Abstract
Narcolepsy, a disorder characterized by fragmented bouts of sleep and wakefulness during the day and night as well as cataplexy, has been linked in humans and non-human animals to the functional integrity of the orexinergic system. Adult orexin knockout mice and dogs with a mutation of the orexin receptor exhibit symptoms that mirror those seen in narcoleptic humans. As with narcolepsy, infant sleep-wake cycles in humans and rats are highly fragmented, with consolidated bouts of sleep and wakefulness developing gradually. Based on these common features of narcoleptics and infants, we hypothesized that the development of sleep-wake fragmentation in orexin knockout mice would be expressed as a developmental divergence between knockouts and wild-types, with the knockouts lagging behind the wild-types. We tested this hypothesis by recording the sleep-wake patterns of infant orexin knockout and wild-type mice across the first three postnatal weeks. Both knockouts and wild-types exhibited age-dependent, and therefore orexin-independent, quantitative and qualitative changes in sleep-wake patterning. At 3 weeks of age, however, by which time the sleep and wake bouts of the wild-types had consolidated further, the knockouts lagged behind the wild-types and exhibited significantly more bout fragmentation. These findings suggest the possibility that the fragmentation of behavioral states that characterizes narcolepsy in adults reflects reversion back toward the more fragmented sleep-wake patterns that characterize infancy.
Keywords: orexin, hypocretin, atonia, development, knockout, mouse
Narcolepsy has emerged in recent years as a newly recognized neurodegenerative disorder (van den Pol, 2000; Siegel et al., 2001; Taheri et al., 2002). Central to this reclassification has been the recent discovery of a neurotransmitter, orexin (or hypocretin) (de Lecea et al., 1998; Sakurai et al., 1998), that is produced by neurons situated within the caudal hypothalamus that project to the locus coeruleus and other nuclei implicated in the regulation of sleep and wakefulness (Peyron et al., 1998). A narcoleptic phenotype is exhibited by dogs with a mutation of the orexin receptor (Lin et al., 1999) and in orexin knockout mice (Chemelli et al., 1999). Also, in humans, narcolepsy has been linked to deficient functioning of the orexinergic system (Peyron et al., 2000; Thannickal et al., 2000). Importantly, adult orexin knockout mice exhibit patterns of sleep and wakefulness that mirror those seen in narcoleptic humans (Chemelli et al., 1999; Willie et al., 2003; Mochizuki et al., 2004).
The development of narcolepsy has received relatively little attention. In humans, onset of the disorder occurs in early adulthood, with the presenting symptom in 90% of cases being excessive daytime sleepiness (Taheri et al., 2002). Cataplexy, a sudden loss of muscle tone in an otherwise awake individual, is a symptom specific to narcolepsy that usually appears later. In Doberman pinschers with an orexin receptor mutation that predisposes them to narcolepsy, cataplexy is first detected at 4 weeks of age and its frequency increases dramatically over the next several months (Riehl et al., 1998; John et al., 2004). In orexin knockout mice, video analysis of 5 subjects suggested the presence of cataplexy as early as 3 weeks of age, but the incidence was very low and sporadic (Chemelli et al., 1999). In none of these studies, however, were patterns of sleep and wakefulness measured across early development. Given that cataplexy is a later-developing symptom of narcolepsy than is excessive daytime sleepiness, it is possible that measures of sleep and wake behavior can provide a more reliable and sensitive measure of the onset of narcolepsy in orexin knockout mice.
As with narcolepsy, the sleep and wake bouts of infant humans (Kleitman & Engelmann, 1953) and rats (Gramsbergen et al., 1970; Blumberg et al., 2005b) are highly fragmented, characterized by rapid transitions between short-duration states. The question addressed here is whether the fragmented sleep and wake bouts observed in both narcoleptics and infants reflects, at least in part, deficient function of the orexinergic system. Specifically, we hypothesized that infant orexin knockout mice would lag behind wild-types with regard to the developmental consolidation of sleep and wake bouts.
The examination of sleep and wakefulness in early infancy, especially in altricial infants such as rats and mice, has been limited by technical problems as well as concerns over the absence of state-dependent neocortical activity (Blumberg et al., 2005a). Recently, we have shown that the nuchal electromyogram (EMG) alone provides a reliable measure of sleep and wakefulness during early infancy in rats and, moreover, provides an adequate foundation for revealing the neural substrates of infant sleep (Karlsson & Blumberg, 2005; Karlsson et al., 2005; Seelke et al., 2005; Mohns et al., 2006). Building on work in adult mammals examining the statistical distributions of sleep and wake bouts (Lo et al., 2002; Lo et al., 2004), we have also described orderly developmental transitions in the statistical distributions of sleep and wake bouts in infant rats between P2 and P21 (Blumberg et al., 2005b). Here, we adapt these techniques for use in infant mice over the first three postnatal weeks to describe a developmental divergence in sleep-wake patterning between orexin knockouts and wild-types.
MATERIALS AND METHODS
All experiments were performed under National Institutes of Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Subjects
All subjects were the male and female infant offspring of a founder population of orexin knockout mice (Mochizuki et al., 2004). Heterozygote breeding pairs were used to produce litters comprising knockout and wild-type infants. For this study, pups were tested on P2-4 (hereafter P4), P10-13 (hereafter P12), or P20-22 (hereafter P21). In total, 29 litters were produced from 12 fathers and 16 mothers, with no more than 3 litters being used from a given mother. A total of 19 P4, 21 P12, and 27 P21 subjects were included in the analyses presented here. At the time of testing, litter sizes were 2-9 pups (average: 5-6 pups/litter) and body weights were 1.6-2.8 g at P4, 3.7-9.1 g at P12, and 6.0-13.4 g at P21. Mothers and their litters were housed and raised in standard laboratory cages (28 × 18 × 14 cm) in the animal colony at the University of Iowa. Food and water were available to the animals ad libitum. All animals were maintained on a 12 hour light and dark schedule with lights on at 6:00 am. All experiments were conducted during the light period, with the exception of a group of P21 subjects that was tested during the dark period.
Surgery
On the day of testing, a pup was removed from the litter and weighed. Care was taken to ensure that the P4 and P12 subjects exhibited a milk band upon removal from the nest (milk bands are not detectable in the older subjects). Under isoflurane anesthesia, bipolar stainless steel hook electrodes (50 μm diameter; California Fine Wire, Grover Beach, CA) were inserted bilaterally into the nuchal muscle and secured with collodion. After surgery, the pup recovered for at least 1 h before testing, either in a humidified incubator (maintained at 35 °C) or in the testing chamber.
For the P21 wild-type and knockout (N=6 per group) subjects tested during the dark period, surgery was performed identically as during the light period, except under red-light illumination. Care was taken to ensure that pups were never exposed to white light after lights-off. Surgery typically began around 8:00 pm and recovery was identical to that of P21 subjects tested during the light period.
Procedure and data acquisition
All pups were tested unrestrained inside an electrically shielded double-walled glass chamber (height = 17.0 cm; i.d. = 12.5 cm) with a Plexiglas lid. Air temperature inside the chamber was regulated at thermoneutrality for each age using a temperature-controlled water circulator (P4: 37°C; P12: 36°C; P21: 34°C). Access holes in the side of the chamber allowed for the passage of electrodes. A round platform constructed of polyethylene mesh was fitted inside the chamber and a perforated felt pad was placed on top of the mesh. For P21 (and one P12) subjects, a Plexiglas insert was constructed that divided the chamber into two halves and thereby allowed for the simultaneous testing of two subjects.
Nuchal EMG electrodes were connected to differential amplifiers (A-M Systems, Carlsborg, WA) and their signals were amplified (×10,000) and filtered (300-5000 Hz). EMG data were visualized by the experimenter during the test using a data acquisition system (BioPac Systems, Santa Barbara, CA). For subjects tested during the day, a microcamera was placed above the chamber lid for monitoring and recording of behavior. EMG data were recorded directly to hard disk and at least 1 h of EMG and video data were recorded to digital videotape using a data recorder (DV8; WinTron Technologies, Rebersberg, PA).
All subjects acclimated to the testing chamber for at least 30 min before data recording began. For P4 and P12 subjects, data were recorded for 1 h. For P21 subjects, data were recorded for 6 h. (For subjects tested during the dark period, data recording typically began at 9:30 pm.) In general, longer tests are needed for older subjects in order to acquire a sufficient number of sleep-wake cycles (Blumberg et al., 2005b). Also, for the P21 subjects, we were able to genotype more than 1 week before testing, thus allowing us to test only subjects of known genotype. For the P4 and P12 subjects, however, genotyping occurred after testing, thus requiring multiple littermates to be tested for 1 h and the discarding of data from heterozygotes. One P12 subject was genotyped before testing and was therefore tested for 3 h.
Genotyping
Tail snips for genotyping were acquired either after testing (for all P4 subjects and all but one P12 subjects) or at least 6 days before testing. DNA was extracted and mice were genotyped using PCR with a neo primer (knockout forward), 5'-TGATATTGCTGAAGAGCTTGGCGG, or a genomic primer (wild-type forward), 5'-GACGACGGCCTCAGACTTCTTGGG, and a genomic primer common to knockouts and wild-types (reverse primer), 3'-ACTAGCCCTTCCCTCCACAGA.
Data analysis
As described previously (Karlsson et al., 2004; Blumberg et al., 2005b), EMG signals were digitized at 1 or 2 kHz using a data acquisition system (BioPac Systems Inc., Santa Barbara, CA). Digitized signals were integrated and full-wave rectified. Then, the EMG signal was dichotomized into bouts of sleep (i.e., atonia/hypotonia) and wake (high muscle tone) as follows: The amplitude of 5 1-s segments of noise-free, uninterrupted atonia and high-tone periods was measured for each pup, averaged, and the midpoint between the two was calculated. A bout of sleep and wake was defined as a period in which muscle tone was below or above, respectively, the midpoint value for at least 1 s. For P21 subjects, we found that increasing the sleep-bout criterion from 1 to 3 s more accurately reflected behavior at this age (as judged by comparing video and EMG records) and improved inter-rater reliability.
Three experienced scorers, typically blind to the subject's age and genotype, performed the analyses. When two scorers analyzed the same record, interrater reliabilities were high, usually exceeding 80% and often exceeding 90%. When a record was deemed too unclear to score or when two scorers were in substantial disagreement (i.e., interrater reliability < 80%), that record was excluded.
For the analyses described above, data were imported into Statview 5.0 (SAS, Cary, NC), JMP 5.0 (SAS, Cary, NC), and DeltaGraph 5.5 (SPSS, Chicago, IL). Because genotyping was performed after testing for the P4 and P12 subjects, data from littermates of the same genotype were often collected. Because it is inappropriate to treat these littermates as independent subjects within the same experimental group (Abbey & Howard, 1973; Holson & Pearce, 1992), their data were always averaged before statistical analyses were performed. At P4 and P12, as many as 4 same-genotype littermates were tested. Because the P21 subjects were genotyped before testing, no more than one wild-type and one knockout pup was selected from each litter.
Two-factor analysis of variance (ANOVA), with age and genotype as factors, was used to test for differences in sleep and wake variables. When appropriate, unpaired t tests were used for post hoc tests. Additional ANOVAs were performed to test for light-dark differences in sleep and wake behavior at P21. Post hoc tests included planned comparisons (i.e., unpaired t tests) to examine within-genotype circadian differences.
In order to assess the statistical distributions of all sleep and wake bouts, log-survivor analyses were performed (Blumberg et al., 2005b). Survivor distributions were produced from each pup and from pooled data at each age. Data were plotted using log-log and semi-log coordinates and regression analyses were performed on the data for each subject to assess the r2 values and, therefore, the degree of fit of the data to power-law and exponential distributions, respectively (Blumberg et al., 2005b). Three-factor ANOVAs, with age, genotype, and distribution (i.e., exponential or power-law) as factors, were used to test for differences in r2 values. When appropriate, paired t tests were used for post hoc tests to assess within-age, between-distribution differences in r2 values.
For all tests, alpha was set at 0.05. Means are presented with their standard errors (s.e.).
Results
As shown in Figure 1A, mean sleep bout durations during the light period increased dramatically between P4 and P12 and then leveled off between P12 and P21. ANOVA revealed a significant effect of age (F2,30 = 43.6, P < 0.0001) and genotype (F1,30 = 7.0, P < 0.05), but not a significant age × genotype interaction (F2,30 = 1.0). Post hoc tests revealed that at P21, but not P12, wild-type mice were exhibiting significantly longer mean sleep durations than were the orexin knockouts.
Figure 1.
Mean sleep (A) and wake (B) bout durations for wild-type (WT) and knockout (KO) mice at P4, P12, and P21. Mean number of sleep-wake cycles per min (C) and percentage of time awake (D) for the same subjects. * significant between-genotype difference. Mean + s.e.
As shown in Figure 1B, mean wake bout durations did not increase substantially until P21, and then only in the wild-type mice. Although ANOVA revealed a significant effect of age (F2,30 = 7.5, P < 0.005), there was no significant effect of genotype (F1,30 = 2.1) and no significant age × genotype interaction (F2,30 = 1.9). The effects of shortened sleep and wake bouts in the knockout mice at P21 combined to produce significantly more sleep-wake cycles per min, as shown in Figure 1C. For this measure, ANOVA revealed a significant effect of age (F2,30 = 107.8, P < 0.0001) and genotype (F1,30 = 7.3, P < 0.05), but not a significant age × genotype interaction (F2,30 = 0.7). As shown in Figure 1D, the mean percentage of time spent awake decreased significantly with age (F2,30 = 46.7, P < 0.0001) but, importantly, did not differ between genotypes (genotype: F1,30 = 0.7; age × genotype: F2,30 = 0.6).
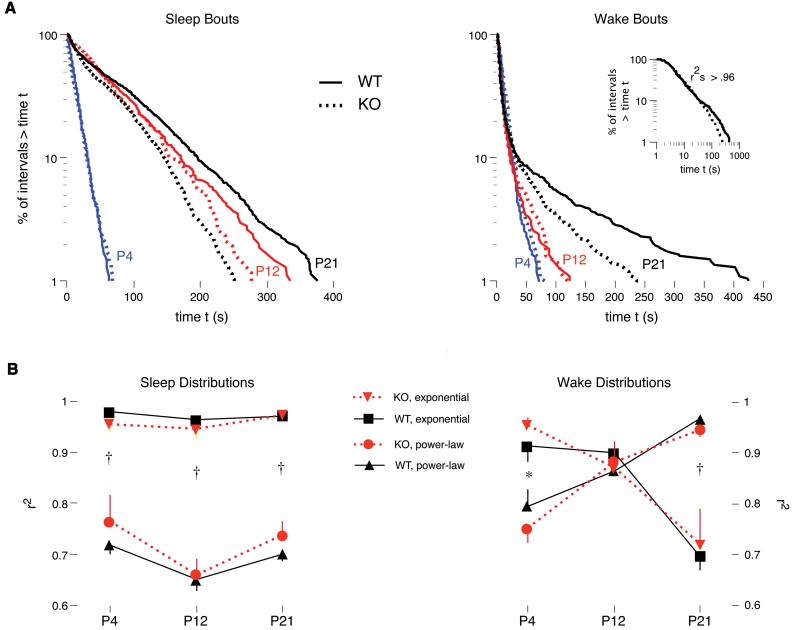
Survivor distributions for pooled sleep bout data at P4, P12, and P21 are presented in Figure 2A (left panel) for wild-type (solid lines) and knockout (dotted lines) mice. It can be seen that the data at all three ages are best described by an exponential function [such that the frequency distribution f(t) of bout durations of duration t was proportional to e(-t/τ), where τ is the characteristic time scale], as they fall along straight line on the semi-log plot. By P21, the data for the wild-types and knockouts have diverged significantly from each other such that the 95% confidence intervals for the two distributions do not overlap at intervals greater than 25 s (data not shown).
Figure 2.
(A) Survivor plots of sleep and wake bout durations for wild-type (WT; solid lines) and knockout (KO; dashed lines) mice at P4 (blue), P12 (red), and P21 (black). Straight lines on these plots indicate that the data follow an exponential distribution. The inset is a replotting of the P21 wake data using log-log coordinates; straight lines on these plots indicate that the data follow a power-law distribution. Individual data points were pooled across all subjects: P4, N = 1086-1398; P12, N = 424-545; P21, N = 1674-2002. (B) Values of r2 produced using regression analysis of survivor data from the wild-type (WT; black) and knockout (KO; red) mice at P4, P12, and P21. For each individual pup, the degree of fit of the data to power-law and exponential distributions was determined, yielding a value of r2 that was then averaged across subjects at each age. † Significant within-age, between-distribution difference in r2 values for both wild-types and knockouts. * Significant within-age, between-distribution difference in r2 values for knockouts only. Mean +/- s.e.
Figure 2B (left panel) presents r2 values based on regression analyses performed for each pup when data were plotted using semi-log or log-log coordinates. Most importantly, the three-factor ANOVA revealed a significant effect of distribution (F1,60 = 410.9, P < 0.0001) on r2 values. Moreover, paired t tests revealed main effect that an exponential distribution provided a significantly better fit to the data than did a power-law distribution at each age and for each genotype.
Whereas survival data that follow an exponential distribution fall on a straight line in a semi-log plot, those that follow a power-law distribution [such that f(t) ~ t-α, where α is a characteristic power-law exponent] fall on a straight line in a log-log plot. As shown in Figure 2A (right panel), survivor distributions for pooled wake data exhibit different distributions depending on age. First, at P4, and to a lesser extent at P12, the data for both wild-types and knockouts fall on overlapping straight lines, indicative of an exponential distribution. By P21, the data are now skewed at intervals greater than 30 s and, in addition, the data for the wild-types and knockouts are no longer overlapping. The 95% confidence intervals for these two distributions do not overlap at intervals greater than 75 s, thus indicating that the two distributions arise from separate populations (data not shown).
Figure 2B (right panel) presents r2 values based on regression analyses performed for each pup when wake bout data were plotted using semi-log or log-log coordinates. The three-factor ANOVA revealed only a significant age x distribution interaction (F2,60 = 34.2, P < 0.0001) on r2 values. In particular, although the data distribute exponentially at P4, paired t tests revealed that, by P21, the data now follow a power-law distribution. This transition is illustrated in the inset in Figure 2A (right panel), which presents the P21 data replotted on log-log coordinates to reveal that they now fall on a straight line.
Figure 3A presents again the data for P21 subjects during the light period but now contrasted with the data for P21 subjects tested during the dark period. ANOVA revealed a significant effect of genotype (F1,23 = 5.8, P < 0.05) but neither a significant effect of light cycle (F1,23 = 3.3, P = 0.08) nor a significant genotype x light-cycle interaction (F1,23 = 2.6). Planned comparisons did indicate, however, that mean sleep duration for the wild-types, but not the knockouts, was significantly higher during the day than during the night.
Figure 3.
Mean sleep (A) and wake (B) bout durations for wild-type (WT) and knockout (KO) mice at P21 for subjects tested under light and dark conditions. * significant between- and within-genotype difference. Mean + s.e.
Figure 3B contrasts the light-dark P21 data for mean wake duration. None of the ANOVAs reached statistical significance (genotype: F1,23 = 3.7, P < 0.07; light cycle: F1,23 = 0.0; genotype x light-cycle interaction: F1,23 = 0.0). ANOVA did reveal that knockouts cycled more rapidly between sleep and wakefulness than did wild-types; though the main effect of genotype was significant (F1,23 = 6.7, P < 0.05), there was no effect of light cycle (F1,23 = 1.8) and no genotype x light-cycle interaction (F1,23 = 0.1). Finally, the percentage of time awake was indifferent to genotype and light cycle (genotype: F1,23 = 0.1; light cycle: F1,23 = 0.3; genotype x light-cycle interaction: F1,23 = 0.1).
DISCUSSION
As every attentive parent is aware, newborns cycle rapidly between sleep and wakefulness and only gradually, over the first several months, do ultradian rhythms consolidate and circadian patterns strengthen (Kleitman & Engelmann, 1953; Jenni et al., 2006). Similar processes occur in rats over the first three postnatal weeks (Gramsbergen et al., 1970; Frank & Heller, 1997; Blumberg et al., 2005b). Because narcolepsy, a disorder typically found in adults, is also characterized by increased sleep and wake fragmentation (Taheri et al., 2002), we hypothesized that sleep-wake fragmentation in narcoleptics and infants reflects in part a shared deficit in neural function. With the discovery of orexin's causative role in narcolepsy (Siegel et al., 2001), the advent of orexin knockout mice (Chemelli et al., 1999), and recent advances in our ability to monitor and analyze behavioral states in altricial infants (Blumberg et al., 2005b), we were in a position to test this hypothesis directly. We predicted that the knockouts would lag behind the wild-types with regard to their consolidation of sleep and wake bouts across early development.
We report here that both knockout and wild-type mice exhibited decreasing fragmentation of sleep and wake bouts between P4 and P21. Moreover, similar to what was shown recently in infant rats (Blumberg et al., 2005b), mice of both strains exhibited orderly changes in the statistical distributions of sleep and wake bouts, including the wake-bout transformation from exponential to power-law behavior (see Figure 2). Against this backdrop of orderly, orexin-independent developmental change, by P21 the knockouts were lagging behind the wild-types with regard to the developmental consolidation of sleep and wake bouts. These knockouts also appeared to lag behind the wild-types in the onset of circadian rhythmicity, exhibiting similar mean sleep bout durations during the light and dark periods as the wild-types exhibited increased sleep bout durations during the light period (see Figure 3A). Given the interrelations of circadian rhythmicity and sleep-wake consolidation (Dijk & Czeisler, 1995), it is possible that some aspects of decreased sleep-wake fragmentation in infancy are related to the development of circadian regulation of behavioral states (Frank & Heller, 1997).
The mean percentage of time awake decreased significantly with age, from a value of 47% at P4 to 14% and 22% at P12 and P21, respectively (Figure 1D). In contrast, a value of approximately 40% has been reported for adult knockout and wild-type mice during the light phase (Mochizuki et al., 2004); in addition, P2-21 rats were found to be awake 25-35% of the time (Blumberg et al., 2005b). It is not clear what is responsible for the more variable wake percentages seen here or the relatively high value at P4. Testing over a range of air temperatures may help to resolve this issue. It should be stressed, however, that mean wake percentage did not differ between the knockouts and wild-types.
In humans, excessive daytime sleepiness is the presenting symptom of narcolepsy in 90% of cases and cataplexy is the presenting symptom in only 10% of cases (Taheri et al., 2002). Nonetheless, cataplexy has received exclusive attention in previous developmental studies in dogs and mice (Riehl et al., 1998; Chemelli et al., 1999; John et al., 2004). Interestingly, from the perspective of cataplexy, narcolepsy entails the onset of a distinct, pathological symptom. In contrast, we have shown here that, from the perspective of sleep and wakefulness, narcolepsy in orexin knockout mice (and likely mutant Doberman pinschers as well) entails retention of the more fragmented patterns of sleep and wakefulness normally expressed by infants. Accordingly, we hypothesize that adult-onset human narcolepsy entails reversion back toward the infantile pattern of fragmented sleep and wakefulness. It must be stressed, however, that because significant sleep-wake bout consolidation occurs in the absence of a functional orexinergic system, individuals neither retain nor revert to their earliest infantile sleep-wake patterns. Finally, it should be noted that the cascade of mechanisms that might link sleep-wake fragmentation with cataplexy remains to be examined.
In both rats and mice in vitro, orexin is able to modulate synaptic activity as early as the day of birth, at which time orexin receptor mRNA is detectable (van den Pol et al., 2001; van den Pol et al., 2002). But the orexin system exhibits substantial developmental changes postnatally in rats. Specifically, prepro-orexin mRNA is only weakly expressed in the hypothalamus through P15, increases dramatically through P20, and largely stabilizes thereafter (de Lecea et al., 1998; Yamamoto et al., 2000). Importantly, as shown in Figure 4, the timing of sleep-wake developmental events seen here in mice is similar to that found in a previous study in rats (Blumberg et al., 2005b). Thus, we hypothesize that increased availability of orexin during the third postnatal week accounts for the differences in sleep-wake patterning observed here between orexin knockout and wild-type mice.
Figure 4.
Survivor plots of sleep and wake bout durations for the P2-4 and P20-22 wild-type mice (solid lines) in the present study and P2 and P21 Norway rats (dotted lines) from a previous study (Blumberg et al., 2005b). The data for the mice are replotted from Figure 2. All plots were constructed using data pooled from multiple subjects. Recall that the gestation length of mice is 3 days shorter than that of rats. Regardless, the distributions are remarkably similar, including the development of wake-related power-law behavior by P21.
In a previous study using 11-week-old mice from the same population of animals used in the present study, it was concluded that the fragmented sleep and wakefulness of orexin knockouts reflects behavioral state instability produced by lowered transition thresholds between states (Mochizuki et al., 2004). Our results with infants are broadly consistent with that conclusion. It should be noted, however, that comparisons between that earlier study and the present one make clear that the sleep-wake patterns of knockouts and wild-types diverge further after P21. A substantial proportion of this further divergence will likely be due to the nighttime increase in mean wake bout durations in wild-types (Mochizuki et al., 2004), which was not yet detected at P21. In future work, as behavioral state development in these animals is explored beyond P21, it will be important to use uniform testing procedures and methods of analysis.
The most salient circadian difference detectable at P21 was the flattening of the light-dark difference in sleep bout duration in the orexin knockouts in relation to the wild-types. This flattening is consistent with (a) the effects seen in adult rats after orexin-specific (Gerashchenko et al., 2001) and non-specific (Chou et al., 2003) lesions of the orexin-concentrating lateral and dorsomedial hypothalamus (DMH), and (b) the notion that the DMH, through its interactions with the suprachiasmatic nucleus (SCN), modulates circadian rhythmicity (Aston-Jones et al., 2001; Saper et al., 2005).
The decreasing fragmentation of sleep-wake organization across early infancy reflects, at least in part, the increasing modulatory control of hypothalamic mechanisms over the basic brainstem circuit that governs sleep and wakefulness in newborns (Karlsson et al., 2004; Karlsson & Blumberg, 2005; Karlsson et al., 2005; Mohns et al., 2006). These hypothalamic mechanisms likely include the DMH, the SCN, and the ventrolateral preoptic area (Aston-Jones et al., 2001; Saper et al., 2001; Saper et al., 2005). In particular, based on work in adult animals and the evidence from individuals with narcolepsy, it has been suggested that the orexinergic system plays a critical role in the stabilization of the sleep-wake “flip-flop” switch (Saper et al., 2001). One impression given by this model is that stabilization of states do not occur in the absence of orexin. The present results, however, indicate that a functioning orexinergic system is not necessary for infants to consolidate sleep and wakefulness over levels seen in newborns. Nor is the orexinergic system necessary for the development of the power-law behavior that characterizes wake bouts in all adult mammalian species studied thus far (Lo et al., 2002; Lo et al., 2004). Thus, the interpretation that is most consistent with the available information, including that from the present study, is that orexin is an important modulator of sleep and wakefulness, enhancing stability beyond that attained during early infancy.
ACKNOWLEDGMENTS
Supported by grants from the National Institute of Mental Health (MH50701, MH66424) to M.S.B. We thank Tom Scammell for generously providing adult heterozygotes for breeding and Takatoshi Mochizuki for technical advice. Eric Devor and Becky Dill-Devor of Integrated DNA Technologies provided invaluable PCR support and guidance. We thank Jessica Middlemis-Brown, Jeremy Duncan, and Jeff Anderson for technical assistance. Adele Seelke kindly provided many helpful comments and suggestions. We are also very grateful to Jerry Siegel and Adele Seelke for helpful comments on an earlier draft of this manuscript.
ABBREVIATIONS
- DMH
dorsomedial hypothalamus
- EMG
electromyogram
- KO
knockout
- P
postnatal day
- SCN
suprachiasmatic nucleus
- WT
wild-type
REFERENCES
- Abbey H, Howard E. Statistical procedure in developmental studies on species with multiple offspring. Developmental Psychobiology. 1973;6:329–335. doi: 10.1002/dev.420060406. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nature Neuroscience. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Karlsson KÆ, Seelke AMH, Mohns EJ. The ontogeny of mammalian sleep: A response to Frank and Heller (2003) Journal of Sleep Research. 2005a;14:91–101. doi: 10.1111/j.1365-2869.2004.00430_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Sciences. 2005b;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. Journal of Neuroscience. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. Journal of Neuroscience. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. American Journal of Physiology. 1997;273:R472–R478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. Journal of Neuroscience. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Developmental Psychobiology. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Deboer T, Achermann P. Development of the 24-h rest-activity pattern in human infants. Infant Behavior and Development. 2006;29:143–152. doi: 10.1016/j.infbeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Maidment NT, Lam HA, Boehmer LN, Patton M, Siegel JM. Developmental changes in CSF hypocretin-1 (orexin-A) levels in normal and genetically narcoleptic Doberman pinschers. Journal of Physiology. 2004;560:587–592. doi: 10.1113/jphysiol.2004.070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3:891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Kreider JC, Blumberg MS. Hypothalamic contribution to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Engelmann TG. Sleep characteristics of infants. Journal of Applied Physiology. 1953;6:269–282. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lo CC, Amaral LAN, Havlin S, Ivanov PC, Penzel T, Peter JH, Stanley HE. Dynamics of sleep-wake transitions during sleep. Europhysics Letters. 2002;57:625–631. [Google Scholar]
- Lo CC, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov PC. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proceedings of the National Academy of Sciences. 2004;101:17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. Journal of Neuroscience. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Karlsson KÆ, Blumberg MS. The preoptic area and basal forebrain play opposing roles in the descending modulation of sleep sleep and wakefulness in infant rats. European Journal of Neuroscience. 2006;23:1301–1310. doi: 10.1111/j.1460-9568.2006.04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Medicine. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl J, Nishino S, Cederberg R, Dement WC, Mignot E. Development of cataplexy in genetically narcoleptic Dobermans. Experimental Neurology. 1998;152:292–302. doi: 10.1006/exnr.1998.6847. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends in Neurosciences. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements, and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Moore R, Thannickal R, Nienhuis R. A brief history of hypocretin/orexin and narcolepsy. Neuropsychopharmacology. 2001;25:S14–S20. doi: 10.1016/S0893-133X(01)00317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annual Review of Neuroscience. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Narcolepsy: a neurodegenerative disease of the hypocretin system? Neuron. 2000;27:415–418. doi: 10.1016/s0896-6273(00)00050-7. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. Journal of Physiology. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: Early developmental expression and response to hypocretin (orexin) Journal of Comparative Neurology. 2001;433:349–363. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ueta Y, Hara Y, Serino R, Nomura M, Shibuya I, Shirahata A, Yamashita H. Postnatal development of orexin/hypocretin in rats. Brain Research Molecular Brain Research. 2000;78:108–119. doi: 10.1016/s0169-328x(00)00080-2. [DOI] [PubMed] [Google Scholar]