars) or conditioned control mice (black bars) before (i.e. 16 or 18 weeks after BMT) (plain bars) and 8 days after i.p. immunization with rabbit RBC (i.e. 17 or 19 weeks post BMT) (hatched bars) and specific anti-αGal IgM titer was analyzed by Elisa. Sera from mixed chimeras mice receiving an i.p. injection of either WT (C) or GalT KO (D) pig RBC and BMT on D0 were analyzed by FCM at the indicated time points. WT (■) or GalT KO (□) pig RBC were incubated with sera and counterstained with anti-IgM-biotin and a streptavidin-conjugated fluorochrome.
Discussion
Natural anti-αGal Abs (NAbs) are a major impediment to pig-to-human xenotransplantation since αGal is the major epitope targeted by NAbs present in human and baboons. Other NAbs (non-Gal) have been detected in healthy-human sera and can target GalT-KO pig endothelial cells (5). Anti-non-αGal Abs have also been detected in baboons after GalT KO pig heart transplantation. These non-αGal NAbs most likely present an additional barrier to organ transplantation from GalT-KO pigs to humans and non-human primates (17,18). GalT KO mice, which lack a functional α1,3 galactosyltransferase (α1,3 GT), produce natural anti-αGal IgM (19). We previously reported that establishment of mixed chimerism with allogeneic GalT+ BMT in GalT KO recipient mice conditioned with a non-myeloablative regimen was associated with a rapid reduction of serum anti-αGal Nab levels and tolerance that was demonstrable in ELISPot assays (8). Mixed chimeras were also tolerant to GalT+ heart allografts (8). However, murine cells express lower levels of αGal epitopes than the more relevant porcine species (2,11). Thus, we were concerned that this difference in αGal Ag-density may preclude the tolerization of pre-existing anti-αGal-producing B cells by induction of porcine mixed chimerism in humans. Indeed, αGal epitopes on xenografts elicit a much stronger immune response than the same epitopes on allografts (2,20).
In mice, NAbs are produced by B-1b B cells which reside in the spleen but may originate in the peritoneal cavity. Specific anti-αGal B cells have been identified as Mac-1+ B-1b cells in the PerC of GalT-KO mice and have been shown to migrate to the spleen while becoming Mac-1neg and antibody-secreting cell following immunization with αGal+ cells (21). Initial B cell tolerance in this model involves anergy, while the more long-term tolerance appears to involve receptor editing and/or deletion (22). B cell tolerance has also been reported to be dependent on antigen density (10), although less is known about tolerance induction of B-1 B cells, which frequently have low affinities for their ligands. Stromal CR1/CR2+ cells, possibly follicular dendritic cells, are involved in the tolerance of αGal-producing B cells by mixed chimerism, probably by picking up immune complexes via CR1/CR2 receptors (23). Furthermore, we have previously demonstrated that immunization of mice with rabbit RBC (which increased the level of circulating anti-αGal Ab) 4 weeks prior to BMT did not preclude tolerization of anti-Gal-producing cells when high doses of wild-type (GalT+) allogeneic BMT were administrated (9). We now report that the presence of high levels of αGal epitopes on the day of BMT does not preclude the development of tolerance of pre-existing anti-αGal-producing B cells.
While pig RBC injected i.v. in WT mice are rapidly engulfed by macrophages one hour after injection (24), pig RBC injected i.p. into SCID mice were still detectable in the PerC one week after injection (25). Here we show that i.p. injection of 109 pig RBC into GalT KO mice induces a potent xenoantibody response in GalT KO mice. Our data also suggest that the increase in the anti-αGal Ab level when mice are immunized with WT pig RBC is T cell dependent. Prevention of this response with our non-myeloablative conditioning regimen allowed αGal+ marrow engraftment and the achievement of B cell tolerance to αGal.
T cells have been shown to be dispensable for the antigen responsiveness of B1b cells (26), but there is still some controversy regarding the regulation of NAbs production by T cells (20,27). Indeed it has been previously shown that mice deficient for TCRαβ and GalT do not show high levels of anti-αGal Nab, even following pig RBC immunization (20). On the other hand, in vivo depletion of T cells in GalT KO mice (not immunized with αGal+ cells) led to significant increases in both natural anti-αGal and total IgM (9). This enhancement was associated with an increased frequency of natural anti-αGal IgM producing B cells (9). B cell responses to xeno-Ag have also been shown to be dependent on T helper cells that are activated by xenopeptides (2,20). NK cell help has recently been reported to promote T-cell-independent xenoantibody production by marginal zone B cells. However, B-1b B cells were not shown to be dependent on this type of help (28). Our demonstration that conditioning for BMT, abrogated anti-pig and anti-αGal IgM responses suggests that T cell help is required for the induced IgM xenoantibody response, consistent with previous studies involving porcine thymic transplantation in GalT KO mice (29). Our conditioning regimen impaired the ability of pig RBC immunization to induce anti-αGal and anti-pig xenoantibody responses. While suppression of the T cell response by our conditioning regimen is likely to explain this result, our studies allow us to conclude that exposure to αGal at high Ag density does not preclude the ability to achieve mixed chimerism from αGal+ donors or, most importantly, tolerance of anti-αGal natural antibody-producing B cells. Our studies also clearly show that the injection of pig RBC to conditioned GalT KO mice, without the induction of chimerism, was in itself insufficient to tolerize anti-αGal producing cells.
In conclusion, we have demonstrated that BMT following non-myeloablative conditioning results in the tolerance of αGal-reactive B-1b B cells even if there is exposure to cells expressing a high density of αGal Ag on the day of BMT. Therefore, high αGal Ag density on pig cells might not preclude the ability to achieve tolerance of pre-existing αGal-reactive human B cells via induction of mixed chimerism.
Acknowledgements
We thank Drs. Vanetta Levesque, Hideki Ohdan and Joshua Mollov for critical review of this manuscript. In addition, we thank Mr. Orlando Moreno for outstanding animal husbandry and technical assistance and Ms. Kelly Walsh for expert assistance with the manuscript.
Abbreviations
- BM
bone marrow
- BMT
bone marrow transplantation
- BMCs
bone marrow cells
- TBI
total body irradiation
- Nabs
natural antibodies
- RBC
Red Blood Cells
- GalT
α1,3-galactosyltransferase
- αGal
Galα1-3Galβ1–4GlcNAc-R
Footnotes
This work was supported by NIH grant PO1 HL18646 and 1S10RR23440-01A1. Fabienne Haspot was supported by a research fellowship of the FRM (Fondation pour la Recherche Medicale, France) and a research fellowship of the AST/ASBMT (American Society of Transplantation / American Society of Blood and Marrow Transplantation). Philip D Bardwell was supported by NIH training grant 5T32-HL07623-18.
The authors have no financial interests to declare.
Reference List
- 1.Good AH, Cooper DKC, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 2.Tanemura M, Yin D, Chong AS, Galili U. Differential immune responses to α-gal epitopes on xenografts and allografts: implications for accomodation in xenotransplantation. J Clin Invest. 2000;105:301–310. doi: 10.1172/JCI7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai L, Kolber-Simonds D, Park KW, et al. Production of {alpha}-1,3- Galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 5.Saethre M, Baumann BC, Fung M, Seebach JD, Mollnes TE. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal-deficient endothelial cells. Transplantation. 2007;84:244–250. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 6.Aksentijevich I, Sachs DH, Sharabi Y, Sundt TMI, Sykes M. Humoral tolerance in mixed xenogeneic chimeras prepared by a non-myeloablative conditioning regimen. Transplant Proc. 1991;23:880–882. [PubMed] [Google Scholar]
- 7.Lee LA, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci USA. 1994;91:10864–10867. doi: 10.1073/pnas.91.23.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohdan H, Yang Y-G, Shimizu A, Swenson KG, Sykes M. Mixed bone marrow chimerism induced without lethal conditioning prevents T cell and anti-Gal α1,3Gal-mediated graft rejection. J Clin Invest. 1999;104:281–290. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohdan H, Swenson KG, Kitamura H, Yang Y-G, Sykes M. Tolerization of Gal α 1,3Gal-reactive B cells in presensitized α 1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Xenotransplant. 2001;8:227–238. doi: 10.1034/j.1399-3089.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 11.Tanemura M, Maruyama S, Galili U. Differential expression of α-gal epitopes (Galα 1–3Galβ 1–4GlcNAc-R) on pig and mouse organs. Transplantation. 2000;69:187–190. doi: 10.1097/00007890-200001150-00034. [DOI] [PubMed] [Google Scholar]
- 12.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dialynas DP, Quan ZS, Wall KA, et al. Characterization of murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to human Leu3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 14.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665. [PubMed] [Google Scholar]
- 15.Yang Y-G, deGoma E, Ohdan H, et al. Tolerization of anti-gal α 1–3gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187:1335–1342. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaTemple DC, Galili U. Adult and neonatal anti-Gal response in knock-out mice for α1,3-galactosyltransferase. Xenotransplantation. 1998;5:191–196. doi: 10.1111/j.1399-3089.1998.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng YL, Moran K, Dor FJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006;81:1058–1062. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 19.Thall AD, Maly P, Lowe JB. Oocyte galα1,3gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270:21437–21440. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- 20.Cretin N, Bracy J, Hanson K, Iacomini J. The role of T cell help in the production of antibodies specific for Gal alpha 1–3Gal. J Immunol. 2002;168:1479–1483. doi: 10.4049/jimmunol.168.3.1479. [DOI] [PubMed] [Google Scholar]
- 21.Ohdan H, Swenson KG, Kruger-Gray HW, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Galα1,3Gal epitopes in α 1,3-galactosyltransferase deficient mice. J Immunol. 2000;165:5518–5529. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 22.Kawahara T, Shimizu I, Ohdan H, Zhao G, Sykes M. Differing mechanisms of early and late B cell tolerance induced by mixed chimerism. Am J Transplant. 2005;5:2821–2829. doi: 10.1111/j.1600-6143.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu I, Kawahara T, Haspot F, Bardwell PD, Carroll MC, Sykes M. B-cell extrinsic CR1/CR2 promotes natural antibody production and tolerance induction of anti-alpha-Gal-producing B-1 cells. Blood. 2006;109:1773–1781. doi: 10.1182/blood-2006-02-002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Verhalen J, Madariaga ML, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe M, Cheng J, Qi J, et al. Elimination of porcine hematopoietic cells by macrophages in mice. J Immunol. 2002;168:621–628. doi: 10.4049/jimmunol.168.2.621. [DOI] [PubMed] [Google Scholar]
- 26.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Ohdan H, Yang Y-G, Swenson KG, Thall AD, Sykes M. In vivo T-cell depletion enhances production of anti-galα1,3gal natural antibodies in α1,3-galactosyltransferase-deficient mice. Transplantation. 2000;69:910–913. doi: 10.1097/00007890-200003150-00041. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Yan Y, Lin Y, et al. Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood. 2007;110:3926–3935. doi: 10.1182/blood-2007-01-065482. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Barbosa JI, Zhao Y, Houser S, Zhao G, Sykes M. Fetal porcine thymus engraftment, survival and CD4 reconstitution in αGal knockout mice is impaired in the presence of high levels of antibodies against αGal. Xenotransplant. 2003;10:24–40. doi: 10.1034/j.1399-3089.2003.01104.x. [DOI] [PubMed] [Google Scholar]