SUMMARY
Innate signals underlying the differentiation of tolerogenic dendritic cells (DC) remain ill defined. Here we show that TLR6 associated with TLR2 uniquely induces IL-10 producing DC and type-1 regulatory T cells. In contrast TLR1 associated with TLR2 promotes differentiation of IL-12p40 producing DC and inflammatory IFN-γ+ T cells. These distinct functional properties are supported by opposite patterns of JNK and p38 MAP kinase activation. The Y. pestis virulence factor LcrV, interestingly, hijacks the TLR2/6 pathway to promote IL-10 and block protective inflammatory responses. These results provide an explanation as to why TLR2 can mediate pro- and anti-inflammatory immune responses and place TLR6 as a distinct TLR receptor driving regulatory IL-10 responses. These findings have also important implications in infectious and inflammatory disease pathogenesis.
INTRODUCTION
Dendritic cells (DC) orchestrate the immune response, and in particular control T cell activation and polarization, via integration of environmental and microbial signals that determine expression of costimulatory molecules and production of cytokines (reviewed in (Kalinski et al., 1999)). For instance, differentiation of pro-inflammatory IFN-γ Th1 cell or anti-inflammatory T regulatory type 1 (Tr1) cells producing selectively IL-10 (Groux, 2003) is linked to the ratio of IL-12 to IL-10 produced by DC (reviewed in (Kalinski et al., 1999)). The concept, that production of high levels of IL-10 by DC is not sufficient to induce Tr1 cells, is well illustrated by the observation that DC stimulated by LPS promote differentiation of Th1 cells even though they secrete high levels of IL-10 (Pulendran et al., 2001; Re and Strominger, 2004). In contrast TLR2 ligands, such as zymosan, induce high IL-10 producing tolerogenic DC which promote differentiation of Tr1 cells (Dillon et al., 2006). However, intriguingly, other groups have shown that TLR2 ligands provide a strong inflammatory signal and promote the development of Th1 cells (Cleveland et al., 1996). The signals leading to the differentiation of tolerogenic DC and the induction of Tr1 cells hence remain poorly understood. Interestingly, TLR2 is a promiscuous TLR that can form heterodimers with TLR6 and TLR1 (Takeda et al., 2003; Triantafilou et al., 2006) recognizing triacylated or diacylated lipoproteins, respectively (Takeda et al., 2002; Takeuchi et al., 2001). Whether the ability of TLR2 to induce tolerogenic DC and Tr1 cells is determined by the TLR with which it associates remains to be determined.
An effective evasion strategy by a pathogen would be to target DC and educate them to become tolerogenic and prime regulatory IL-10 response that block inflammation and allow the pathogen to multiply without restraint. Yersinia pestis, the causative agent of bubonic plague, must replicate to a high density in the blood such that fleas can take up sufficient numbers of bacteria and enable transmission to a new host. Interestingly, induction of IL-10 by the Yersinia virulence factor LcrV (V-antigen) is reported to suppress macrophage activation (Overheim et al., 2005b; Sing et al., 2002) and production of inflammatory cytokines (Brubaker, 2003; Nakajima et al., 1995). However the immune-modulatory role of LcrV remains controversial for several reasons. LcrV is a multi-functional virulence factor that is encoded on a 70kB virulence plasmid (pYV), which is part of the type III secretion machinery (T3SS) that allows for the injection of Yersinia Outer Proteins (YOPs) directly into the host cell cytosol. These YOPS inhibit phagocytosis, block NFkB activation and prevent the release of chemoattractants by immune cells (Cornelis, 2002). Thus it is difficult in that context to determine whether LcrV has direct immune-modulatory functions. Furthermore, it is also argued that the synthesis of a lipopolysaccharide (LPS)-lipid A with poor TLR4 stimulating activity is the dominant strategy used by Y. pestis to prevent the development of a protective inflammatory response (Montminy et al., 2006b). Finally, in vitro experiments suggest that LcrV interacts with TLR2 and CD14 to induce IL-10 (Sing et al., 2002). However, TLR2−/− mice show no resistance to plague (Pouliot et al., 2007).
Using complementary approaches we address the controversial issue as to whether LcrV may play immune-modulatory functions that contribute to plague pathogenesis. Furthermore, using LcrV as a model, we establish the molecular basis underlying TLR-mediated education of tolerogenic DC and the apparently contradictory pro- and anti-inflammatory functions of TLR2. Ultimately, our results reveal an unexpected role of TLR6 in the education of tolerogenic DC and Yersinia pestis pathogenesis.
RESULTS
LcrV blocks the development of an inflammatory response during plague infection
To evaluate the role of regulatory cytokines in a model of bubonic plague we subcutaneously (s.c.) infected mice deficient for IL-10, IL-4 and their appropriate littermate controls with 10LD50 plague strain Colorado-92 (CO92). Interestingly, mice lacking IL-10, but not IL-4, were fully protected from disease. This protection correlated with a significant reduction in bacterial burden and splenic IFN-γ levels (Fig. 1A). Altogether these results suggest that IL-10 plays a significant role in plague pathogenesis by blocking the development of a Th1 response.
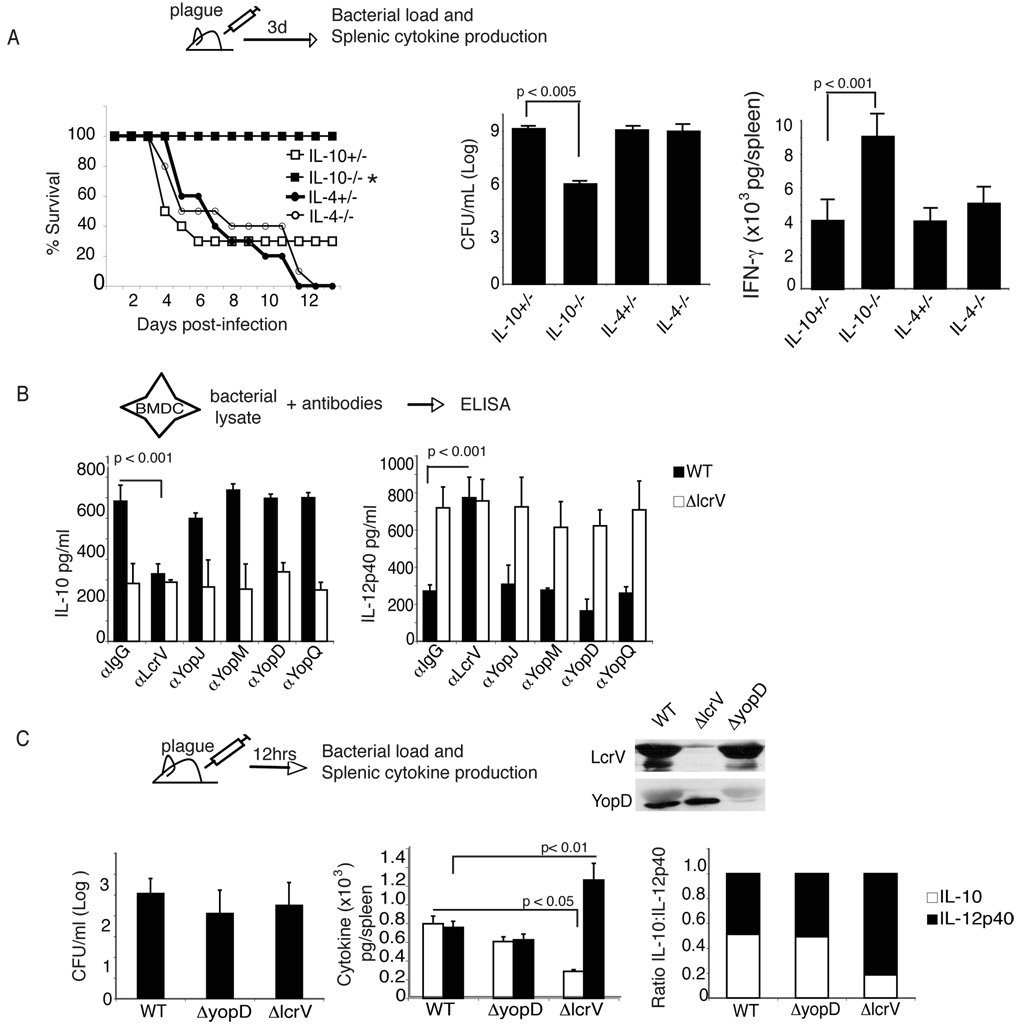
Figure 1. IL-10 induction by Y. pestis LcrV contributes to virulence.
(A) IL-10−/−, IL-4−/− or littermate controls were infected s.c. with 10LD50 (10cfu) Y. pestis strain CO92. Survival was monitored. *, p<0.002 by Wilcoxon rank test. At Day 3 post-infection the amount of plague and IFN-γ levels in the spleen were measured. (B) BMDC from C57BL/6 mice were cultured with bacterial lysate from wild type plague or plague lacking LcrV (Δlcrv Y. pestis). Antibodies against LcrV, Yops and control rabbit IgG were added to cultures and supernatants were measured 24 hours later for levels of IL-10 and IL-12p40. Statistical significance was performed using One-way ANOVA. The data is the average of triplicate wells and is representative of three separate experiments. (C) C57BL/6 mice were infected with 1000cfu wild type plague strain, plague lacking LcrV (Δlcrv) or wild type plague lacking YopD (ΔyopD). Bacterial lysates from WT, ΔlcrV or ΔyopD strains were analyzed for expression of LcrV and YopD by Western Blot. Anti-LcrV was followed by anti-YopD after the membrane was stripped. Twelve hours post-infection the spleens were analyzed for bacterial burden and levels of IL-10 and IL-12p40. The ratio of IL-10 to IL-12p40 was calculated as described in Material and Methods. One-way ANOVA was performed. Significant p values are indicated in the figure. The data is the average of 10 individually assayed mice.
We have previously reported that Y. pestis preferentially target antigen-presenting cells and, in particular, DC during in vivo plague infection (Marketon et al., 2005). To test the hypothesis that LcrV plays a role in the induction of DC with a tolerogenic phenotype characterized by high levels of IL-10 and low levels of IL-12p40, we analyzed the cytokines produced by bone marrow derived DC (BMDC) stimulated with bacterial lysate from wild type plague (Strain KIMD27) or the Δlcrv Y. pestis strain. The use of bacterial lysates allow to bypass issues related to the preparation of recombinant protein. Consistent with the hypothesis that LcrV displays immune-modulatory properties, the Δlcrv Y. pestis induced significantly less IL-10 and higher levels of IL-12p40 than wild type plague (Fig. 1B). To further assess the critical role of LcrV in Y. pestis-mediated IL-10 production and IL-12p40 inhibition, the effects of anti-LcrV and anti-Yop J, M, D and Q polyclonal antibodies on DC polarization were determined. As anticipated, addition of wild type bacterial plague lysate with anti-LcrV antibodies, but not anti-Yops polyclonal antibodies or control IgGs, blocked IL-10 and increased IL2-p40 production by BMDC (Fig. 1B).
To support the immune-modulatory functions of LcrV in vivo, the cytokine pattern induced upon plague infection was analyzed after mice were infected intravenously (i.v.) with 1000 cfu wild type plague (Strain KIMD27), Δlcrv Y. pestis or ΔyopD Y. pestis strain. YopD deletion mutant has a defect in Yop translocation but no defect in LcrV secretion in vivo (Williams J. Bact. 180(2); 1998). As shown in figure 1C, Δlcrv Y. pestis and ΔyopD Y. pestis express comparable levels of YopD and LcrV, compared to wild type plague, respectively. To analyze the impact of LcrV at the initial phase of the disease cytokine responses were analyzed 12 hours after infection. Importantly at this time point wild type, Δlcrv Y. pestis and ΔyopD Y. pestis had a comparable bacterial burden (Fig. 1C). As anticipated, ΔyopD Y.pestis induced levels of IL-10 and IL-12p40 similar to wild type Yersinia. In contrast, Δlcrv Y. pestis, induced a 3-fold decrease and 2-fold increase in IL-10 and IL-12p40 production, respectively, resulting in an overall 6-fold decrease in the IL-10/IL-12p40 ratio. Altogether these results support the conclusion that the change in cytokine pattern associated with Δlcrv Y.pestis is not secondary to a defect in Yops.
These results, in association with the results observed in IL-10 deficient mice, suggest that the ability of plague to induce low inflammatory responses cannot be solely attributed to the synthesis of lipopolysaccharide (LPS)-lipid A with poor toll-like receptor 4 (TLR4)-stimulating activity. They support the hypothesis that LcrV and IL-10 contribute actively to plague pathogenesis by blocking pro-inflammatory responses.
Recombinant LcrV induces IL-10 and suppresses LPS-induced IL-12p40 from DC
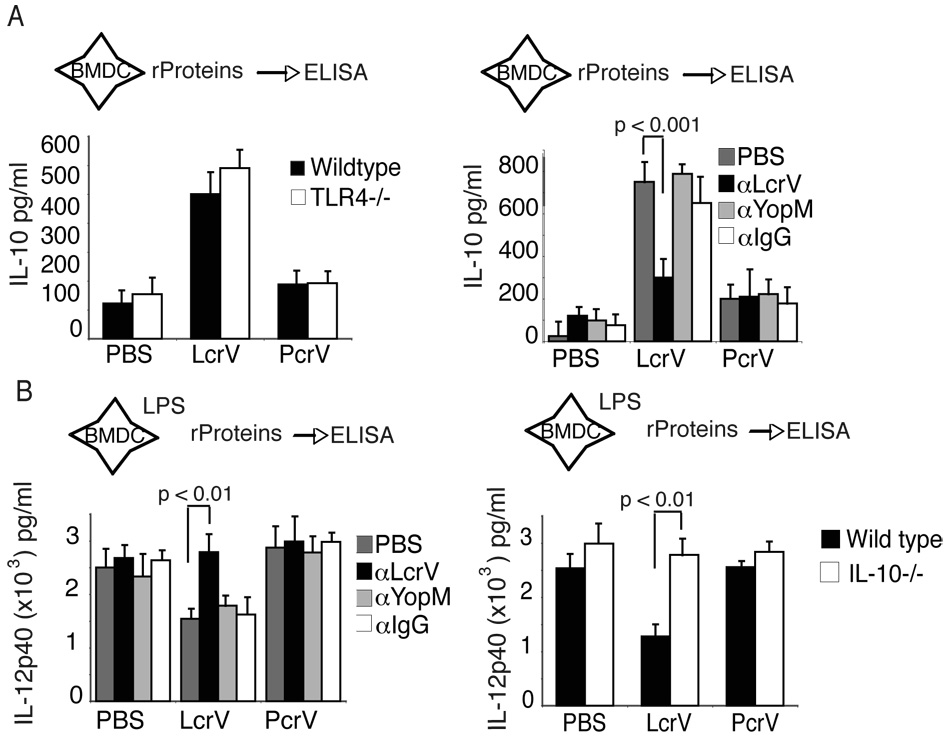
To directly test the role of LcrV on IL-10 induction and IL-12p40 suppression we purified recombinant LcrV, PcrV (LcrV homolog), and a LcrV deletion mutant V10 (Overheim et al., 2005a) (Fig. 2), all of which are grown, purified and treated in the same way. As shown in supplementary figure 1A, LcrV, but not PcrV, induced IL-10 in a dose-dependent manner. As previously reported (Overheim et al., 2005b), the LcrV deletion mutant V10 induced significantly lower levels of IL-10 than wild type LcrV (Supplementary Fig.1A and Fig 2). Importantly, LcrV-mediated IL-10 induction was unchanged in TLR4−/− mice (Fig. 2A) and blocked upon addition of antibodies against LcrV, but not against YopM or control rabbit IgG (Fig. 2A), eliminating the possible role of endotoxin and other TLR contaminants. Finally, in agreement with the data presented in figure 1B and 1C, addition of LcrV, but not V10 (Supplementary Fig. 1A) or PcrV, blocked significantly LPS-induced IL-12p40 production (Fig. 2B and Supplementary Fig. 1A). Importantly, this effect was also reversed by the addition of polyclonal antibodies against LcrV, but not YopM or rabbit IgG and was lost in IL-10 deficient mice. Of note, LcrV displayed similar immune-modulatory properties in splenic DC and peritoneal macrophages (Supplementary Fig 2B and 2C).
Figure 2. LcrV induces IL-10 and suppresses LPS-induced IL-12p40.
(A) Left, Recombinant LcrV, PcrV or V10 (data not shown) was added to BMDC cultured from C57BL/6 mice or TLR4−/− mice and IL-10 levels were measured by ELISA. The data represents the average of 3 individually assayed mice and is representative of two independent experiments. Right, BMDC from wild type mice were cultured with PBS, LcrV, PcrV or V10 (data not shown) with antibodies against LcrV, YopM or Rabbit IgG. Supernatants were assayed by ELISA after 18 hours for levels of IL-10. One-Way ANOVA was performed and significant p values are indicated in the figure. Data is the average of triplicate wells and is representative of 4 separate experiments. (B) Left, BMDC from C57BL/6 mice were pulsed with LPS and two hours later treated with LcrV or PcrV. Twenty-four hours later supernatants were analyzed for IL-12p40. Right, BMDC from wild type or IL-10−/− mice were pulsed with LPS for two hours and then treated with LcrV or PcrV. IL-12p40 was measured by ELISA. The data is representative of 4 separate experiments. One-way ANOVA was performed to determine statistical significance for A and B and significant p values are indicated in the figure.
Altogether these results (Fig. 1, 2 and Supplementary Fig. 1) suggest that LcrV is both necessary and sufficient to induce DC with a tolerogenic phenotype, characterized by high levels of IL-10 and low levels of IL-12p40 production.
TLR6 and CD14 are involved in IL-10 induction and contribute to plague pathogenesis
Sing et al (Sing et al., 2002) showed that induction of IL-10 by LcrV was dependent upon TLR2 and CD14. TLR2 can be expressed as a homodimer, or form receptor complexes with CD14, TLR1 and TLR6. To assess the potential role of CD14, TLR1 and TLR6 in LcrV-mediated immune-modulation and plague pathogenesis, mice deficient in CD14, TLR6 and TLR1 were analyzed (Fig. 3). Interestingly, IL-10 (Fig. 3) and IL-12p40 (data not shown) induction by LcrV was abrogated in BMDC derived from TLR2, TLR6 and CD14 deficient mice, suggesting that LcrV signals through a CD14/TLR2/TLR6 complex. In contrast, LcrV induced similar levels of IL-10 in TLR1−/− mice and wild type mice (Fig. 3A and data). Loss of IL-10 induction in TLR2, 6 and CD14 deficient BMDC was associated with a loss in the ability of LcrV to block LPS-induced IL-12p40 production. Of note, as expected, DC from CD14−/− mice had reduced levels of IL-12p40 production in response to LPS, yet still measurable, reaching around 500pg/ml (Fig. 3A).
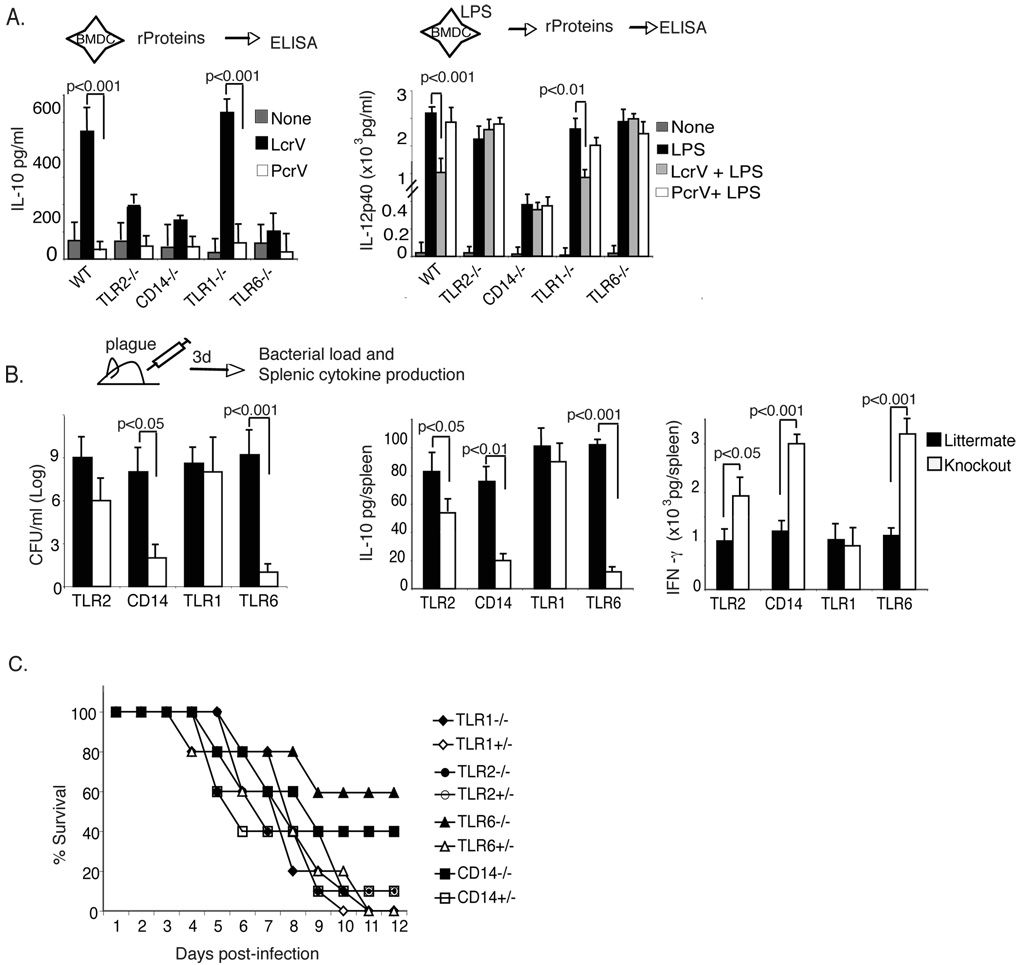
Figure 3. TLR6 is necessary for virulence and IL-10 production by plague.
(A) BMDC from TLR2−/−, CD14−/−, TLR1−/−, TLR6−/− and C57BL/6 mice were incubated with or PcrV (left) or pulsed with LPS, and then treated two hours later with LcrV or PcrV. Data is the average of triplicate wells and is representative of 5 separate experiments. (B) TLR2−/−, CD14−/−, TLR1−/−, TLR6−/− and the littermate controls for each strain were infected subcutaneously with 10LD50 (10cfu) plague strain CO92. At day 3 post-infection bacterial counts from spleen (left), and levels of IL-10 (center) and IFN-γ (right) was determined from splenic supernatants. The data is the average of 5 individually assayed mice and is representative of three separate experiments. For panels A and B, statistical significance was determined using One-way ANOVA and significant p values are indicated in the figure. (C) Survival curve of knockout and littermate control mice infected as described in 3B. The data is representative of two experiments with 5–10 mice/group for each experiment and statistical significance was determined using Wilcoxon log rank test. *, p <0.05.
We next investigated the in vivo role of TLR2, CD14, TLR1, and TLR6 during plague pathogenesis. Each strain of mouse and its littermate control were infected subcutaneously (s.c.) with 10LD50 plague (strain CO92). As reported previously (Pouliot et al., 2007; Reithmeier-Rost et al., 2007) TLR2−/− mice succumbed had little difference in bacterial load as compared to littermate controls (Fig. 3B). However, TLR2−/− mice had a significant reduction in IL-10 (Fig. 3B) and increase in IFN-γ (Fig. 3B), suggesting that TLR2 may actually be involved in immune-modulation. Even more interestingly, mice deficient for either CD14 or TLR6 were partially protected from plague infection compared to their littermate controls (Fig. 3C), had decreased bacteria burden (Fig. 3B), as well as barely detectable levels of IL-10 (Fig. 3B) and a three-fold higher amount of IFN-γ (Fig. 3B). Consistent with our in vitro data, TLR1 did not play a role in plague pathogenesis (Fig. 3B).
Taken together these data demonstrate that CD14 and TLR6 contribute to plague pathogenesis by promoting IL-10 production and blocking inflammatory responses. Furthermore, these observations suggest that LcrV interacts with TLR6 and CD14 for induction of IL-10 and suppression of inflammation.
TLR ligands engaging TLR6 have the unique property of promoting the differentiation of tolerogenic DC and Tr1 cells
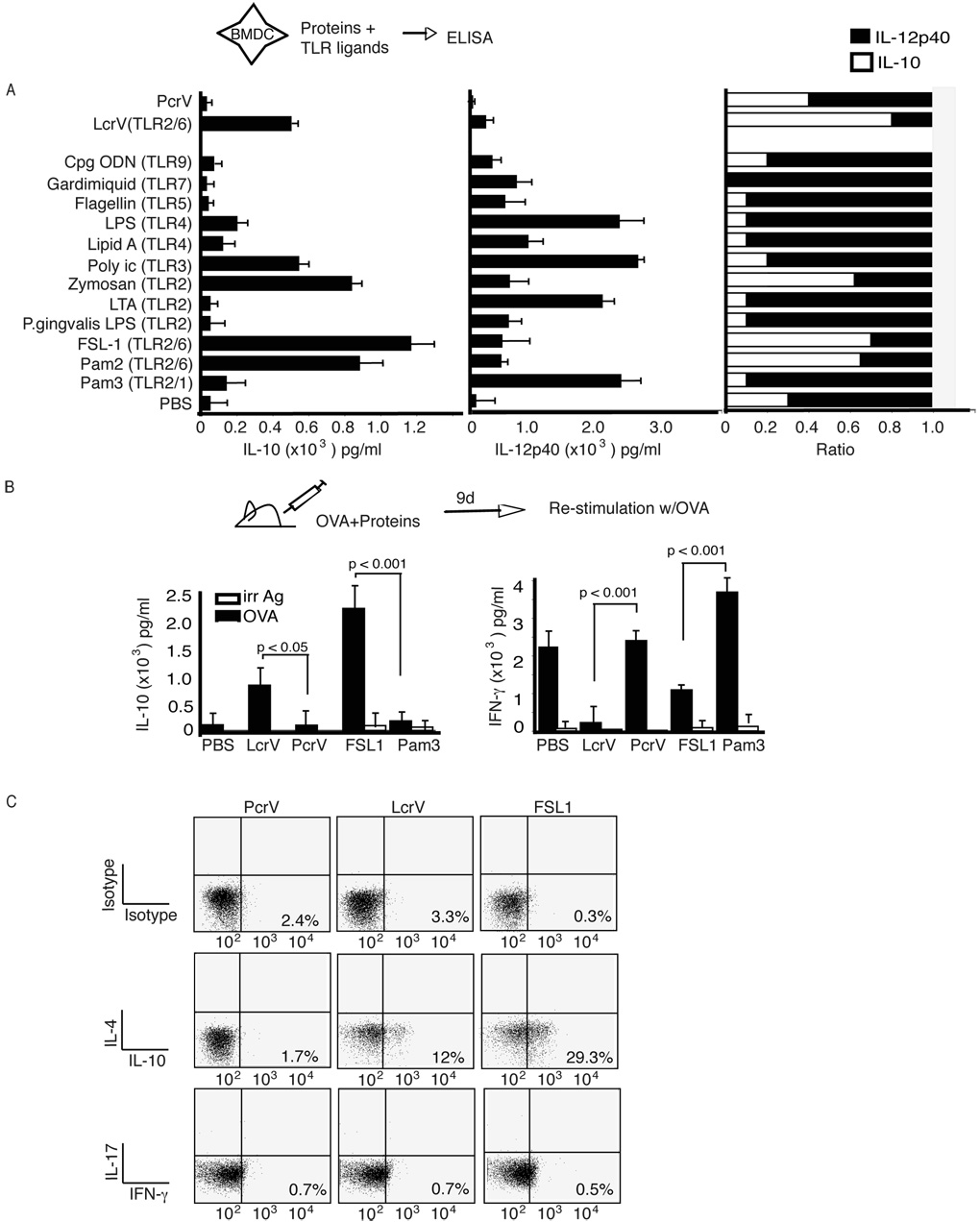
To determine whether the ability to educate tolerogenic DC was a generic or specific property of TLR2/6 ligands, we analyzed a panel of TLR ligands. As indicated previously, the relative amount of IL-10 and IL-12p40 produced by DC is thought to play a critical role in determining T cell polarization (as reviewed in Kalinski et al., 1999). Unexpectedly, the only TLR ligands capable of inducing a cytokine pattern similar to LcrV in DC characterized by an IL-10/IL-12p40 ratio ≥ 0.5, were the diacylated TLR2/6 ligands FSL1 and Pam2CysK4 (Fig. 4A). In contrast, the triacylated TLR2/1 ligand Pam3CysK4 induced a cytokine pattern similar to LPS with an IL-10/IL-12p40 ratio of ≤0.15. Interestingly, the TLR2/TLR6 ligand FSL1, similarly to LcrV (Fig. 1B and Supplementary Fig. 1B), also inhibited LPS-mediated IL-12p40 production (Supplementary Fig. 1B), whereas the TLR2/TLR1 ligand Pam3CysK4, increased it (Supplementary Fig. 1B), as previously reported (Hirata et al., 2008). Other ligands, which induced even higher amounts of IL-10 were zymosan (which also activates Dectin-1 signaling (Dillon et al., 2006)) and poly I/C. However these ligands also induced high levels of IL-12p40 with an IL-10/IL-12p40 ratio of ≈0.2 (Fig. 4A, right).
Figure 4. TLR2/6 ligands induce high levels of IL-10 and can prime an IL-10- dominant CD4 T cell response.
(A) BMDC from C57BL/6 mice were incubated with TLR ligands, LcrV or PcrV. Supernatants were analyzed for IL-10 (left), IL-12p40 (center) and the ratio of IL-10:IL-12p40 (right). (B) C57BL/6 were immunized with OVA323-339 and alhydrogel in association with LcrV, PcrV, FSL1 or Pam3CysK4. Nine days later CD4 T cells were isolated from the lymph nodes and incubated with OVA323-339 and irradiated splenocytes. Forty-eight hours after stimulation supernatants were analyzed for IL-10 (left), IFN-γ (center), and the ratio of IL-10:IFN-γ (right). The data was analyzed using One-Way ANOVA. The data is the average of 5 individually assayed mice and is representative of two separate experiments. (C) Cells were isolated from draining lymph nods of C57BL/6 mice nine days after immunization with OVA and incomplete Freund’s adjuvant in association with LcrV, PcrV or FSL1. Cells were re-stimulated in vitro with OVA and assayed for intracellular IL-4, IL-10, IFN-γ and IL-17 levels.
To determine whether the cytokine pattern expressed by DC in figure 4A was associated with predicted CD4 T cell polarization, we analyzed the cytokine pattern of ova-specific CD4 T cells upon immunization with OVA323-339 peptide in association with LcrV, control PcrV, TLR2/6 (FSL-1) and 2/1 ligands (Pam3CysK4) (Fig. 4B). Alhydrogel (Li et al., 2007) or incomplete Freund adjuvant (Gavin et al., 2006) were used as primary adjuvants because their effects are MyD88 independent. As shown Fig. 4B, adjuvant alone promoted differentiation of Ag-specific T cells producing significant levels of IFN-γ and low levels of IL-10 (Fig. 4B). Control PcrV (Fig. 4B) and V10 (data not shown) did not alter the CD4 T cell cytokine pattern observed with alhydrogel. Importantly, as anticipated by the DC cytokine pattern observed in figure 4A, LcrV and FSL1, induced the differentiation of Ag-specific T cells producing high levels of IL-10 and low levels of IFN-γ (Fig. 4B). Importantly, no IL-4 production could be detected (data not shown). In contrast, mice immunized with the TLR2/1 ligand Pam3CysK4 (Fig. 4B) and TLR ligands such as poly I/C (data not shown) induced Ag-specific T cells producing high levels of IFN-γ and no IL-10. These results could be reproduced using IFA instead of alhydrogel as a primary adjuvant (data not shown). To further confirm that LcrV and TLR2/6 ligands induced T cells with a Tr1 phenotype, i.e. T cells producing IL-10 in absence of IL-4 and inflammatory cytokines, we performed intracellular flow cytometry with anti-IL-10 antibodies in combination with anti-IL4, -IFN-γ or IL-17 antibodies (Fig. 4C). As anticipated, immunization with OVA323-339 and incomplete Freunds adjuvant in association with LcrV and FSL1, but not PcrV, induced in vivo differentiation of OVA-specific T cells with a Tr1 phenotype. The induction of typical Tr1 cells was further supported by the finding that LcrV stimulation, whether in vitro or in vivo, was never associated with foxp3 expression in T cells (data not shown).
Altogether these results suggest that TLR2/6 ligands have the unique property of educating DC to become tolerogenic DC that produce high levels of IL-10 and promote the differentiation of Tr1 cells. In contrast TLR2/1 ligands educate DC to produce high levels of IL-12p40 and low levels of IL-10, hence promoting the differentiation of Th1 cells.
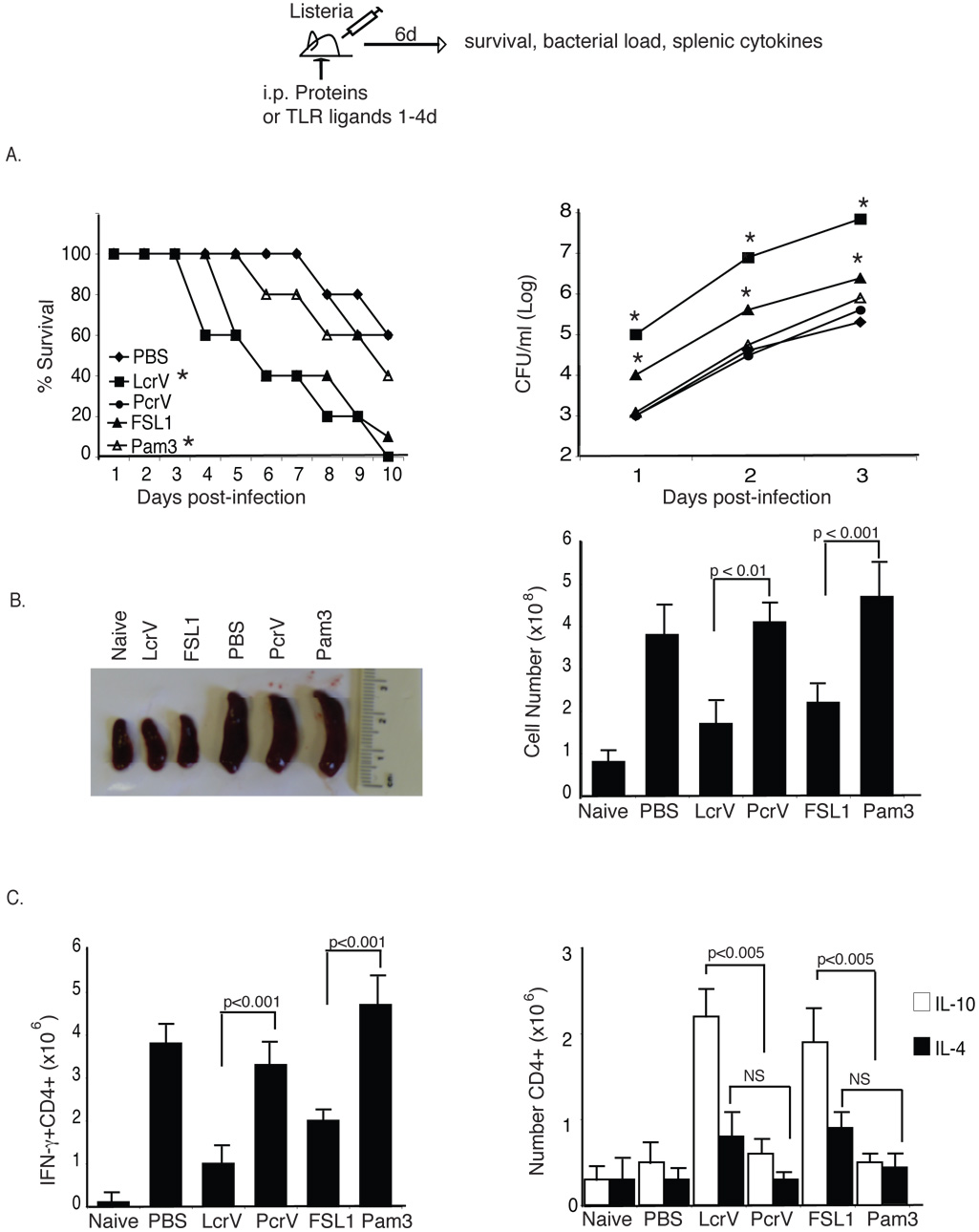
LcrV and TLR2/6 ligands redirect anti-Listeria inflammatory Th1 responses to regulatory Tr1 responses
In order to evaluate whether TLR2/6 ligands could suppress Th1 immunity to microbial infection we studied the impact of LcrV and FSL1 on anti-Listeria responses. PcrV and V10 served as a control for LcrV and the TLR2/1 ligand, Pam3CysK4, as a control for FSL1. We chose Listeria monocytogenes for two reasons. First, clearance and survival of L. monocytogenes infection is dependent on Th1-mediated immunity. Second, L. monocytogenes lacks a type III secretion (TTS) system and thus allows to dissociate the effects of LcrV from TTS.
Survival, bacterial load and CD4 T cell polarization was monitored in mice treated with recombinant proteins or TLR ligands after i.v. infection with 1×103 CFU (LD50) L. monocytogenes. As expected, approximately 50% of the mice treated with PBS survived the infection at day 10. Interestingly, LcrV- and FSL1-treated mice had very significant increased mortality. In contrast, V10 (data not shown), PcrV- and Pam3CysK4-treated mice behaved similarly to PBS-treated mice (Fig. 5A). Upon necropsy of animals at day 6, LcrV- and FSL1-treated mice had small spleens similar in size to those of non-infected mice, correlating with a significantly reduced cellular number as compared to PBS-, PcrV- and Pam3CysK4- treated mice (Fig. 5B). Finally, intracellular cytokine analysis of spleen cells revealed that CD4 T cells in LcrV- and FSL1-treated mice produced predominantly IL-10, whereas CD4 T cells of PcrV- and Pam3CysK4-treated mice produced high levels of IFN-γ and little or no IL-10 or IL-4. Importantly, analysis of mice deficient in IL-10 and IL-4 confirmed, as anticipated, that the immune-modulatory effects were dependent on IL-10 but not on IL-4 (Supplementary Fig. 3 A and B, respectively). In addition, analysis of the impact of LcrV on L. monocytogenes infection in TLR6 deficient mice demonstrates that LcrV exerted its immune-modulatory effects in vivo via TLR6 (Supplementary Fig. 4).
Figure 5. LcrV and TLR2/6 ligand induce immune-suppression in vivo.
C57BL/6 mice were immunized with PBS, LcrV, PcrV, FSL-1 or Pam3CysK4 and then infected with 103 cfu L. monocytogenes intravenously. (A) Left, survival of mice was followed for 10 days. Data is the average of 10 mice/group and is representative of four experiments. p<0.001, Wilcoxon-rank test. Right, bacterial colonization of the spleen was assayed at day 1–3. The data is the average of 5 individual mice and is representative of 3 separate experiments. One-Way ANOVA was used to compare bacterial burden at each time-point. (B) Splenic size (left) and cell number (right) were assayed from 5 individual mice (C) Intracellular levels of IFN-γ, IL-10 and IL-4 was assayed in CD4 T cells isolated from the spleen at day 6 post-infection. Total cell number was determined by multiplying the percent positive for intracellular cytokine by the total cell counts for each mouse. The data is the average of 5 individually assayed mice and is representative of at least three separate experiments. One-Way ANOVA was used to determine statistical significance for (B) and (C).
To further assess that LcrV and TLR2/6 ligands could block bacterial proinflammatory innate signals in a dominant manner, we confirmed these observation in mice infected with the gram negative bacteria Salmonella typhimurium known to express potent proinflammatory LPS (Supplementary Fig. 5).
Taken together, these data demonstrate that during bacterial infection engagement of TLR2/6 induces a shift from a protective inflammatory Th1 response to a dominant IL-10 regulatory response, which results in increased bacterial burden and mortality.
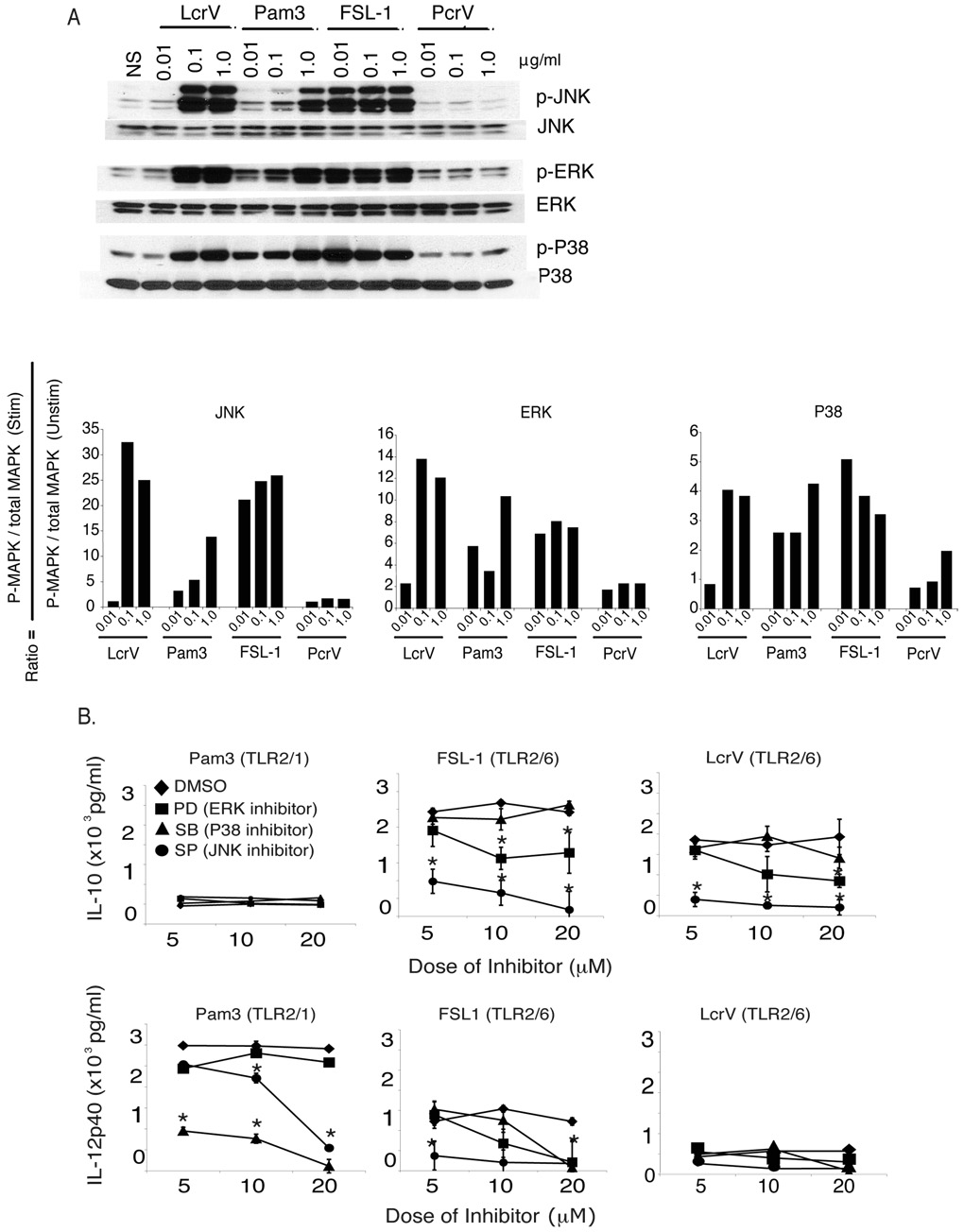
TLR1 and TLR6 induce different MAPK signals in BMDC
The finding that engagement of TLR2/1 promotes IL-12p40 and leads to inflammation and that TLR2/6 promotes IL-10 and gives rise to regulatory responses suggests that these ligands engage different downstream signals. To examine the phosphorylation of Mitogen-activated protein (MAP) kinases c-Jun N-terminal kinases (JNK), extracellular signal-regulated kinases (ERK) and p38 MAPK (p38) BMDC were cultured with increasing doses of LcrV, PcrV, FSL1 and Pam3CysK4 for 15 minutes. This time point was chosen because it was optimal for all molecules tested (data not shown). LcrV, FSL1, and Pam3CysK4 all induced ERK and p38 phosphorylation (Fig. 6A). Of note, whereas Pam3CysK4 consistently induced p38 phosphorylation, including at the lowest concentration, FSL1 and LcrV failed to induce p38 phosphorylation in 2/4 experiments even thought ERK phosphorylation was observed (data not shown). Presented here is the experiment in which the highest level of p38 phosphorylation was observed with LcrV and FSL1 (Fig. 6A and data not shown). The most striking signaling property of LcrV and FSL1 (TLR2/6 ligand) that sets them apart from Pam3CysK4 (TLR2/1 ligand) is their ability to induce consistently and at low concentrations high levels of JNK phosphorylation (30-fold increase at 0.1µg/ml) (Fig. 6A).
Figure 6. Engagement of TLR2/6 leads to differential MAP kinase phosphorylation patterns.
(A) BMDC from C57BL/6 mice were treated with increasing doses of LcrV, PcrV, FSL1 or Pam3CysK4. After 15 minutes cell lysates were examined for phosphorylation of MAP kinases. The fold increase was determined using scanning electron densitometry and the equation indicated in the figure. (B) BMDC were pre-treated for 30 minutes with PD98059 (MEK1/2 inhibitor), SP600125 (JNK inhibitor), SB203580 (P38 inhibitor) or DMSO and then incubated with LcrV, FSL1 or Pam3CysK4. IL-10 (top panels) and IL-12p40 (bottom panels) were examined 18 hours later by ELISA. Data shown is the average of triplicate wells and is representative of three individual experiments. *, p<0.05 as compared to DMSO control.
To test the physiological relevance of these findings, BMDC were treated with LcrV, FSL1 and Pam3CysK4 in the presence of different concentrations of ERK (PD98059), JNK (SP600125) and p38 (SB203580) inhibitors, or vehicle control (DMSO). After 18 hours, IL-10 and IL-12p40 levels were measured in the supernatants. In accordance with our previous results (Fig. 4A), Pam3CysK4 induced low amounts of IL-10 and high levels of IL-12p40, whereas FSL1 and LcrV produced high levels of IL-10 and relatively low levels of IL-12p40 (Fig. 6B). Interestingly, supporting a major role for p38, but not JNK and ERK, in Pam3CysK4 signaling, loss in IL-12p40 production was selectively observed with p38 inhibition. Conversely, the pharmacological studies revealed that only JNK, and to a lesser degree ERK, played a role in LcrV- and FSL1-mediated IL-10 production. A significant effect of JNK and ERK inhibition on IL-12p40 induction by FSL1 was also observed. Of note, effects of pharmacological inhibitors on IL-10 and IL-12p40 induction by Pam3CysK4 and LcrV, respectively, could not be seen because of the very low levels of cytokines produced.
Altogether these data suggest that JNK, and to a lesser degree ERK, activation play a major role in LcrV and FSL1-mediated IL-10 induction, whereas p38 plays a major role in Pam2CysK4-mediated IL-12p40 induction.
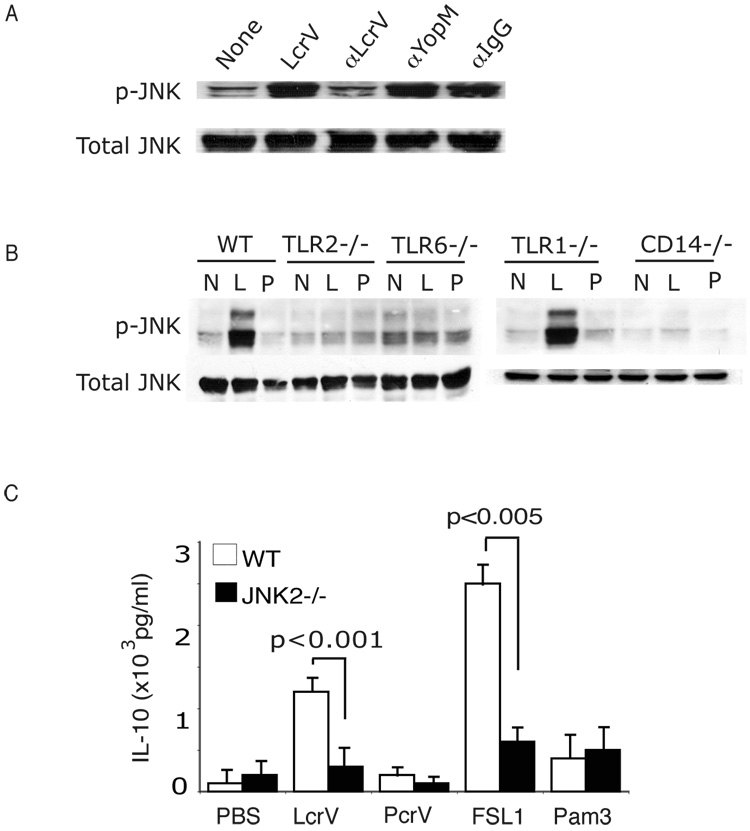
The TLR2/6→JNK pathway critically regulates LcrV- and FSL1–mediated IL-10 production
To test the hypothesis that the TLR2/6→JNK pathway plays a critical role in LcrV-induced IL-10 production, we analyzed JNK phosphorylation in TLR2, −6 and −1 deficient mice and determined whether DC deficient in JNK2 lost the capacity to induce IL-10 in response to LcrV and the TLR2/6 ligand FSL1.
First, we verified that LcrV-induced JNK phosphorylation was inhibited by addition of anti-LcrV polyclonal blocking antibodies, but not by anti-YopM polyclonal antibodies or rabbit IgG (Fig. 7A). This result, in conjunction with the data of figure 1B and 2, demonstrates that the effects observed are specifically mediated by LcrV and not by a contaminant.
Figure 7. TLR2/6 signaling and IL-10 induction is mediated via JNK2.
(A) BMDC were treated with LcrV or PcrV in the presence of anti-LcrV, anti-YopM and anti-Rabbit IgG. After 15 minutes the cell lysate was analyzed by Western Blot for phosphorylation of JNK. These data are representative of two different experiments. (B) BMDC from C57BL/6, TLR2−/−, TLR6−/−, TLR1−/− and CD14−/− were stimulated with LcrV or PcrV for 15 minutes and examined for total and phosphorylated JNK by Western Blot. (C) BMDC were harvested from wild type or JNK2−/− mice and then treated with LcrV, PcrV, FSL1 and Pam3CysK4. After 18 hours supernatants were analyzed for IL-10 levels by ELISA. Data shown is the average of triplicate cultures from three different mice. Two-WAY ANOVA was performed with Bonferroni step downs.
We next determined whether LcrV- and FSL-mediated JNK phosphorylation was mediated by TLR2 and TLR6. As shown in figure 7B, upon BMDC stimulation with LcrV and FSL1 JNK phosphorylation was conserved in TLR1 deficient mice, but lost in TLR6, CD14 and TLR2 deficient mice. The loss of JNK phosphorylation parallels the loss of immune-modulation by LcrV, FSL1 and plague observed in these mutant mice (Fig. 3) and suggests that the TLR2/6→JNK pathway critically regulates the immune-modulatory properties mediated by LcrV and FSL1.
To further confirm the critical role played by JNK, we analyzed IL-10 production in WT and JNK2 deficient BMDC upon LcrV- and FSL-1 stimulation. As anticipated, in absence of JNK2, LcrV and FSL1 lost the ability to induce IL-10, whereas Pam3CysK4 could still induce IL-12p40 (data not shown). These data confirm the results obtained with the JNK inhibitor SP600125 shown in figure 6B.
Altogether these data demonstrate that the TLR2/6→JNK pathway plays a critical role in the immune-modulatory properties of LcrV and FSL1.
DISCUSSION
Our study identifies TLR6 as a critical TLR driving the differentiation of tolerogenic DC and regulatory IL-10 immune responses. This finding, and our observations that IL-10 and TLR6 deficient mice are significantly protected from plague infection, shed a new light on how pro and anti-inflammatory immune responses are determined in vivo by specific innate signals acting on DC. Furthermore, they provide a new molecular basis for LcrV-mediated immune modulation.
Y. pestis has evolved multiple strategies aimed at blocking immune defense mechanisms eventually leading to the rapid death of the host. Immune-modulation by LcrV involving IL-10 induction, via interaction with TLR2 (Foligne et al., 2007; Sing et al., 2005) and CD14 (Sing et al., 2002), was proposed as a potential mechanism to block the induction of protective inflammatory immune responses against Yersinia. This idea has been challenged by two groups who have shown no difference between TLR2−/− and wild type mice during subcutaneous plague infection ((Pouliot et al., 2007; Reithmeier-Rost et al., 2007). Goguen and colleagues (Montminy et al., 2006b) proposed as alternative explanation that Y. pestis LPS, because of its acylation status, is a weak TLR4 agonist incapable of driving Th1 responses. However, if weak TLR4 stimulation were the main reason for defective inflammatory immune response during plague infection, mice deficient in the TLR4 co-receptor CD14 would have decreased inflammatory cytokine production and higher bacterial burden. Our data show the opposite outcome whereby CD14−/− mice are able to induce a protective inflammatory response leading to clearance of plague bacteria. In addition, weak TLR4 stimulation does not account for the finding that IL-10 and TLR6 deficient mice mount an inflammatory immune response and are protected from plague infection. Finally our studies also indicate that LcrV is necessary and sufficient to mediate immune modulatory properties. As anticipated, infection of mice with CO92 Y. pestis strain lacking LcrV (Δlcrv Y. pestis) survived infection up to 1×106 cfu (manuscript in preparation). More interestingly, analysis of the cytokine profile at 24 hours post-infection, when bacterial burden was comparable in wild type and Δlcrv Y. pestis, revealed that, in absence of LcrV, plague was able to induce an inflammatory immune response. These results were in agreement with in vitro data showing that bacterial lysates from Δlcrv Y. pestis had no immune-modulatory properties and that lystates from wild type Y. pestis in presence of blocking anti-LcrV antibodies lost their ability to induce IL-10 and suppress LPS activation. In vitro data also indicated that LcrV was sufficient to induce immune modulation. Intriguingly, LcrV was also able to block induction of inflammatory immune responses against Listeria monocytogenes and Salmonella typhimurium, confirming independently from plague infection that its immune-modulatory effects were dominant over other proinflammatory innate signals. The ability of LcrV to block protective inflammatory immune responses was lost in mice deficient in TLR6, suggesting that LcrV mediates its regulatory effects through the engagement of TLR6. Furthermore, in vitro experiments suggest that LcrV actually signals through a TLR2/TLR6/CD14 complex. In this regard it is interesting to note that LcrV and TLR2/6 ligands have similar functional and signal transduction properties. How LcrV engages the TLR2/TLR6/CD14 complex remains to be determined. Interestingly, mutation of N-terminal residues in LcrV from Y. enterocolitica abrogated IL-10 induction and inhibited TLR2 binding (Sing et al., 2005), suggesting that the N-terminal portion of LcrV may bind TLR2. Which portion of LcrV interacts with TLR6 and CD14 is the object of ongoing studies. Taken together our observations strongly suggest that TLR2/TLR6/CD14 engagement by LcrV plays a critical role in plague pathogenesis via the induction of IL-10 and the inhibition of protective inflammatory immune responses. However, our observations do not preclude a role for decreased LPS acylation (Montminy et al., 2006a), and inhibition of NFκB activation and phagocytosis by Yops (Cornelis, 2002) in plague pathogenesis, but rather suggest that Y. pestis uses redundant strategies to completely block protective inflammatory and phagocytic immune responses.
Intriguingly, our studies are also in concordance with previous literature showing no role for TLR2 in plague pathogenesis. The different outcome of plague infection in TLR2 and TLR6 deficient mice suggests that TLR2 could promote opposing functional outcomes depending on which TLR it partners with. This hypothesis is supported by an abundant and apparently contradictory literature reporting that TLR2 has both pro- and anti-inflammatory functions (Cleveland et al., 1996; Dillon et al., 2006; Jiang et al., 2005; Re and Strominger, 2004; Revets et al., 2005). TLR2 can be expressed as a homodimer and as a heterodimer in association with TLR1 or TLR6. Our observations taken as a whole suggest that TLR6, but not TLR1, induces differentiation of tolerogenic DC and Tr1 cells. These findings provide an explanation as to why TLR2 can have pro- and anti-inflammatory properties, and TLR6- but not TLR2-deficient mice are resistant to plague infection. In that regard its is interesting to note that, despite the fact that TLR2 deficient mice show no resistance to plague, they have lower IL-10 and higher IFN-γ than their wild type littermate controls. We cannot exclude at this point whether TLR6 can be expressed in absence of TLR2. Furthermore, TLR6 may have additional functions that remain unclear. How CD14 comes into play and whether CD14 alters TLR2 and −6 expression and signaling remains to be determined. Interestingly, Beutler and colleagues have reported a mutation within CD14 that can effect the responses of TLR2/6 ligands (Jiang et al., 2005).
To our surprise, analysis of a comprehensive panel of TLR ligands, revealed that TLR6 was the only TLR able to induce DC with a tolerogenic phenotype. Of note, some TLR ligands, such as poly i/c, induced DC producing high levels of IL-10 in conjunction with high levels of IL-12. Importantly, these DC promoted the differentiation of Th1 but not Tr1 cells (data not shown). Future studies will determine whether TLR6 plays a critical role in intestinal homeostasis. Such a role is supported, but not demonstrated, by the observation that lactobacillus bacteria engineered to express LcrV produced high levels of IL-10 in a TLR2-dependent manner and protected mice from developing colitis (Foligne et al., 2007).
The opposing functional properties of TLR2/6 and TLR2/1 ligands led us to examine whether engagement of TLR1 or TLR6 in association with TLR2 induced different MAPK signaling pathways. ERK activation was induced by all TLR2 ligands and was critical, depending on the TLR2 ligand, for IL-10 and IL-12p40 production. The key difference between TLR2/6 and 2/1 ligands was the ability of the former, but not the latter, to induce strong levels of JNK phosphorylation. In contrast, TLR2/1 ligands induced more readily p38 phosphorylation. Moreover, p38 inhibition resulted in loss of TLR2/1 proinflammatory functions, whereas JNK inhibition was associated with a loss of IL-10 induction by TLR2/6 ligands. The critical role of JNK in TLR2/6 signaling was further demonstrated by the loss of IL-10 induction in JNK2 deficient mice. The relative contribution of individual MAPK to TLR signaling remains a complex issue and may depend on the nature of the ligand and the effector response.
In this paper we have identified TLR6 and JNK activation as critical elements for the generation of tolerogenic DC. These results also suggest that TLR6 may be a very interesting therapeutic target, because in contrast to TLR2 and CD14 it is uniquely associated with regulatory IL-10 responses. Targeting TLR6 may be highly relevant in infectious diseases, such as tuberculosis (Kursar et al., 2007) and disseminated candidiasis (Netea et al., 2008), where the infectious agents harbor TLR2/6 ligands and increased IL-10 is associated with defective protective responses. Furthermore, stimulation of TLR6 may constitute a new therapeutic avenue for chronic inflammatory bowel disease. In this regard, it is interesting to point out that IL-10 deficient mice develop spontaneous colitis (Rennick and Fort, 2000), and that a genetic study identified a link between TLR6 and colitis (Pierik et al., 2006).
EXPERIMENTAL PROCEDURES
Mice
C57BL/6, IL-10−/− (C57BL/6 background n=8 backcrosses), IL-4−/− (C57BL/6 background n=8 backcrosses), TLR2−/− (n=8 backcrosses) and CD14−/− (n=8 backcrosses) mice were purchased from Jackson laboratory (Bar Harbor, ME). TLR1−/− and TLR6−/− mice were generously donated by S. Akira. All mice were bred to C57BL/6 mice for one generation to obtain F1. Heterozygote F1 mice were bred with each other to obtain F2 knockouts and littermates. OT2 (C57BL/6 background n=8). All mice were maintained at University of Chicago, and all animal experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee
Bacterial strains and infections
Two strains of plague were used for in vivo studies. Colorado 92 (CO92) is a fully virulent strain which when injected subcutaneously mimics the early stages of bubonic plague, but rapidly enters the blood causing a septicemic infection and death. KIMD27 also referred to as KIMD5 is a non-pigmented, attenuated strain which when injected i.v. mimics the septicemic stage of plague and is lethal. KIMD5 and KIMD27 are used interchangeably for the same strain the plague field (Brubaker, 1969; Overheim et al., 2005b).
Yersinia pestis CO92 is a clinical isolate routinely used in experimental models of plague (Davis et al., 1996) and obtained from Dr. Susan Welkos (United States Army Medical Research Institute of Infectious Disease, Bacteriology Division, Fort Detrick, Maryland). For the bubonic plague model, mice were challenged by subcutaneous injection with 0.1-ml aliquots of 50cfu/ml. For this experiment, Y. pestis CO92 was grown in HIB at 26°C overnight. The plague bacilli were washed and diluted in sterile PBS to the required concentration. Mice observed for morbidity, mortality, and recovery over a course of 10–14 days
LcrV mutant Y. pestis (KIM D27 Δlcrv) has been previously described (Quenee et al., 2008). Y. pestis KIM D27 and Δlcrv were grown in heart infusion broth (HIB; Difco) at 26°C overnight, diluted 1:20 in fresh HIB, and grown for an additional 3 h at 26°C. Mice were infected by retro-orbital injection with 100ul of bacterial suspensions (containing 100 or 1,000 cfu) and observed for 14 days, and the deaths were recorded. Surviving animals were euthanized at the end of the observation period.
Protein expression purification
LcrV, PcrV, and V10 were prepared as previously described (Overheim et al., 2005a). Lipopolysaccharide (LPS) contamination of purified proteins was assayed with Limulus amebocyte lysate (QCL-1000, Cambrex, NJ) and determined to be less than 1 ng/100µg purified protein. Protein concentrations were determined by the bicinchoninic acid assay (Pierce Technology, Rockford, IL).
BMDC culture and stimulation
Culture of BMDC has been modified from Inaba et al (Inaba et al., 1992) and described previously (Marketon et al., 2005). The phenotype of BMDC on day 8 before stimulation is CD11chiCD11b+B220lo ClassIIint, CD80int, CD86int, CD40lo (data not shown). BMDC were stimulated with TLR ligands obtained by (Invivogen San Diego, CA). For studies using bacterial lysate, bacteria were adjusted to 108 cfu/ml and 5×106 cfu-equivalents were used to stimulate 5×105 BMDC.
Immunization
C57BL/6 mice were immunized subcutaneously with 50µg OVA peptide 323–339 (Peptides International, Louisville KY) and 50µg LcrV, V10, PcrV, FSL-1 (Invivogen) or Pam3CysK4 (Invivogen) emulsified in IFA (Sigma, St. Louis, MO) or alhydrogel (Sigma) at three sites on the rear flank. On day 8 following immunization, mice were euthanized and draining lymph nodes were harvested.
Cytokine assays
CD4 T cells from immunized mice were isolated by magnetic sorting from either spleen or draining lymph nodes. Cells were incubated at 37°C in a humidified atmosphere containing 7.5% CO2 with irradiated splenocytes (300 RADS) with OVA peptide in RPMI-1640 containing 10% fetal calf serum, 100 U/ml penicillin (Invitrogen, Carlsbad, CA), 100 µg/ml streptomycin (Invitrogen), 5 × 10−5 M 2-mercaptoethanol. Supernatants were harvested at 48 and 72 hours and analyzed for cytokine production (IL-10, IL-4, and IFN-γ) by ELISA (BD Pharmingen, San Jose CA). For studies using spleen homogenates, 1 spleen was homogenized in 1ml of PBS.
Flow cytometry
Cells (0.5–1 × 106) were washed and incubated with anti-mouse FcRII/III (CD16/32 clone 2.4G2; BD PharMingen) for 15 min at 4°C to prevent nonspecific Fc binding. Cells were washed in PBS with 10% BSA (Sigma) and labeled directly with mAbs specific for lymphocyte surface markers (CD80-PE (BD 16-1OA1), CD86-PE (BD GL1), CD40 PE (BD 3/23), MHC class II (FITC AF6-120.1), CD11c FITC (BD HL3), CD4 PeCy5 (BD L3T4), CD11b APC (BD M1/70) at 4°C for 15 min. Expression was determined on a FACSCalibur flow cytometer (BD Biosciences) using CellQuest software (BD Biosciences). Intracellar flow cytometry was performed using anti-IL-10 APC (eBioscience JES5-16E3), -IL-4 PE (BD 11B11), -IFN-γ APC (BD XMG1.2) and -Foxp3 APC (eBioscience FJK-16s) and analyzed as described above.
Intravenous challenge with Listeria monocytogenes
L. monocytogenes strain EGD was grown overnight at 37°C. Bacteria were prepared by inoculating 50ml BHI with 1ml of the overnight culture and grown at 37°C for 3 hours. The concentration was evaluated by optical density at 600nm. Serial dilutions were prepared for use in animals and the inoculum was confirmed by plating on BHI plates.
Mice were infected retro-orbitally (RO) with either 1×103 or 1×105 cfu L. monocytogenes and treated i.p. for four days with 50µg LcrV, PcrV, V10, FSL1, Pam3CysK4 or PBS. Three days after infection bacterial loads were assessed in the spleens of mice. Homogenized spleens were diluted and plated in triplicate on BHI plates and grown at 37°C for 48 hours. Surviving mice were euthanized on day +6 and spleens were mechanically disrupted and plated at a density of 5×106/well in a 24 well plate. The cells were treated with brefeldin A for 5 hours and intracellular levels of IL-10, IL-4 and IFN-γ were assessed by flow cytometry. Parallel cultures were set up and stimulated with heat killed L. monocytogenes (107/ml) and supernatants were harvested and analyzed for cytokine by ELISA.
Cell signaling
To look at MAPK (JNK, ERK and P38) phosphorylation, 1–2×106 cells were serum starved and stimulated for the indicated time with LcrV, PcrV and different TLR ligands. When used, MAPK inhibitors were added to the cells 30 minutes prior to stimulation. Cells were lysed for 20 min in ice-cold lysis buffer containing fresh protease and phosphatase inhibitors (50 mM Tris-Hcl [pH 7.5]; 150 mM NaCl; 1% Triton-X100; 1 mM EDTA; 1 mM Na3VO4; 1 mM NaF; and protease inhibitor cocktail tablets). Cellular debris was removed by centrifugation at 15,000 rpm for 20min at 4°C. Total lysates were subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes (Biorad). Proteins were then detected by using the indicated antibodies followed by HRP-conjugated goat anti-mouse (HRP-GAM) or donkey anti-rabbit (HRP-DAR) Abs (Jackson Immunoresearch Laboratories) using the enhanced chemiluminescence (ECL) kit from Amersham Pharmacia Biotech.
Scanning densitometry was performed using the NIH Image J (version 1.1.) software.
Statistics
One-way and Two way ANOVA’s were used when noted in figure legends. Tests that had an interaction of p<0.05 were further analyzed using Bonferroni step down tests, comparing each group to LcrV. Wilcoxon rank test was used for analysis of survival.
Supplementary Material
ACKNOWLEDGMENTS
All authors acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (GLRCE, NIH Award 1-U54-AI-057153), support from NIH training grant T32 AI07090 and postdoctorals NRSA F32-AI-068370 and Digestive Disease Research Core Center of the University of Chicago (DK42086). The authors would like to thank Dr. Robert Brubaker for insightful discussions, Shizou Akira for the generous donation of TLR1−/− and TLR6−/− mice, and Emily Kistner for providing assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brubaker RR. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969;98:1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker RR. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen) Infect Immun. 2003;71:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis GR. Yersinia type III secretion: send in the effectors. J Cell Biol. 2002;158:401–408. doi: 10.1083/jcb.200205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KJ, Fritz DL, Pitt ML, Welkos SL, Worsham PL, Friedlander AM. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops) Arch Pathol Lab Med. 1996;120:156–163. [PubMed] [Google Scholar]
- Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, Simonet M, Daniel C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology. 2007;133:862–874. doi: 10.1053/j.gastro.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation. 2003;75:8S–12S. doi: 10.1097/01.TP.0000067944.90241.BD. [DOI] [PubMed] [Google Scholar]
- Hirata N, Yanagawa Y, Ebihara T, Seya T, Uematsu S, Akira S, Hayashi F, Iwabuchi K, Onoe K. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Mol Immunol. 2008;45:2734–2742. doi: 10.1016/j.molimm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006a;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nature Immunology. 2006b;7:1066–1073. doi: 10.1038/ni1386. [see comment] [DOI] [PubMed] [Google Scholar]
- Nakajima R, Motin VL, Brubaker RR. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008;52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- Overheim KA, Depaolo RW, Debord KL, Morrin EM, Anderson DM, Green NM, Brubaker RR, Jabri B, Schneewind O. LcrV plague vaccine with altered immunomodulatory properties. Infection & Immunity. 2005a;73:5152–5159. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overheim KA, Depaolo RW, Debord KL, Morrin EM, Anderson DM, Green NM, Brubaker RR, Jabri B, Schneewind O. LcrV plague vaccine with altered immunomodulatory properties. Infect Immun. 2005b;73:5152–5159. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Vermeire S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- Pouliot K, Pan N, Wang S, Lu S, Lien E, Goguen JD. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect Immun. 2007;75:3571–3580. doi: 10.1128/IAI.01644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- Quenee LE, Cornelius CA, Ciletti NA, Elli D, Schneewind O. Yersinia pestis caf1 variants and the limits of plague vaccine protection. Infect Immun. 2008;76:2025–2036. doi: 10.1128/IAI.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger JL. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol. 2004;173:7548–7555. doi: 10.4049/jimmunol.173.12.7548. [DOI] [PubMed] [Google Scholar]
- Reithmeier-Rost D, Hill J, Elvin SJ, Williamson D, Dittmann S, Schmid A, Wilharm G, Sing A. The weak interaction of LcrV and TLR2 does not contribute to the virulence of Yersinia pestis. Microbes Infect. 2007;9:997–1002. doi: 10.1016/j.micinf.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–G833. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- Revets H, Pynaert G, Grooten J, De Baetselier P. Lipoprotein I, a TLR2/4 ligand modulates Th2-driven allergic immune responses. J Immunol. 2005;174:1097–1103. doi: 10.4049/jimmunol.174.2.1097. [DOI] [PubMed] [Google Scholar]
- Sing A, Reithmeier-Rost D, Granfors K, Hill J, Roggenkamp A, Heesemann J. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc Natl Acad Sci U S A. 2005;102:16049–16054. doi: 10.1073/pnas.0504728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002;196:1017–1024. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res. 2002;8:459–463. doi: 10.1179/096805102125001073. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.