Abstract
Background
Current biological concepts of borderline personality disorder (BPD) emphasize the interference of emotional hyperarousal and cognitive functions. A prototypical example is episodic memory. Pre-clinical investigations of emotion–episodic memory interactions have shown specific retrograde and anterograde episodic memory changes in response to emotional stimuli. These changes are amygdala dependent and vary as a function of emotional arousal and valence.
Method
To determine whether there is amygdala hyper-responsiveness to emotional stimuli as the underlying pathological substrate of cognitive dysfunction in BPD, 16 unmedicated female patients with BPD were tested on the behavioural indices of emotion-induced amnesia and hypermnesia established in 16 healthy controls.
Results
BPD patients displayed enhanced retrograde and anterograde amnesia in response to presentation of negative stimuli, while positive stimuli elicited no episodic memory-modulating effects.
Conclusion
These findings suggest that an amygdala hyper-responsiveness to negative stimuli may serve as a crucial aetiological contributor to emotion-induced cognitive dysfunction in BPD.
INTRODUCTION
More than any other species, humans are beneficiaries and victims of emotion. A lack of emotional equilibrium is a common denominator across psychiatric conditions, with particular significance in borderline personality disorder (BPD) (Skodol et al. 2002a,b). The BPD clinical phenotype is characterized by an emotionally unstable and impulsive cognitive and behavioural style (Domes et al. 2006), which has led to suggestions that the core pathology of BPD is a hyperarousal-dyscontrol syndrome (Lieb et al. 2004).
To date, no specific cognitive dysfunction in BPD has been identified (Kunert et al. 2003; Ruocco, 2005). However, current theories of BPD emphasize the disruptive potential of negative emotion on cognition (Fertuck et al. 2006). One cognitive domain where the interference with emotion can be characterized is episodic (autobiographical) memory (Dolan, 2002; McGaugh, 2004; LaBar & Cabeza, 2006; Phelps, 2006). Functional imaging studies of the associated neural circuitry have revealed amygdala–hippocampal interactions during emotional episodic memory encoding, with hippocampal circuits being modulated by amygdala input (Kilpatrick & Cahill, 2003; Dolcos et al. 2004 a; Kensinger & Corkin, 2004; Richardson et al. 2004). One classic experimental design to study amygdala–hippocampal connectivity is the subsequent memory paradigm, which demonstrates variations in recall of emotional items as a function of amygdala activation at encoding of these items (Cahill et al. 1996; Hamann et al. 1999; Canli et al. 2000; Kilpatrick & Cahill, 2003; Dolcos et al. 2004 a; Kensinger & Corkin 2004). A key neurochemical mediator of this effect is norepinephrine (NE), which has its major source in the locus coeruleus (LC) (Berridge & Waterhouse, 2003; Aston-Jones & Cohen, 2005). Notably, the amplification of emotional memory encoding by endogenous LC–NE release can be further augmented by NE agonists such as reboxetine (Harmer et al. 2003, 2004) and blocked by NE antagonists such as propranolol (Strange et al. 2003; Strange & Dolan, 2004; van Stegeren et al. 2005).
Amygdala lesion eliminates the episodic memory advantage of emotional stimuli as does amygdala suppression with propranolol, indicating a key intra-amygdalar role for LC–NE in enhancing emotional memory encoding (Strange et al. 2003; Strange & Dolan, 2004; van Stegeren et al. 2005). The psychological ‘costs and benefits’ of such enhancement have been modelled with free recall tests of episodic memory, in which facilitated encoding of a heterogeneous item (oddball) presented within a train of homogeneous standard items (the von Restorff effect) (von Restorff, 1933; Wallace, 1965) interferes with the encoding of preceding and following standard items, particularly if the oddball is emotional (Tulving, 1969; Angelini et al. 1994; Hurlemann et al. 2005). In keeping with a taxonomy of emotion along orthogonal dimensions of valence and arousal (Russell, 1980; Lang, 1995), retrograde interference is valence dependent in that emotionally negative items elicit amnesia and emotionally positive items elicit hypermnesia. By contrast, anterograde interference is arousal dependent, with emotionally negative and positive items eliciting amnesia. As for the emotional oddball effect per se, both the retrograde and anterograde episodic memory changes driven by this effect are under amygdala and LC–NE control (Hurlemann et al. 2005, 2006).
In BPD patients, episodic memory formation is biased towards enhanced encoding of emotionally negative items, possibly resulting from LC–NE (Skodol et al. 2002b) and amygdala hyper-responsiveness to negative emotion (Herpertz et al. 2001; Donegan et al. 2003). Moreover, emotional instability and impulsivity increase under treatment with the NE agonist reboxetine (Anghelescu et al. 2005) and decrease under treatment with the NE antagonist clonidine (Philipsen et al. 2004). Furthermore, challenge studies with the NE agonist yohimbine in controls have linked elevated noradrenergic tone to increased impulsivity (Swann et al. 2005). These observations provide the basis for our experimental hypothesis that BPD patients would display greater retrograde and anterograde amnesia in response to negative emotion as compared to controls. Such a finding would indicate LC–NE hyperactivation of the amygdala as an aetiological contributor to emotion-induced cognitive dysfunction in BPD. Thus, we tested 16 unmedicated female patients with BPD on a robust laboratory index of emotion-induced amnesia and hypermnesia established in healthy controls.
METHOD
Subjects
Informed written consent was obtained from all patients and controls after a complete and extensive description of the study, which was approved by the Ethics Committee of the University of Bonn in accordance with the principles of the Declaration of Helsinki (Rickham, 1964). The study included 16 self-harming female out-patients (age range 19.5–34.5 years; mean age 25.2¡4.6 years) treated at the Department of Psychiatry, University of Bonn. In clinical populations, BPD occurs predominantly in females (Lieb et al. 2004). Patients fulfilled both DSM-IV diagnostic criteria for BPD and BPD criteria from the Revised Diagnostic Interview for BPD (DIP-R; Gunderson & Zanarini, 1983). Structured Clinical Interviews for DSM-IV Diagnoses (SCID I and II; Wittchen et al. 1997) were performed to exclude lifetime diagnosis of Axis I and II co-occurring disorders as a potential confounding factor in the assessment of emotion–episodic memory interactions. The Global Assessment of Functioning (GAF) Scale as part of the DSM-IV Axis V assessment served to evaluate patients' overall level of psychological, social and occupational functioning (APA, 1994).
Cutting, as the most common form of self-harm, provided an objective index of impulsive behaviour (Herpertz et al. 1997) and allowed us to recruit a homogeneous patient sample (Berlin et al. 2005; Domes et al. 2006). BPD patients were drug-naïve (n =6) or free of psychotropic medication for 4 weeks (n= 10). Sixteen female control subjects (age range 20.3–34.2 years; mean age 25.6¡4.3 years), matched for age and educational status and determined to be free of personal as well as first-degree family history of DSM-IV Axis I and II disorders, were enrolled in the study. Neither patients nor controls were included if they had current substance or alcohol abuse or a history of neurological or severe somatic disorder. Controls had not been on psychotropic medication for 12 weeks.
To ensure that potential abnormalities in our test of emotion–episodic memory interactions could not be attributed to deficits in visual, verbal and cognitive functions, both patients and controls underwent brief neuropsychological screening. This included the Verbaler Lern- und Merkfähigkeitstest (VLMT), a German version of the Rey Auditory Verbal Learning Test, to assess immediate verbal learning span, new learning, susceptibility to interference, and recognition memory (Helmstaedter et al. 2001). The Rey–Osterrieth Complex Figure Test (CFT) was used to test incidental visual memory and the visuospatial constructional ability (Rey, 1941; Osterrieth, 1944). Motor speed and visual attention were examined with the Trail Making Test, Part A (TMT-A; Raitan, 1958). The Wortschatztest (WST), a vocabulary test implemented in the Hamburg–Wechsler Intelligenztest für Erwachsene (HAWIE-R), a German adaptation of the Wechsler Adult Intelligence scale, provided an estimate for intelligence (IQ) (Tewes, 1991). The demographic and neuropsychological data for the patient and control groups are reported in Table 1.
Table 1. Demographics, neuropsychological and diagnostic data for the samplea.
| CTL (n= 16) |
BPD (n= 16) |
||
|---|---|---|---|
| Demographics | |||
| Age, years | 25·6 (4·6) | 25·2 (4·3) | |
| Female sex | 16 | 16 | |
| Height, cm | 168·4 (6·7) | 167·1 (5·4) | |
| Weight, kg | 64·4 (7·1) | 68·1 (14·5) | |
| Education, years | 11·9 (1·5) | 11·6(1·3) | |
| IQ (WST) | 105·6 (9·3) | 106·8 (10·5) | |
| With partner | 10 | 8 | |
| Employed | 15 | 14 | |
| Neuropsychological data | |||
| VLMT Trial 1–5 | 51·94 (3·26) | 52·49 (3·44) | |
| Rey-Osterrieth CFT Copy | 33·95 (3·54) | 34·36 (3·92) | |
| Rey–Osterrieth CFT Delay | 22·81 (2·97) | 23·16 (3·21) | |
| TMT-A | 25·33 (4·92) | 25·91 (4·68) | |
| Diagnostic data | |||
| GAF | — | 76·63 (5·72) | |
| DIB-R scaled | — | 7·80 (0·80) | |
| HAMD-21 | — | 6·10 (3·10) | |
| FAF total | — | 23·19 (7·77) | |
| BIS-11 total | — | 85·63 (11·48) | |
| BDHI total | — | 43·40 (10·50) | |
| FDS | — | 20·66 (15·86) | |
| CTQ | — | 82·32 (17·97) | |
| Medication-free/-naïve | 0/16 | 10/6 | |
| Psychiatric hospitalization | 0 | 7 | |
| Self-injury | 0 | 16 | |
| Previous suicide attempt | 0 | 7 | |
Data are given as mean (standard deviation) unless otherwise specified.
CTL, Controls; BPD, borderline personality disorder; IQ (WST), Wortschatztest, a vocabulary test implemented in the HAWIE-R (German adaptation of the Wechsler Adult Intelligence Scale); VLMT, Verbaler Lern- und Merkfähigkeitstest (German version of the Rey Auditory Verbal Learning Test); CFT, Rey–Osterrieth Complex Figure Test; TMT-A, Trail Making Test (part A); GAF, Global Assessment of Functioning; DIB-R, Revised Diagnostic Interview for Borderline personality disorder; HAMD-21, Hamilton Depression Scale; FAF, Questionnaire for Measuring Factors of Aggression; BIS-11, Barrat Impulsiveness Scale; BDHI, Buss–Durkee Hostility Inventory; CTQ, Childhood Trauma Questionnaire; FDS, Fragebogen für Dissoziative Symptome (German version of the American Dissociative Experiences Scale).
Diagnostic instruments and questionnaires
BPD patients were characterized using a battery of clinician- and self-rating tools. State euthymia was confirmed by a score <10 on the Hamilton Depression Scale (HAMD-21; Hamilton, 1960). The Questionnaire for Measuring Factors of Aggression, Fragebogen zur Erfassung von Aggressivitätsfaktoren (FAF; Hampel & Selg, 1975), the Barrat Impulsiveness Scale (BIS-11; Patton et al. 1995) and the Buss–Durkee Hostility Inventory (BDHI; Buss & Durkee, 1957) were administered to assess aggressive and impulsive behaviour. The Fragebogen für Dissoziative Symptome (FDS), a German adaptation of the American Dissociative Experiences Scale, served to determine the frequency of dissociative symptoms (Freyberger et al. 1998). The Childhood Trauma Questionnaire (CTQ) was used to investigate prevalence of childhood trauma and relations between childhood trauma and dissociation in adult life (Bernstein et al. 1994). The diagnostic data for the patient group are reported in Table 1.
Episodic memory test
The experimental design and stimulus set-up have been detailed elsewhere (Hurlemann et al. 2005) and are described in abbreviated form here. Subjects were exposed to 36 study-distraction-test sequences. During each 40-s study phase, a von Restorff list composed of one oddball and seven standard stimuli in the form of picture items paired with their verbal descriptors was presented. Standard stimuli included black-and-white line drawings of living and non-living entities (Snodgrass & Vanderwart, 1980; Cycowitz et al. 1997), while oddballs included images primarily selected and edited from the International Affective Picture System (IAPS; Lang et al. 2005). Of 36 oddballs implemented in the paradigm, 12 were perceptual-neutral (P), and 24 were perceptual-emotional (ExP), including 12 positive (EposP) and 12 negative (EnegP) oddballs. EposP and EnegP oddballs differed from each other in terms of valence, but were matched for arousal. ExP oddballs differed from P oddballs in terms of valence and arousal. Each von Restorff list was followed by a 30-s arithmetic distraction task, before episodic memory was tested by free recall.
Recall profiles were pooled according to the three oddball types, thus yielding a neutral, positive and negative condition. As outcome parameter, memory performance was determined condition-wise by calculating the percentage of correct recall (i.e. the output/input ratio) for the following three list positions: oddball ×1, oddball, and oddball + 1. Additionally, a list index (LI) based on the seven non-oddball list positions was calculated for each condition (e.g. LIp). Contrasting the emotional conditions (ExP) with the neutral condition (P) (according to ExP – P = Ex) allowed us to isolate retrograde and anterograde effects generated by positive (Epos) and negative (Eneg) emotion on the adjacent standard item (E x± 1) corresponding to a time window of ± 5 s. The design and timeline of the episodic memory test are illustrated in Fig. 1 a, b.
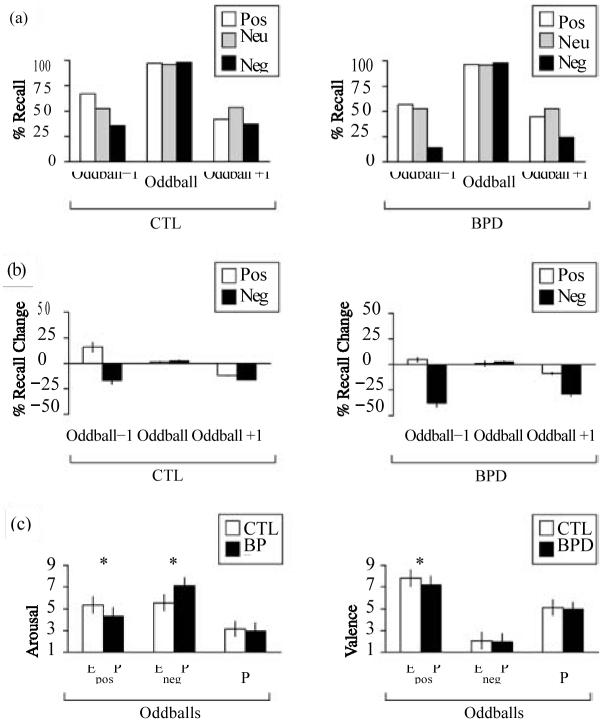
Fig. 1.
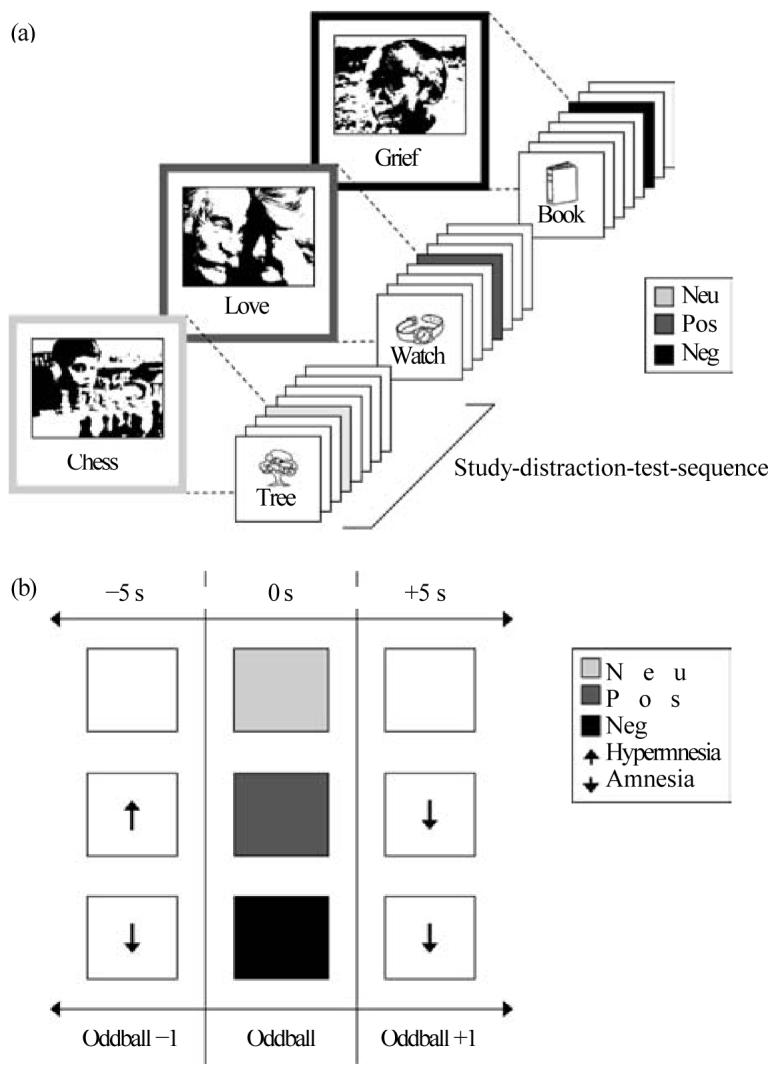
Episodic memory test. (a) Timeline. Participants were exposed to 36 study-distraction-test sequences. During each 40-s study phase, they were presented with a list of eight items, including seven standard items and one oddball inserted on list position 3, 4, 5 or 6. After a 30-s arithmetic distraction task, encoding strength for the eight list items was tested by free recall. (b) Analysis. In each list, one oddball, either emotional (Ex: positive, EposP; negative, EnegP) or neutral (P), was temporally flanked by a preceding (oddballx1) and following (oddball +1) standard item. Results from the list recall were pooled according to the three oddball types, yielding a positive, negative and neutral condition. Contrasting the positive and negative conditions with the neutral condition (according to ExP × P = Ex) allowed us to quantify retrograde and anterograde episodic memory-modulating effects of positive and negative emotion within a time window of Ex¡1 items or ¡5s. Positive oddballs (EposP) interfere with Ex¡1 encoding in the form of retrograde memory increments (hypermnesia) and anterograde memory decrements (amnesia), while negative oddballs (EnegP) provoke both retrograde and anterograde memory decrements (amnesia). Neg, negative; neu, neutral; pos, positive.
While our previous paradigm was devised to investigate Eneg−1 retrograde effects as a function of emotional oddball effects (Strange et al. 2003), the present paradigm was optimized to dissociate the contributions of emotional valence and arousal to Ex−1 retrograde and Ex+ 1 anterograde effects by using a subtractive design (Hurlemann et al. 2005). As BPD might be associated with potential changes in the cognitive appraisal of emotion, patients and controls both performed paper-and-pencil valence and arousal ratings to ExP and P oddballs on a nine-point scale after the episodic memory test.
Statistics
Demographic and neuropsychological data obtained from the patient and control groups were compared on the basis of two-sample t tests. The episodic memory test resulted in different recall scores (Ex, Ex± 1, LIx) for the emotional (positive, negative) conditions, which were analysed in relation to the corresponding recall scores (P, P ± 1, LIP) for the neutral condition. Two-factor within-subject and three-factor mixed analyses of variance (ANOVAs) were followed by two-tailed one-sample and two-sample t tests to determine the source of significance. The Greenhouse–Geisser correction for inhomogeneity of variance was applied whenever a sphericity assumption was violated. To account for an inflation of the type I error attributable to multiple post hoc testing, the threshold for significance was Bonferroni adjusted.
RESULTS
As summarized in Table 1, patients (BPD, n =16) were euthymic, medication-naive (n =6) or medication-free for o4 weeks (n =10), and demonstrated a high level of psychological, social and occupational functioning, when tested on the behavioural measures of Ex± 1 modulatory effects established in controls (CTL, n =16). The lack of between-group differences with respect to demographic or neuropsychological variables (two-sample t tests, p>0.05) confirmed careful matching of patients and controls.
Analysis of the episodic memory test
The percentages of mean recall (± S.D.) for oddballs and standard items (collapsed across the positive, negative and neutral conditions) were as follows: CTL, 96.88 (4.15) and 56.47 (7.21); BPD, 96.70 (2.42) and 51.27 (7.87). One-sample t tests confirmed the presence of near-ceiling von Restorff effects within each group: CTL, t(15)=24.372, p<0.0001; BPD, t(15)=19.455, p<0.0001. A one-way ANOVA with group (CTL, BPD) as the between-subjects factor showed no difference in SIP scores between groups [F(1, 31) = 0.8 19, p >0.05], that is BPD did not compromise an ability to recall standard items in the neutral condition.
An exploratory condition (positive, negative, neutral) × position (oddball, oddball ¡1) 3 × 3 ANOVA restricted to the CTL group yielded effects of valence [F(2, 30)= 13.408, p<0.0001], position [F(2, 30)=369.463,p<0.0001] and condition × position interaction [F(4, 60) = 14.235, p <0.0001) effects. As depicted in Fig. 2 a, b, post hoc one-sample t tests confirmed the presence of retrograde Eneg–1 amnesic (−16.67 %) [t(15) = −3.130, p=0.007] and Epos–1 hypermnesic (+ 15.62%) [t(15)=3.101, p=0.007] as well as anterograde Eneg + 1 (−16.15%) [t(15) =−3.725, p = 0.002] and Epos+ 1 (−11.97%) [t(15) = −3.360, p = 0.004] amnesic effects. Additional session (first, second, third) × condition 3 × 3 within-subjects ANOVAs showed that recall performance for both oddballs [F(2, 30) = 1.328, p >0.05] and standard items [F(2, 30) = 1.542, p0.05] remained stable throughout the experiment. The pattern of memory increments and decrements measured in the CTL group paralleled those of previous investigations and served as baseline for subsequent comparisons.
Fig. 2.
Results of the episodic memory test and oddball ratings. (a) Percentage correct recall as determined in controls (CTL) and patients (BPD). Equal (near-ceiling) von Restorff effects were obtained in both the emotional and the neutral conditions. (b) Percentage of recall change in the emotional conditions relative to the neutral condition. Controls (CTL) displayed a characteristic pattern of emotion-induced memory increments and decrements: negative emotion elicited retrograde (−16.67%) and anterograde (−16.15%) amnesia, whereas positive emotion elicited retrograde (+ 15.62%) hypermnesia and anterograde (− 11.97%) amnesia. In patients (BPD), no episodic memory-modulating effects of positive emotion were present, whereas enlarged retrograde (−41.15%) and anterograde (−27.08%) amnesic effects of negative emotion were measured. (c) Oddball arousal and valence ratings. Increased arousal scores for negative oddballs (EnegP) in combination with decreased arousal and valence scores for positive oddballs (EposP) in patients (BPD) relative to controls (CTL) are consistent with a substantial negativity bias in the cognitive appraisal of emotion in BPD. Asterisks indicate significant rating differences between controls (CTL) and patients (BPD). neg, negative; neu, neutral; pos, positive; oddball ¡1, standard items preceding or following the oddball; EposP, positive oddball; P, neutral oddball; EnegP, negative oddball. Error bars indicate 1 S.E.
Analysing the influence of BPD diagnosis, a group (CTL, BPD) × condition × position 2 × 3 × 3 ANOVA yielded group [F(1, 30) = 11.972, p =0.002], condition [F(2, 60) = 60.160, p <0.0001], position [F(2, 60) = 793.660, p <0.000 1], two-way condition × group [F(2, 60) = 6.405, p = 0.003], position × group [F(2, 60) = 6.433, p =0.003], condition × position [F(4, 120) = 31.617,p < 0.0001), and three-way condition × position × group [F(4, 120) =4.090, p =0.004] interaction effects. Post hoc two-sample t tests demonstrated that Eneg−1 [t(30) =4.945, p <0.0001], Eneg+ 1 [t(30)= −3.196, p=0.003] and Epos–1 [t(30)=3.250, p=0.003] recall was different between BPD and CTL groups. A condition × position 3 × 3 ANOVA restricted to the BPD group yielded effects of valence [F(2, 30) =53.553, p <0.0001], position [F(2, 30) = 545.084, p <0.0001] and condition × position interaction [F(4,60)=21.769, p<0.0001] effects. Post hoc one-sample t tests revealed enhanced retrograde Eneg−1 (−41.15%) [t(15)=−8.399;p<0.0001] and anterograde Eneg+ 1 (−27.08%) [t(15)= −6.343, p<0.0001] amnesic effects in the BPD group. By contrast, neither retrograde nor anterograde episodic memory changes in response to positive oddballs were present. The percentage recall differences relative to the CTL group negative condition (relative to the CTL group neutral condition) were as follows: Eneg−1, −21.35 (−38.02); Eneg+ 1, −12.50 (−28.65) (Fig. 2a,b).
Analysis of emotion ratings
The paper-and-pencil oddball arousal and valence ratings (mean ±S.D.) obtained after memory testing resulted in the following scores: CTL group: EposP oddballs (5.35±0.76, 7.81 ± 0.75); EnegP oddballs (5.56±0.73, 2.06±0.77); P oddballs (3.15±0.69, 5.13±0.72). BPD patients: (4.31±0.79, 7.21±0.80); EnegP oddballs (7.15±0.75, 1.94±0.77); P oddballs (2.94±0.77, 4.96±0.65) (Fig. 2c). Separate group (CTL, BPD) × oddball type (positive, negative, neutral) 2 × 3 ANOVAs were calculated, yielding significant between-group effects on arousal scores [F(1, 1)=14.222, p=0.001) and valence scores [F(1, 1)=4.879, p=0.035]. Post hoc two-sample t tests demonstrated increased arousal scores for EnegP oddballs [t(30) = − 6.112, p <0.0001] as well as decreased arousal [t(30)=3.771, p=0.001] and valence [t(30)=2.209, p=0.035] scores for EposP oddballs in patients with BPD. This constellation of changes provides evidence for a robust negativity bias in the cognitive appraisal of emotional oddballs.
DISCUSSION
In the absence of uniquely defined and identifiable biological markers in BPD (Herpertz et al. 1999; Posner et al. 2002; Berlin et al. 2005), the current experiment focused on emotion-induced amnesia and hypermnesia as a potential cognitive index of a hyperarousal-dyscontrol syndrome in BPD. Our findings indicate enhanced Eneg − 1 retrograde and Eneg + 1 anterograde amnesia, relative to controls, in response to items that elicit negative emotion. This profile contrasts with a lack of predicted Epos−1 Epos+ 1 anterograde amnesic effects in response to items that elicit positive emotion. This constellation of hyper-responsiveness to negative emotion and hypo-responsiveness to positive emotion provides biological evidence that BPD is indeed characterized by enhanced processing of emotionally negative stimuli and relative lack of processing of emotionally positive stimuli. As the effects we observed were unrelated to task demands per se, they suggest that this aberrant processing is an obligatory feature in BPD.
In previous studies,Ex+ 1 anterograde effects have been interpreted as reflecting the psychological ‘cost’ of devoting attentional resources to preferential encoding of Ex emotional items (Hurlemann et al. 2005, 2006). Specifically, it has been suggested that during serial attentive and mnemonic processing, as required in the present paradigm, an amygdala-dependent Ex emotional item-induced capture of attention evokes a transient refractory period, of at least 5-s duration, which disrupts attentional reorienting and causes an inertia in encoding a following Ex+ 1 non-emotional item. Enhanced Eneg+ 1 anterograde amnesia in BPD may reflect such a process and would be in keeping with a role for the amygdala in focusing attention on Eneg emotional items. Enhanced attention to emotionally negative events thus may provide a basis for less processing capacity for encoding a competing non-emotional event. By contrast, BPD patients exhibit no Epos+ 1 anterograde amnesic effect (as seen in controls), a finding that may reflect a bias away from processing emotionally positive events. We note that near-ceiling von Restorff effects are preserved across valences in BPD patients, indicating no global deficit in directing attention to the perceptual ‘pop-out’ features of oddballs. This is consistent with reports of intact attentional faculties in non-depressed BPD patients under emotionally neutral experimental conditions (Posner et al. 2002; Lenzenweger et al. 2004).
While Ex+ 1 anterograde effects are thought to reflect an amygdala-driven attentional modulation of episodic encoding, Ex− 1 retrograde effects are most likely to result from a direct modulation of hippocampal circuits by amygdala and prefrontal cortex (PFC) inputs (Strange et al. 2003; Hurlemann et al. 2005, 2006). This is consistent with a prevailing concept that during emotional memory formation, the amygdala communicates Ex emotional item-evoked arousal to the hippocampus, thus rendering it susceptible to valence transfer from the PFC (Dolcos et al. 2004b; Kensinger, 2004; Kensinger, & Corkin, 2004). According to this model, PFC input is crucial for a differential expression of Eneg − 1 retrograde amnesic versus Epos−1 retrograde hypermnesic effects, whereas the magnitude of these effects is amygdala dependent.
The enhancement of Eneg¡1 modulatory effects in the present study is compatible with abnormal low-threshold and high-amplitude amygdala responses to aversive facial expressions (Donegan et al. 2003) and IAPS items (Herpertz et al. 2001) in BPD. We suggest that this negative emotion response bias in BPD is compatible with diminished reactivity of the PFC circuits involved in producing positive emotion and/or regulating negative emotion. Evidence for this view comes from neuroimaging studies in BPD patients indicating PFC alterations at baseline (Goyer et al. 1994; De La Fuente et al. 1997; Lyoo et al. 1998; Juengling et al. 2003) as well as in response to negative emotion (Herpertz et al. 2001; Donegan et al. 2003) and serotonergic (5-HT) challenges (Soloff et al. 2000; Leyton et al. 2001). Further evidence links BPD to functional and structural abnormalities in PFC subareas such as the orbital prefrontal cortex (OFC) (Berlin et al. 2005), specifically hypometabolism (Goyer et al. 1994; De La Fuente et al. 1997; Soloff et al. 2003) and decreased volume (Lyoo et al. 1998; Tebartz van Elst et al. 2003). OFC dysfunction in BPD would be compatible with deficient computation of positive emotion in this subregion (Dolcos et al. 2004b). Together, these lines of evidence provide support for the hypothesis of dual amygdala and PFC pathology as the aetiological contributor to a hyperarousal-dyscontrol syndrome in BPD (Lieb et al. 2004).
The degree of inhibitory control over negative emotion is a potent modulator of its psychological impact. To date, research on controllability has largely focused on the monoamine brainstem nuclei such as the 5-HT dorsal raphénucleus (DRN) and the LC (Amat et al. 2005). As LC–NE signalling is under PFC top-down inhibitory control (Arnsten & Goldman-Rakic, 1984; Weiss & Simson, 1986; Jodo et al. 1998; Berridge & Waterhouse, 2003; Aston-Jones & Cohen, 2005) and determines the magnitude of Ex¡1 effects (Strange et al. 2003; Hurlemann et al. 2005), enhanced Eneg−1 retrograde amnesic effects in the present study may reflect deficient regulation of LC–NE bottom-up input to the amygdala. We suggest that such phasic hyperactivation of the amygdala is crucial for emotion-induced cognitive dysfunction in BPD.
On the basis of our results we speculate that interventions aimed at normalizing negativity bias and increasing PFC top-down inhibitory control provide an effective treatment strategy to influence amygdala hyper-responsiveness to negative emotion in BPD. We note that NE antagonists such as clonidine (Philipsen et al. 2004) are reported as exerting beneficial effects on emotional instability and impulsivity in BPD, underscoring the link between these symptoms and increased noradrenergic tone (Arnsten et al. 1999; Swann et al. 2005). By contrast, further exogenous elevation of NE levels with NE agonists such as reboxetine may provoke an exacerbation of emotional instability and impulsivity in BPD (Anghelescu et al. 2005). Deficient PFC capacity to down-regulate negative emotion in BPD is also susceptible to psychotherapeutical strategies aimed at controlling attention to, and changing the subjective interpretation of, aversive events (Lieb et al. 2004). Such improved cognitive control in BPD may significantly decrease LC–NE hyperactivation of the amygdala in response to negative emotion and neutralize its disruptive effects on cognition (Jackson et al. 2000; Ochsner & Gross, 2005).
ACKNOWLEDGEMENTS
We thank M. Pankonin for excellent assistance and K. Vogeley for inspiring discussions. R. Hurlemann was supported by BONFOR and the German Federal Ministry of Education and Research (BMBF).
REFERENCES
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Angelini R, Capozzoli F, Lepore P, Grossi D, Orsini A. Experimental amnesia induced by emotional items. Perceptual and Motor Skills. 1994;78:19–28. doi: 10.2466/pms.1994.78.1.19. [DOI] [PubMed] [Google Scholar]
- Anghelescu I, Janen B, Schindler F, Lammers CH. Worsening of borderline symptoms under reboxetine treatment. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:9–560. doi: 10.1176/jnp.17.4.559. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. 4th edn American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Arnsten AF, Goldman-Rakic P. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nucleus in the rhesus monkey. Brain. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biological Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. American Journal of Psychiatry. 2005;162:2360–2373. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of Consulting Psychology. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Rothstein M, Snodgrass JG. Picture naming by young children: norms for name agreement, familiarity, and visual complexity. Journal of Experimental Child Psychology. 1997;65:171–237. doi: 10.1006/jecp.1996.2356. [DOI] [PubMed] [Google Scholar]
- De La Fuente JM, Goldman S, Stanus E, Vizuete C, Morlan I, Bobes J, Mendlewicz J. Brain glucose metabolism in borderline personality disorder. Journal of Psychiatric Research. 1997;31:531–541. doi: 10.1016/s0022-3956(97)00001-0. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004a;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004b;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Winter B, Schnell K, Vohs K, Fast K, Herpertz SC. The influence of emotions on inhibitory functioning in borderline personality disorder. Psychological Medicine. 2006;36:1163–1172. doi: 10.1017/S0033291706007756. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Fertuck EA, Lenzenweger MF, Clarkin JF, Hoermann S, Stanley B. Executive neurocognition, memory systems, and borderline personality disorder. Clinical Psychology Review. 2006;26:346–375. doi: 10.1016/j.cpr.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Freyberger HJ, Spitzer C, Stieglitz RD, Kuhn G, Magdeburg N, Bernstein-Carlson E. Questionnaire on dissociative symptoms. German adaptation, reliability and validity of the American Dissociative Experience Scale (DES) Psychotherapie, Psychosomatik, Medizinische Psychologie. 1998;48:223–229. [PubMed] [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, Schulz SC, Cohen RM. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Zanarini MC. The Revised Diagnostic Interview for Borderlines (DIB-R) McLean Hospital; Belmont, MA: 1983. [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel R, Selg H. FAF– The Questionnaire for Measuring Factors of Aggression [in German] Hogrefe; Göttingen: 1975. [Google Scholar]
- Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. American Journal of Psychiatry. 2003;160:990–992. doi: 10.1176/appi.ajp.160.5.990. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest (VLMT) Hogrefe; Gottingen: 2001. [Google Scholar]
- Herpertz S, Sass H, Favazza A. Impulsivity in self-mutilative behavior: psychometric and biological findings. Journal of Psychiatric Research. 1997;31:451–465. doi: 10.1016/s0022-3956(97)00004-6. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Kunert HJ, Schwenger UB, Sass H. Affective responsiveness in borderline personality disorder: a psychophysiological approach. American Journal of Psychiatry. 1999;156:1550–1556. doi: 10.1176/ajp.156.10.1550. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, Vogeley K, Maier W, Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Vogeley K, Wagner M, Pieperhoff P, Amunts K, Oros-Peusquens AM, Shah NJ, Maier W, Dolan RJ. Amygdala control of emotion-induced forgetting and remembering: evidence from Urbach-Wiethe syndrome. Neuropsychologia. 2006;45:877–884. doi: 10.1016/j.neuropsychologia.2006.08.027. Published online 5 October 2006. PMID: 17027866. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Schmahl C, Hesslinger B, Ebert D, Bremner JD, Gostomzyk J, Bohus M, Lieb K. Positron emission tomography in female patients with borderline personality disorder. Journal of Psychiatric Research. 2003;37:109–115. doi: 10.1016/s0022-3956(02)00084-5. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: the contribution of valence and arousal. Reviews in the Neurosciences. 2004;15:241–251. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kunert HJ, Druecke HW, Sass H, Herpertz SC. Frontal lobe dysfunctions in borderline personality disorder? Neuropsychological findings. Journal of Personality Disorders. 2003;17:497–509. doi: 10.1521/pedi.17.6.497.25354. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe. Studies of motivation and attention. The American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. University of Florida; Gainesville, FL: 2005. (Technical Report A-6). [Google Scholar]
- Lenzenweger MF, Clarkin JF, Fertuck EA, Kernberg OF. Executive neurocognitive functioning and neurobehavioral systems indicators in borderline personality disorder: a preliminary study. Journal of Personality Disorders. 2004;18:421–438. doi: 10.1521/pedi.18.5.421.51323. [DOI] [PubMed] [Google Scholar]
- Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, Young SN, Blier P, Benkelfat C. Brain regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. American Journal of Psychiatry. 2001;158:775–782. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Han MH, Cho DY. A brain MRI study in subjects with borderline personality disorder. Journal of Affective Disorders. 1998;50:235–243. doi: 10.1016/s0165-0327(98)00104-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Philipsen A, Richter H, Schmahl C, Peters J, Rusch N, Bohus M, Lieb K. Clonidine in acute aversive inner tension and self-injurious behavior in female patients with borderline personality disorder. Journal of Clinical Psychiatry. 2004;65:1414–1419. doi: 10.4088/jcp.v65n1018. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Vizueta N, Levy KN, Evans DE, Thomas KM, Clarkin JF. Attentional mechanisms of borderline personality disorder. Proceedings of the National Academy of Sciences USA. 2002;99:16366–16370. doi: 10.1073/pnas.252644699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitan RM. Validity of the trail making test as an indication of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. Archives de Psychologie. 1941;30:286–340. [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Rickham PP. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. British Medical Journal. 1964;5402:177–178. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco AC. The neuropsychology of borderline personality disorder: a meta-analysis and review. Psychiatry Research. 2005;137:191–202. doi: 10.1016/j.psychres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002 a;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Siever LJ, Livesley WJ, Gunderson JG, Pfohl B, Widiger TA. The borderline diagnosis II: biology, genetics, and clinical course. Biological Psychiatry. 2002b;51:951–963. doi: 10.1016/s0006-3223(02)01325-2. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology. Human Learning. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Research. 2003;123:153–163. doi: 10.1016/s0925-4927(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biological Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences USA. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biological Psychiatry. 2005;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Tewes U. Hamburg- Wechsler-Intelligenztest fur Erwachsene – Revision 1991. Hogrefe; Gottingen: 1991. [Google Scholar]
- Tulving E. Retrograde amnesia in free recall. Science. 1969;164:88–90. doi: 10.1126/science.164.3875.88. [DOI] [PubMed] [Google Scholar]
- von Restorff H. Ueber die Wirkungen von Bereichsbildung im Spurenfeld. Psychologische Forschung. 1933;18:299–342. [Google Scholar]
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JP, Rombouts SA. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Wallace WP. Review of the historical empirical and theoretical status of the von Restorff phenomenon. Psychological Bulletin. 1965;63:410–424. doi: 10.1037/h0022001. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PG. Depression in an animal model: focus on the locus ceruleus. Ciba Foundation Symposium. 1986;123:191–215. doi: 10.1002/9780470513361.ch11. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Zaudig M, Fydrich T. Structured Clinical Interviews for DSM-IV Diagnoses (SCID I and II) [in German] Hogrefe; Göttingen: 1997. [Google Scholar]