Abstract
Recent functional brain imaging studies in humans suggest that the neural generator(s) for tinnitus may reside in the central nervous system and involve both auditory as well as nonauditory centers. The contribution of nonauditory centers in the pathogenesis and regulation of tinnitus is reinforced by studies showing that many patients have somatic tinnitus whereby movements and manipulations of the eyes, head, neck, jaw, and shoulder can modulate the loudness and pitch of their tinnitus. In most cases, the maneuvers lead to increases in tinnitus loudness or pitch rather than decreases. Our results indicate that most tinnitus patients experience only a modest change in loudness or pitch when performing these maneuvers. However, some patients report that these maneuvers significantly modulate the loudness or pitch, sometimes by a factor of 2 to 3. The high prevalence of somatic tinnitus serves to illustrate the complex multimodal interactions that exist between the auditory pathway and other sensory-motor systems innervating the head, neck, shoulders, and eyes.
Keywords: Somatic tinnitus, positron emission tomography, gaze-evoked tinnitus, trigeminal nerve
Most audiologists and otolaryngologists studying the normal or pathological aspects of hearing usually focus their efforts on the anatomical, biochemical, or physiological properties of the cochlea and classical auditory pathway while largely ignoring the multitude of secondary or tertiary inputs from other sensory, motor, or autonomic systems that interact with the auditory systems at a number of locations. This tunnel vision approach to hearing loss and tinnitus fails to take into account the numerous anatomical interactions that need to occur to perform such common everyday acts such as localizing a sound in space or speaking.
Multimodal Interactions
When auditory neuroscientists discuss the intricacies and nuances of sound localization, attention is mainly directed at identifying the acoustic cues that the auditory system uses to compute the location of a sound source, specifically the external ear transfer function that shapes the acoustic spectrum that reaches the tympanic membrane and interaural differences in sound intensity and interaural time differences. 1–4 These acoustic cues, however, would be of little value unless the central nervous system had a frame of reference for interpreting the acoustic data. For example, the acoustic cues must be evaluated in relationship to the position of the head and pinna (e.g., the cat) and its relationship to the rest of the body (e.g., head position, deviated right, left, up or down relative to the torso) as well as knowledge about the location of the head and body with respect to the environment (e.g., lying down, standing up, standing in an open versus closed room). The significance of nonauditory cues in sound localization is illustrated by animal studies showing that early visual experience plays an important role in the development of sound localization ability.5,6 In animals such as cats and bats that can move their pinna, the brain must calculate the effect of the pinna orientation and shape on the spectrum and amplitude of sounds arriving at the two ears.7 When we speak, the tensor tympani and stapedius middle ear muscles, innervated by the trigeminal (5th cranial nerve) and facial (7th cranial) nerve, respectively, begin to contract before the onset of the vocalization, thereby attenuating self-generated sounds.8,9 These carefully orchestrated neurophysiological interactions occur subconsciously without a moment’s thought.
These examples illustrate the complex multimodal interactions that occur between other sensory and motor systems and the auditory system and the potential role these complex neural networks could have on the perception of tinnitus. In this article we review some of the studies from our laboratory that illustrate how nonauditory centers affect the perception of tinnitus in unexpected ways.
Imaging Somatic Tinnitus
The experimental design of this study emerged by chance while attempting to recruit subjects for a brain imaging study of tinnitus. The original plan was to compare the brain activity patterns in tinnitus patients with those of normal subjects; however, between-subjects comparisons can be confounded by extraneous variables such as age, gender, hearing loss, and other uncontrolled variables. During a meeting with a local tinnitus support group, several patients stood up and announced they could modulate the loudness of their tinnitus to a significant degree by moving their head, neck, jaw, or tongue, a phenomenon now generally referred to as somatic tinnitus.10,11 At the time, we were unaware that patients could voluntarily modulate their tinnitus and were skeptical of these claims. However, it occurred to us that if the patients could significantly modulate the loudness of their tinnitus, they could serve as their own controls. That is, the brain activity patterns observed when the tinnitus was loud could be subtracted from the brain activity patterns when the tinnitus was quiet.
Four patients, two men and two women (47 to 53 years of age), were identified through a local tinnitus support group who indicated they could significantly modulate the loudness of their tinnitus by movements of the head, neck, jaw, tongue, and face.12 The common oral facial maneuver (OFM) that significantly changed the loudness of their tinnitus among all four patients was a jaw clench. In addition to severe tinnitus, these four patients had a high-frequency sensorineural hearing loss for between group comparison addition, six normal hearing subjects without tinnitus served as controls. To identify the regions of the brain involved in somatic tinnitus, regional cerebral blood flow (rCBF), a surrogate marker of neural activity, was measured using 15 O radiolabeled water as a tracer. Positron emission tomography (PET) imaging of the brain and statistical parametric mapping (SPM) software were used to identify regions of the brain where rCBF changed significantly during sound stimulation or during the OFM relative to activity in the resting state. During the PET scans, subjects wore insert earphones and active noise-reduction ear muffs; this reduced the background noise from the scanner and other equipment so hearing thresholds in the scanner were equivalent to those measured in an audiometric booth, except at 125 Hz.13 As expected, when the normal subjects made the OFM, there was a significant increase in neural activity in the left and right sensory-motor cortex and supplementary motor areas known to be involved in clenching the jaw; however, no change in activity was seen in auditory areas. In contrast, in two patients who reported an increase in the loudness of their tinnitus during the OFM, an increase in neural activity was seen in the same sensory-motor areas as the control subjects, but in addition, neural activity increased significantly in the left primary auditory cortex and the region between the medial geniculate bodies. Thus the increase in tinnitus loudness was associated with increased neural activity in regions of the central auditory pathway. The other two patients with right-ear tinnitus reported a decrease in tinnitus loudness during the OFM. When results from the two patients who experienced a decrease in tinnitus loudness were compared with normal controls, the decrease in tinnitus loudness in these two patients was associated with decreased neural activity in the left middle temporal gyrus (primary and association areas of auditory cortex) and the left hippocampus, a part of the limbic system involved with memory and spatial navigation. An important feature of these results is that an increase or decrease in tinnitus loudness was always associated with a change in neural activity in the auditory cortex on just one side of the brain. In contrast, when a real sound was presented to just one ear, it activated both the left and right auditory cortex.14 Based on the results that the OFM only changed activity on one side of the brain, unlike a real sound that activates the cochlea and both sides of the brain, we concluded that the tinnitus generator in these four patients must be located in the central auditory pathway (medial geniculate or auditory cortex). This interpretation was confirmed in later PET imaging studies using other subjects who could modulate their tinnitus with eye movements or intravenous administration of lidocaine.15,16
Tinnitus Modulation by Eye Movement
One of the earliest reports of tinnitus modulation appeared in a case report by House, who stated that a patient who had undergone acoustic neuroma surgery to remove a tumor from the 8th nerve developed gaze evoked tinnitus whereby tinnitus was induced by shifting eye gaze from straight ahead to either side or up or down.17 Over the next 20 years, several more case reports appeared in the literature suggesting that gaze-evoked or gaze-modulated tinnitus was an extremely rare complication of acoustic neuroma surgery.18–20
In an effort to recruit acoustic neuroma patients with gaze-evoked tinnitus for our imaging studies, we placed a single notice in the Acoustic Neuroma Association newsletter asking patients to contact us if they could modulate their tinnitus by moving their eyes. In addition, a notice was placed in Tinnitus Today asking patients if they could modulate their tinnitus by shifting their eye gaze or by other motor acts such as clenching the jaw or movements of the head or neck. Because the literature suggested gaze-evoked tinnitus was a rare phenomenon, we expected a small response. Instead, 159 patients contacted us. A detailed questionnaire was sent to the respondents and 113 returned the questionnaire; 5 of these did not have gaze-evoked tinnitus.21 Eighty-seven patients who had undergone acoustic neuroma surgery reported that they developed gaze-evoked or gaze-modulated tinnitus. In most cases (95%), tumor removal resulted in total loss of hearing in the affected ear. The average length of time since surgery was 7.6 years (standard deviation, 6.5 years). These results indicate that gaze-evoked/modulated tinnitus is much more common than previously suspected.
Most of these acoustic neuroma patients (77%) indicated that they heard the tinnitus in the ear or side of the head on which the tumor was removed; none heard the tinnitus exclusively in the ear or side of the head opposite to the side of tumor removal.21 In this sample, lateral gaze caused the loudness of the tinnitus to increase in ~99% of the acoustic neuroma patients. Approximately 44% of patients reported a doubling in loudness with lateral gaze, and ~37% indicated it was three times louder. Most patients (~89%) reported that the pitch of their tinnitus increased with eye movements; only ~7% reported a decrease and ~4% reported both increases and decreases. About a third of the subjects reported a tripling of their pitch, and ~47% indicated it doubled. These results demonstrate that the pitch and loudness changes induced by eye movements were substantial in acoustic neuroma patients with gaze-evoked/modulated tinnitus.
Approximately 22% of our acoustic neuroma patients with gaze-evoked/modulated tinnitus reported that jaw movements (somatic tinnitus) altered the loudness of their tinnitus. 22 Most (~90%) patients reported an increase in loudness. Approximately 69% reported that jaw movements doubled the loudness of their tinnitus; the remainder reported small to moderate changes in loudness. Jaw movements increased tinnitus pitch in nearly (~95%) of all patients with gaze-evoked tinnitus; the change in pitch doubled in 72% of patients.
Surprisingly, 17 respondents indicated they had not undergone acoustic neuroma surgery; nevertheless they reported that they had gaze-evoked/modulated tinnitus. Most (~94%) reported that eye movements caused the loudness of their tinnitus to increase, and in half the cases the loudness doubled with eye movement. In most cases (80%), eye movement caused the pitch of the tinnitus to increase, and in two thirds of the cases, there was a doubling of the pitch percept. These results indicate that acoustic neuroma surgery is not a prerequisite for developing gaze-evoked tinnitus. Approximately 80% of these patients also reported that jaw movements altered the loudness of their tinnitus; in most cases, tinnitus loudness increased. Jaw movements also tended to increase the pitch of the tinnitus substantially in most patients.
In the past few years, several retrospective surveys have been conducted on patients who had undergone acoustic neuroma resection. One study reported that 36% of patients had gaze-evoked tinnitus at 15 months postsurgery, and this number dropped to 19% at 62 months postoperatively.23 A significant risk factor for developing gaze-evoked tinnitus was the presence of tinnitus preoperatively. Another recent survey of acoustic neuroma patients reported a prevalence of 19% with gaze-evoked tinnitus. Interestingly, 14% of these patients also reported they had somatic tinnitus that could be modulated by movements of the head and neck regions.22
Tinnitus Modulation by Jaw Clench
Tinnitus has long been associated with temporomandibular joint (TMJ) disorders.24,25 Moreover, some reports indicate that about a third of patients with TMJ disorders can modulate the loudness of their tinnitus. However, at the time of OFM PET imaging studies, it was unclear how common tinnitus modulation by jaw clench was in the general population. In an effort to identify patients who could modulate the loudness of their tinnitus with a jaw clench, we posted two notices in Tinnitus Today (American Tinnitus Association newsletter, 1996) requesting that patients contact us if they could modulate their tinnitus by pressing or moving their jaw, tongue, face, teeth, neck, head, or eyes.26 Questionnaires were sent to 142 respondents; 93 questionnaires were returned, most of which were from men (80%). In this sample, 90% indicated that their tinnitus became louder with a jaw clench; 41% reported that clenching their jaw caused their tinnitus to double in loudness, and 26% indicated that it tripled in loudness. About half reported that jaw clench altered the pitch of their tinnitus. In most of these cases (90%) the pitch increased; the pitch doubled in 40% of these subjects and tripled in 14%. A small proportion of respondents (12%) indicated that eye movements changed the loudness of their tinnitus; all indicated that loudness increased, and more than half reported a doubling in loudness. Only 9% of the sample reported that eye movements altered the pitch of their tinnitus; in the majority of cases the pitch increased. These results indicate that some tinnitus patients can substantially alter the loudness and pitch of their tinnitus by jaw clench and/or eye movements. Unfortunately, it is impossible to estimate the prevalence of tinnitus patients who can modulate their tinnitus by jaw clench or eye movement, but clearly it is not a rare phenomenon.
Clinical Characteristics of Somatic Tinnitus
Although there is no universally agreed on definition or test battery for somatic tinnitus, a growing body of clinical data indicates that many patients can modulate their tinnitus by both movements and pressure applied to the head, neck, face, and shoulders.10,11,27,28 Levine noted that many of these patients have somatic disorders of the head and neck, normal hearing (noncochlear tinnitus), and tinnitus localized to the side of the somatic disorder.10 The degree to which somatic modulation alters the loudness or pitch of tinnitus has varied across studies. Some studies indicate the modulation effects are relatively small, whereas some of our results reported earlier from subjects selectively recruited for somatic tinnitus showed large changes (two to three times) in loudness and pitch. It has been previously posited that forceful maneuvers were almost universally necessary to elicit a noticeable change in tinnitus perception, that head and neck maneuvers were the most consistent for modulating tinnitus, and that modulation typically worsened the subject’s tinnitus for a short period of time.29 These conclusions have led to more questions regarding which maneuvers are most effective in modulating tinnitus and the degree to which the pitch and loudness of tinnitus can alter the perception of tinnitus.
To begin to address these questions, we sent letters to 200 members of the Tinnitus Support Group at the University at Buffalo. To date, 45 respondents have enrolled and completed the protocol (mean age, 65.2 years; 67% male, 33% female). Participants completed a comprehensive questionnaire (medical condition, current medications, tinnitus characteristics, ability to modulate tinnitus). Afterward, participants performed 42 maneuvers of the head and neck under the guidance of the investigator that evaluated specific cranial or cervical nerves (Table 1). To evaluate the somatic modulation of tinnitus loudness consistently, each subject was told to rate his or her baseline tinnitus with a value of 5 on a 1 to 10 scale. Then, a somatic maneuver was performed for 5 seconds, and immediately afterward the subject was asked to rescore the tinnitus on the 1 to 10 scale (>5 indicates increased loudness or pitch where 10=severe tinnitus or <5 indicates decreased loudness or pitch; 1=tinnitus gone). When possible, subjects were asked if the change in tinnitus quality was in pitch or volume.
Table 1.
Cranial Nerves and Corresponding Movements of the Head, Neck, and Eyes
| Nerve | Movement/Maneuver |
|---|---|
| CN 3, 4, and 6 | Movement of eyes horizontally to the left and right, vertically up and down, and diagonally to the upper and lower corners of the visual field. |
| CN 5 | Jaw with force bilaterally, and on either the left or right side. Jaw thrusting in the midline, to the left, and to the right. |
| CN 7 | Movements of facial expression including eye closure, eyebrow raise, puffing the cheeks, baring the teeth, and pursing the lips. |
| CN 11 | Shrugging the shoulders and rotating the head left and right (bringing the chin closer to the shoulder). |
| CN 12 | Thrusting of the tongue in the midline, to the left, and to the right. |
| C1 and C2 | Lateral flexion of the neck (bringing the ear to the shoulder), forward flexion of the neck (bringing the chin to the chest), extension of the neck (moving the head toward the back). |
CN, cranial nerve; C, cervical nerve.
Most subjects reported their tinnitus was bilateral (64%), but some localized it to the left (20%) or right ear (16%). Tinnitus duration ranged from 2 to 60 years (mean, 18.1 years). Subjects reported that the tinnitus sounded like a “hiss” (24%), “ring” (16%), ‘‘buzz’’ (15.5%; n=7), or “tone” (13.3%) and was relatively constant (89%). Before making any maneuvers, patients were asked whether they could improve or worsen their tinnitus with movements of the head and neck. Remarkably, only a few subjects were aware that they could modulate their tinnitus; two of 45 stated that jaw movements worsened their tinnitus, one indicated that it improved tinnitus and one indicated that head movements worsened tinnitus.
Somatic Modulation
Of the 45 participants, 78% (35 of 45) were able to modulate their tinnitus with at least one movement of the head or neck; the remaining 22% reported no change in their tinnitus. Among the 78% of participants who could modulate their tinnitus, the average number of head or neck movements that modulated tinnitus was 8.3 movements per subject (Fig. 1). Of the 1890 total movements tested (42 movements × 45 subjects), 300 (15.9%) resulted in tinnitus modulation. The maneuvers that showed the greatest probability of modulating the tinnitus perception were the midline jaw thrust (35.6%), right jaw clench (31.1%), active neck extension (28.9%), and right shoulder rotation with resistance (28.9%). The maneuvers least likely to modulate tinnitus were active shoulder shrug (4.4%), eyebrow raise (6.7%), shoulder shrug against resistance (6.7%), and left and right tongue protrusion (6.7% each).
Figure 1.
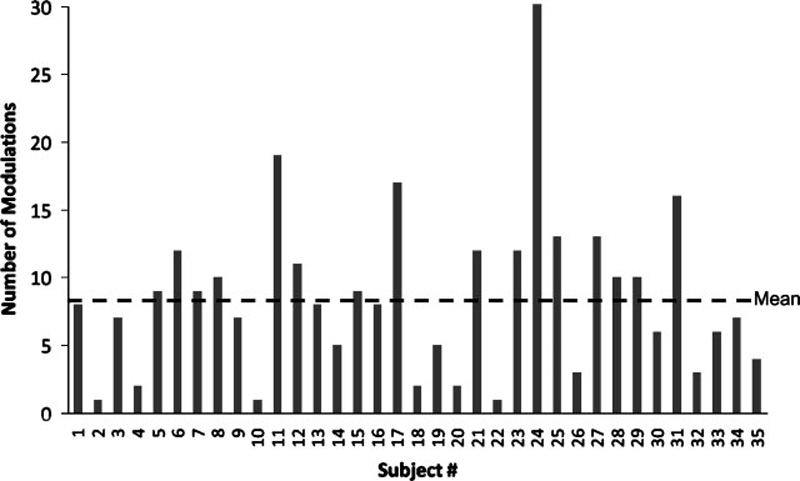
Thirty-five of 45 subjects were able to modulate their tinnitus with head, neck, jaw, and shoulder movements. Figure shows number of movements that resulted in a modulation for each of the 35 subjects. Horizontal dashed line shows mean value.
When the maneuvers were grouped by cranial/cervical nerve, clear trends emerged. Cranial nerve (CN) V and cervical nerves 1 and 2 modulated the perception of tinnitus in 64.4% and 60% of participants, respectively (Fig. 2). In contrast, movements of the tongue (CN XII), the eyes (CN III, IV, and VI), and the face (CN VII) showed limited ability to modulate tinnitus with 13.3%, 26.7%, and 28.8%, respectively.
Figure 2.
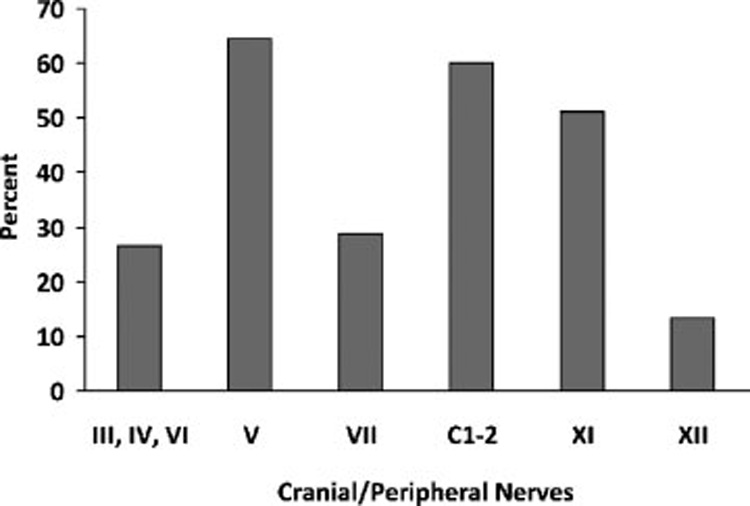
Percentage of all movements that evoked a modulation broken down by cranial nerves or peripheral nerve (cervical nerves 1 and 2). Cranial nerves controlling eye movements are grouped as one category because they are not easily isolated from one another.
The majority (67%) of subjects experienced a worsening of their tinnitus with modulation; however, the magnitude of the effect was small. Of the 305 tinnitus modulations recorded, 53.8% changed from 5 to 6 and 23.3% changed from 5 to 4, resulting in 77.1% of modulations moving 1 point up or down on the 10-point measurement scale (Fig. 3). Of all subjects who reported an increase in their tinnitus, 80.7% changed from 5 to a 6. Among the subjects who reported a decrease in their tinnitus, 69.6% changed from 5 to a 4. Interestingly, two subjects reported a dramatic reduction of their tinnitus from a 5 to 1, and one reported a dramatic increase in tinnitus from 5 to 10.
Figure 3.
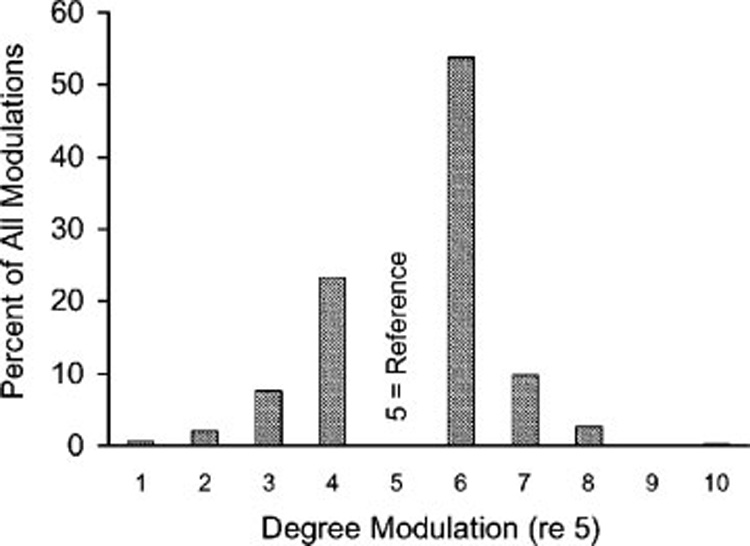
Percentage of all modulations versus degree of modulation from the reference value of 5. Data from subset of patients that could modulate their tinnitus (>5 indicates increased loudness or pitch; <5 indicates decreased loudness or pitch).
Force of Maneuvers
Twenty maneuvers of the neck and shoulders were performed for each subject using passive (moved by the investigator), active (moved by the patient), and resistive force (moved by the patient against resistance). Of the 900 neck and shoulder movements performed (20 movements × 45 subjects), 152 movements resulted in modulation; 30 maneuvers (19.7%) were passive, 54 (35.5%) were active, and 68 (47.4%) were against resistance (Fig. 4). Although passive and active movements of the neck and shoulder modulated the perception of tinnitus in many subjects, forceful maneuvers were clearly more effective. It is unclear why this is the case, but one possible explanation is that forceful maneuvers unconsciously activate other neural networks involved in breath-holding and flexion of the abdominal muscles, similar to what occurs during the Valsalva maneuver, which increases intracranial pressure and venous return to the heart as well as normalizing middle ear pressure by opening the eustachian tube.
Figure 4.
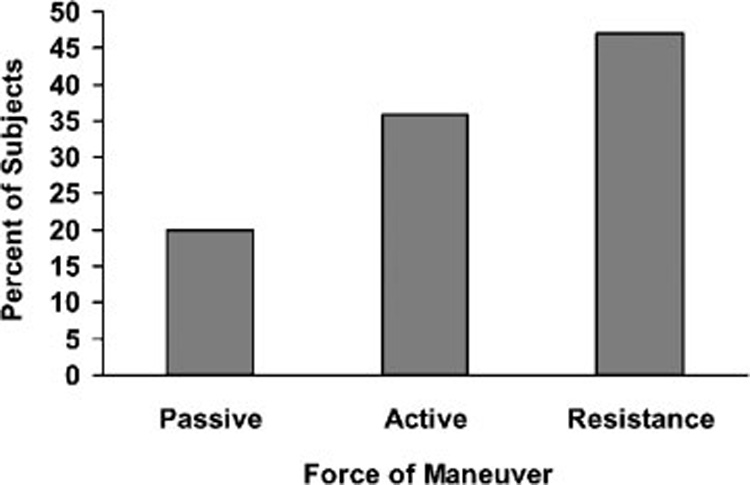
Maneuvers involving the neck that resulted in modulation. Figure shows percentage of modulation elicited by force of the maneuver.
SUMMARY
The modulation of tinnitus by the nonauditory sensory-motor system was initially thought to be an uncommon phenomenon, but over the past 20 years, there has been a growing awareness that tinnitus can be modulated in many individuals by sensory-motor inputs mainly from the region of the head, neck, and shoulder and in some rare instances from movements or stimulation of upper limbs.20,30 Careful evaluation of 45 members of our tinnitus support group, using a comprehensive neurological test battery that assesses the involvement of CN III, IV, V, VI, VII, XI, and XII and cervical nerves 1 and 2 showed that 78% of tinnitus subjects could modulate their tinnitus. The high percentage of patients who could modulate their tinnitus with this test battery is consistent with more recent surveys of tinnitus patients involving similar but not identical evaluation schemes.10,11
In our survey of patients in our tinnitus support group, the magnitude of somatic modulation was small to modest, typically a ± 1-point deviation from a baseline of 5 on a 1 to 10 scale. Most of these subjects reported that the maneuvers increased the loudness of their tinnitus consistent with previous reports.11 However, in a highly selected sample of subjects recruited though newsletters seeking patients who could modulate their tinnitus with eye, head, neck, and face movements, a high percentage of patients were found who could double or triple the loudness of their tinnitus. The large increases in loudness reported by these patients would seem to make them ideal candidates for brain imaging studies seeking to identify regions of the brain involved in the perception of tinnitus.12,31 In contrast, two remarkable patients evaluated from our support group were able to suppress their tinnitus completely. These individuals would not only be ideal candidates for imaging studies but might benefit from somatotherapy and repetitive exercises aimed at alleviation of tinnitus.27,32,33 Although maneuvers that increase tinnitus loudness may seem to be ones that should be avoided in developing a treatment strategy for tinnitus, some reports suggest that repetitive movements that increase tinnitus loudness may actually lead to a reduction in the severity of tinnitus.32
Efforts are currently underway by individual investigators, as well as research teams such as the Tinnitus Research Initiative, to develop standardized methods to define, assess, and categorize patients with somatic tinnitus.10,11,27,29 Some assessment techniques employed here may help define those maneuvers and neural pathways that are likely to modulate tinnitus versus those that have minimal importance. Our results lend support to previous contentions that movements of the jaw and neck (involving the trigeminal nerve, spinal accessory nerve, and cervical nerves 1 and 2) are the most consistent and most efficacious methods of modulating tinnitus. Conceptually, a scheme for categorizing patients based on specific movements and neural pathways may provide a frame work for developing effective therapeutic approaches for treating certain forms of tinnitus.
ACKNOWLEDGMENTS
Research supported in part by past or present grants from the American Tinnitus Association, Tinnitus Research Consortium, Tinnitus Research Initiative, and NIH (R01DC00909101, R01DC009219).
ABBREVIATIONS
- rCBF
regional cerebral blood flow
- OFM
oral facial maneuver
- PET
positron emission tomography
- SPM
statistical parametric mapping
- TMJ
temporomandibular joint
Footnotes
Learning Outcomes: As a result of this activity, the learner will be able to (1) describe how movements of the head, neck, jaw, shoulder, and eyes can modulate the loudness and pitch of tinnitus, and (2) describe which cranial and cervical nerves are likely involved in somatic tinnitus.
REFERENCES
- 1.Caird D, Klinke R. Processing of interaural time and intensity differences in the cat inferior colliculus. Exp Brain Res. 1987;68(2):379–392. doi: 10.1007/BF00248803. [DOI] [PubMed] [Google Scholar]
- 2.Musicant AD, Butler RA. Influence of monaural spectral cues on binaural localization. J Acoust Soc Am. 1985;77(1):202–208. doi: 10.1121/1.392259. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins DB, Wightman FL. Interaural time discrimination ability of listeners with sensorineural hearing loss. Audiology. 1980;19(6):495–507. doi: 10.3109/00206098009070081. [DOI] [PubMed] [Google Scholar]
- 4.Hafter ER, Dye RH, Jr, Gilkey RH. Lateralization of tonal signals which have neither onsets nor offsets. J Acoust Soc Am. 1979;65(2):471–477. doi: 10.1121/1.382346. [DOI] [PubMed] [Google Scholar]
- 5.Knudsen EI. Early auditory experience aligns the auditory map of space in the optic tectum of the barn owl. Science. 1983;222:939–942. doi: 10.1126/science.6635667. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen EI. Experience shapes sound localization and auditory unit properties during development in the barn owl. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory Function, Neurobiological Bases of Hearing. New York, NY: John Wiley; 1988. pp. 137–152. [Google Scholar]
- 7.Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol. 1995;73(2):743–765. doi: 10.1152/jn.1995.73.2.743. [DOI] [PubMed] [Google Scholar]
- 8.Olsen CC, Brandt JF. Middle ear muscle activity during speech in stapedectomized and laryngectomized subjects. J Am Audiol Soc. 1976;1(15):215–220. [PubMed] [Google Scholar]
- 9.Borg E, Zakrisson JE. The activity of the stapedius muscle in man during vocalization. Acta Otolaryngol. 1975;79(5–6):325–333. doi: 10.3109/00016487509124694. [DOI] [PubMed] [Google Scholar]
- 10.Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20(6):351–362. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez TG, Guerra GC, Lorenzi MC, Brandao AL, Bento RF. The influence of voluntary muscle contractions upon the onset and modulation of tinnitus. Audiol Neurootol. 2002;7(6):370–375. doi: 10.1159/000066155. [DOI] [PubMed] [Google Scholar]
- 12.Lockwood AH, Salvi RJ, Coad ML, et al. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50(1):114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Burkard RF, Lockwood AH, Salvi RJ. Effects of background noise on audiometric thresholds during positron emission tomography: passive and active noise-reduction. Scand Audiol. 2000;29(4):211–216. doi: 10.1080/010503900750022835. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood AH, Salvi RJ, Coad ML, et al. The functional anatomy of the normal human auditory system: responses to 0.5 and 4.0 kHz tones at varied intensities. Cereb Cortex. 1999;9(1):65–76. doi: 10.1093/cercor/9.1.65. [DOI] [PubMed] [Google Scholar]
- 15.Reyes SA, Salvi RJ, Burkard RF, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171(1–2):43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood AH, Wack DS, Burkard RF, et al. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56(4):472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- 17.House WF. Letter to the editor. Am J Otol. 1982;4:188. [Google Scholar]
- 18.Wall M, Rosenberg M, Richardson D. Gaze-evoked tinnitus. Neurology. 1987;37(6):1034–1036. doi: 10.1212/wnl.37.6.1034. [DOI] [PubMed] [Google Scholar]
- 19.Cacace AT, Lovely TJ, Winter DF, Parnes SM, McFarland DJ. Auditory perceptual and visual-spatial characteristics of gaze-evoked tinnitus. Audiology. 1994;33(5):291–303. doi: 10.3109/00206099409071889. [DOI] [PubMed] [Google Scholar]
- 20.Cacace AT, Cousins JP, Parnes SM, et al. Cutaneous-evoked tinnitus. I. Phenomenology, psychophysics and functional imaging. Audiol Neurootol. 1999;4(5):247–257. doi: 10.1159/000013848. [DOI] [PubMed] [Google Scholar]
- 21.Coad ML, Lockwood A, Salvi R, Burkard R. Characteristics of patients with gaze-evoked tinnitus. Otol Neurotol. 2001;22(5):650–654. doi: 10.1097/00129492-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Baguley DM, Phillips J, Humphriss RL, et al. The prevalence and onset of gaze modulation of tinnitus and increased sensitivity to noise after translabyrinthine vestibular schwannoma excision. Otol Neurotol. 2006;27(2):220–224. doi: 10.1097/01.mao.0000172412.87778.28. [DOI] [PubMed] [Google Scholar]
- 23.Biggs ND, Ramsden RT. Gaze-evoked tinnitus following acoustic neuroma resection: a deafferentation plasticity phenomenon? Clin Otolaryngol Allied Sci. 2002;27(5):338–343. doi: 10.1046/j.1365-2273.2002.00591.x. [DOI] [PubMed] [Google Scholar]
- 24.Morgan DH. Tinnitus of TMJ origin: a preliminary report. Cranio. 1992;10(2):124–129. doi: 10.1080/08869634.1992.11677900. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein B, Axelsson A, Carlsson GE. Prevalence of signs and symptoms of craniomandibular disorders in tinnitus patients. J Craniomandib Disord. 1990;4(3):186–192. [PubMed] [Google Scholar]
- 26.Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Modulation of tinnitus by voluntary jaw movements. Am J Otol. 1998;19(6):785–789. [PubMed] [Google Scholar]
- 27.Sanchez TG, da Silva Lima A, Brandao AL, Lorenzi MC, Bento RF. Somatic modulation of tinnitus: test reliability and results after repetitive muscle contraction training. Ann Otol Rhinol Laryngol. 2007;116(1):30–35. doi: 10.1177/000348940711600106. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein B. Tinnitus and craniomandibular disorders—is there a link? Swed Dent J Suppl. 1993;95:1–46. [PubMed] [Google Scholar]
- 29.Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153(4):643–648. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
- 30.Moller AR. Pathophysiology of tinnitus. Ann Otol Rhinol Laryngol. 1984;93(1 Pt 1):39–44. [PubMed] [Google Scholar]
- 31.Lockwood AH, Burkard RF, Salvi RJ, et al. Positron emission tomographic (PET) studies of gaze-evoked tinnitus. Assoc Res Otolaryngol. 1999;22:472. (Abstr) [Google Scholar]
- 32.Levine RA, Nam EC, Oron Y, Melcher JR. Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Prog Brain Res. 2007;166:195–207. doi: 10.1016/S0079-6123(07)66017-8. [DOI] [PubMed] [Google Scholar]
- 33.Levine RA. Somatic modulation appears to be a fundamental attribute of tinnitus. In: Hazel JPW, editor. Proceedings of the Sixth International Tinnitus Seminar; Cambridge, UK. Tinnitus and Hyperacusis Center; 1999. pp. 193–196. [Google Scholar]


