Abstract
In recent years, research applying functional neuroimaging to the study of cue-elicited drug craving has emerged. This research has begun to identify a distributed system of brain activity during drug craving. A review of this literature suggested that expectations regarding the opportunity to use a drug affected the pattern of neural responses elicited by drug cues. Using functional magnetic resonance imaging (fMRI), we examined the effects of smoking expectancy on the neural response to neutral (e.g., roll of tape) and smoking-related (a cigarette) stimuli in male cigarette smokers deprived of nicotine for 8 hr. As predicted, several brain regions (e.g., the anterior cingulate cortex) exhibited differential activation during cigarette versus neutral cue exposure. Moreover, we found that subregions of the prefrontal cortex (i.e., ventromedial, ventrolateral, and dorsolateral prefrontal cortices) showed cue-elicited activation that was modulated by smoking expectancy. These results highlight the importance of perceived drug use opportunity in the neurobiological response to drug cues.
Introduction
Drug craving remains a construct of central interest to addiction researchers. Efforts to elucidate craving have been improved in recent years with the advent of functional neuroimaging technologies. Methods such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) provide a means for directly investigating the neural substrates of craving in humans. Within the past decade, brain imaging studies examining craving have proliferated. The majority of these studies have measured the blood flow response in individuals addicted to drugs while they are presented with drug-related stimuli designed to elicit craving (e.g., pictures of drug paraphernalia). Thus far, a distributed system of brain regions has been associated with cue-elicited urge, including medial temporal lobe structures (amygdala, hippocampus, parahippocampus), orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC) (Franken, 2003; Hommer, 1999; See, 2002; Wilson, Sayette, & Fiez, 2004).
It has been demonstrated behaviorally that craving may be modulated by the context associated with cue presentation, including whether participants anticipate using the drugs to which they are being exposed (i.e., perceived drug use opportunity; Wertz & Sayette, 2001b). When instructed that drugs are available for consumption during an experiment, individuals produce distinct affective (Carter & Tiffany, 2001; Sayette et al., 2003) and physiological (Carter & Tiffany, 2001; Lazev, Herzog, & Brandon, 1999; Zinser, Fiore, Davidson, & Baker, 1999) responses, and report substantially higher craving (Carter & Tiffany, 2001; Droungas, Ehrman, Childress, & O'Brien, 1995; Juliano & Brandon, 1998; Sayette et al., 2003) than when instructed that drugs are not available for an extended period of time. For instance, smokers told that they could smoke soon showed greater attentional bias to smoking-related words than did smokers told they could not (Wertz & Sayette, 2001a).
To date, neuroimaging studies of craving have not explicitly manipulated perceived drug-use opportunity, making it difficult to assess the degree to which regions observed in previous studies may respond to the perception of drug availability as opposed to other factors affecting craving. (Participants in a study by Grant and colleagues [1996] were told that they would be allowed to self-administer the cocaine presented to them following completion of experimental procedures in order to increase craving elicited during drug cue exposure. However, drug availability was not of central interest in the study.) One factor that has varied across cue exposure studies has been the treatment status of participants. We have suggested that treatment status affects drug use opportunity (Wertz & Sayette, 2001b). Presumably, those seeking treatment (i.e., abstinence) do not plan on using the drug, whereas active users participating in studies intend to use the drug as soon as possible. Consistent with this position, individuals enrolled in drug treatment programs exhibit responses consistent with low expectations of drug use opportunity, whereas those not in treatment exhibit responses consistent with high expectations of drug use opportunity (Wertz & Sayette, 2001b).
These effects appear to extend to neuroimaging data, with distinct neurobiological responses elicited by drug cue presentation, particularly in subdivisions of prefrontal cortex (PFC), determined by whether or not the individuals under study are undergoing treatment. In a review of neuroimaging studies of drug cue reactivity in humans, we observed that activation of DLPFC and OFC have been reported almost exclusively by studies in which participants were active drug users (Wilson et al., 2004), whereas other regions associated with cue-elicited craving are seemingly unaffected by treatment status. For instance, activation of the ACC—the region most frequently found in previous studies—is approximately equally distributed across studies employing actively using and treatment-seeking participants. We have since located four more recent neuroimaging studies examining drug cue exposure (Brody et al., 2004; Grüsser et al., in press; Kilts, Gross, Ely, & Drexler, 2004; Myrick et al., 2004). Of these studies, one exclusively recruited participants actively using drugs (Myrick et al., 2004) and one recruited only users in treatment (Grüsser et al., in press). The results of these studies generally conform to previously observed patterns and do not alter conclusions drawn in the prior review (Wilson et al., 2004).
Because treatment status represents just one way to affect perceived drug use opportunity, we also posited that other contexts might yield different effects (Wilson et al., 2004). Specifically, the pattern of cue-elicited neural activity in treatment seekers may differ from that produced in actively using addicts who are explicitly told that they may not use drugs for a long period of time. In the former case, individuals are attempting to quit (i.e., they are abstinence-seeking) and presumably do not intend to consume drugs, whereas in the latter circumstance individuals desire to use (i.e., they are abstinence-avoidant) but are prevented from doing so by situational constraints (Tiffany, 1990). Both of these conditions are ones in which drug use opportunity is absent. For abstinence-seekers, this perception is internally motivated, whereas it is imposed externally for abstinence-avoiders. These distinct states could conceivably influence the neural activity observed during drug cue exposure in different ways.
The aim of this preliminary fMRI study was to begin to examine the impact of perceived smoking opportunity on the neural response to drug cues in abstinence-avoidant smokers. Based on our prior review (Wilson et al., 2004), we predicted that the ACC would exhibit cue-elicited activation independent of perceived smoking opportunity. We also hypothesized that cue-evoked activation of DLPFC and OFC would be modulated by smoking expectancy.
Method
Participants
A total of 22 right-handed, male, native-English-speaking cigarette smokers participated in the experiment (mean age=24.4 years, SD=4.9). All participants reported smoking 20–40 cigarettes/day for at least 24 continuous months (mean cigarettes/day=21.6, SD=2.7). Participants were recruited through advertisements in local newspapers. Exclusionary criteria included dependence on any drug other than nicotine or caffeine, illiteracy, or medical conditions that ethically contraindicated nicotine administration. Our decision not to exclude caffeine-dependent participants is consistent with most studies in this area. Nonetheless, research suggests that caffeine consumption can influence neural activity as measured by fMRI (e.g., Laurienti et al., 2002). We did not assess caffeine consumption in our participants and thus cannot evaluate the degree to which it may have influenced our results.
Written informed consent was obtained from all participants. Participants were paid for participation, and all procedures were approved by the institutional review board of the University of Pittsburgh. Data from two participants were excluded from all analyses because of excessive head motion during scanning; subsequent analyses are reported on the remaining 20 participants.
Participants were invited to participate in a 2-hr study. They were randomly assigned to one of two experimental conditions. Half of the participants were told they would be able to smoke during a break at the midpoint of the experimental session (Instructed-Yes; n=10). The other half were told they could not smoke during the experimental session and would have to wait approximately 2 hr before smoking (Instructed-No; n=10). Age, number of cigarettes smoked per day, years smoking, number of quit attempts, and years of education were similar across instructed smoking expectancy conditions (p values >.05; Table 1).
Table 1.
Participant demographic characteristics (means with standard deviation).
| Instructed-Yes (n=10) |
Instructed-No (n=10) |
|
|---|---|---|
| Age (years) | 24.1 (4.3) | 25.3 (5.9) |
| Cigarettes/day | 21.3 (2.2) | 22.0 (3.4) |
| Years smoking | 7.8 (1.9) | 8.1 (4.8) |
| Number of quit attempts |
3.4 (4.9) | 1.0 (1.2) |
| Education (years) | 13.4 (1.7) | 13.1 (1.3) |
Cue exposure procedure
Participants completed two cue exposure runs (Figure 1), during which they were asked to hold and look at either (a) stimuli designed to elicit minimal changes in craving (i.e., neutral objects) or (b) stimuli designed to elicit robust increases in craving (one of their own cigarettes). Each cue exposure run began with a 48-s resting baseline epoch during which no objects were held. After the initial rest period, the first cue of the run was placed in the participant's left hand and instructions identifying the object were delivered over an intercom system. After 74 s, the object was removed. A second resting baseline epoch lasting 74 s followed removal of the object. The second cue of the run and identifying instructions were then presented and the object was held for 74 s. Participants were explicitly instructed to passively view each of the objects while they held them by looking at a reflection of their hand in a mirror positioned above their head. This mirror was adjusted prior to the onset of each scanning run for each participant to ensure that he could clearly see the reflection of his hand. Cues were presented in a fixed order. During the first cue exposure run, participants were presented with a small yellow notepad (neutral object) and a white plastic golf ball (neutral object). This run allowed participants to acclimate to the task. During the second run, participants were presented with a roll of black electrical tape (neutral object) and one of their cigarettes (craving-eliciting object).
Figure 1.
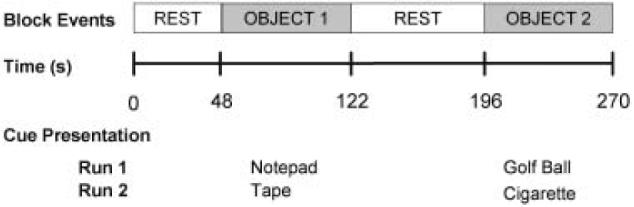
Schematic diagram of the cue exposure procedure. During each cue exposure run, subjects completed the following sequence: an initial 48-s resting baseline epoch during which no objects were held, presentation of first object for a period of 74 s, a second 74-s resting baseline epoch, followed by the second object presented for 74 s. Neutral object 1 (notepad) and neutral object 2 (plastic golf ball) were presented during run 1. Neutral object 3 (roll of electrical tape) and cigarette were presented during run 2.
Urge rating assessment
Participants verbally rated their urge to smoke on a scale from 0 (“absolutely no urge to smoke at all”) to 100 (“strongest urge to smoke I've ever experienced”). Urge ratings were provided immediately before the start of each of the two cue exposure runs. Participants also rated their urge at the completion of each run. Thus, four urge ratings were obtained from each participant. Ideally, each of these ratings would have been obtained during stimulus exposure (i.e., while participants were holding each object); however, we decided to assess urge preceding each run (urge #1 and urge #3) rather than during exposure to the first object of the run in order to avoid eliciting unwanted neural activity and because of practical constraints (e.g., difficulty communicating with participants over scanner noise). Urge ratings obtained at the completion of each run (urge #2 and urge #4) occurred after fMRI data acquisition had terminated and while participants were still holding the second object of the run.
Procedure
Participants who responded to the advertisements completed a preliminary screening interview over the phone. Eligible participants visited the lab for three sessions: a more thorough screening assessment (session 1), a session in which abstinence instructions were provided (session 2), and the experimental session (session 3). Sessions 2 and 3 were conducted 8 hr apart on the same day. During session 1, participants provided an expired-air carbon monoxide (CO) sample (CO #1), which was used to verify smoking status. Session 2 occurred 8 hr before the experimental session, during which subjects returned to the laboratory to smoke one of their cigarettes. After the subject smoked the cigarette, a second CO sample was obtained (CO #2) to provide a baseline for comparison with levels obtained at the start of the experimental session. Subsequently all participants were instructed not to drink alcohol or use tobacco products or other drugs for the 8 hr before they arrived at the laboratory to participate in the experiment. Participants then presented their packs of cigarettes and lighters to the experimenter and were permitted to leave the laboratory. Experimental sessions were scheduled to begin between 16.00 hr and 18.00 hr. To check compliance with deprivation instructions, participants reported the last time they smoked a cigarette and a third CO sample was obtained (CO #3). For the third CO assessment, samples exceeding half of the CO #2 value or 16 parts per million resulted in exclusion from the study.
Immediately before scanning began, participants were given instructions regarding whether they would be permitted to smoke during the experimental session. Because all participants were informed that the experimental session would last for 2 hr, Instructed-No participants expected a significant delay before having the opportunity to smoke (Juliano & Brandon, 1998). For Instructed-Yes participants, smoking expectancy instructions were delivered in a room close to that housing the MRI scanner by an experimenter standing in front of a sign designating the room as a “smoking area for research purposes” (actual smoking took place outside). This approach was used to enhance the likelihood that these participants would anticipate the opportunity to smoke almost immediately after cigarette cue exposure (i.e., that they would be able to smoke after a short trip down the hall). At this point, participants completed the first of two cue presentation runs. Participants then completed a guessing task involving monetary gains or losses (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000) for approximately 45 min (data from this task are not presented herein).
Participants then completed the second cue presentation run. While holding the cigarette during the second run, Instructed-Yes participants were told that in 40 s they would be removed from the scanner and would be permitted to immediately smoke the cigarette they were holding. Instructed-No participants were told they would be removed from the scanner in 40 s but would not be able to smoke the cigarette they were holding. Following self-reported craving assessment, all participants were removed from the scanner for a brief break (about 5 min), and participants who were told they would be permitted to smoke were escorted outside, where they were permitted to smoke a cigarette at their own pace. Afterward, participants were returned to the scanner to complete approximately 45 additional minutes of the guessing task (data not presented) and were then debriefed.
fMRI data acquisition and processing
Participants were scanned using a conventional 1.5-T GE Signa whole-body magnet and standard radio frequency coil. A structural series of 36 contiguous oblique-axial slices (3.75×3.75×3.8 mm voxels) parallel to the AC-PC line was collected using a standard T1-weighted pulse sequence. Functional images were acquired in the same plane as the structural series with coverage limited to the 20 center slices using a T2*-weighted one-shot spiral pulse sequence (TE=35 ms, TR=1500 ms, FOV=24 cm, flip angle=70°). fMRI data analysis was conducted using the Neuroimaging Software package (NIS 3.5), developed at the University of Pittsburgh and Princeton University, as implemented in the Functional Imaging Software Widgets graphical computing environment (Fissell et al., 2003). Following reconstruction, single-subject data were corrected for motion using Automated Image Registration (AIR 3.08; Woods, Cherry, & Mazziotta, 1992) and adjusted for drift between runs. After stripping to remove skull, structural images from each participant were coregistered to a common reference anatomy (Woods, Mazziotta, & Cherry, 1993). To form a composite dataset for group-level statistical analyses, functional images were transformed into the same space, globally mean-normalized to minimize differences in image intensity between participants, and smoothed using a three-dimensional Gaussian filter (6-mm FWHM) to account for between-subject anatomical differences. Group-based statistical images were visualized and transformed into standard stereotaxic space (Talairach & Tournoux, 1988) using the Analysis of Functional NeuroImages software package (AFNI 2.6; Cox, 1996).
fMRI data analysis
The set of coregistered functional data was used in all voxel-based statistical analyses, although single-subject data were inspected to confirm consistency of results across subjects. The fMRI signal averaged over the final 48 s of cue exposure for each object was the blood oxygen level-dependent (BOLD) response of interest. The initial 26 s of each object exposure epoch was removed to allow for stabilization of responses corresponding to instruction delivery. The first cue exposure run allowed participants to acclimate to holding the objects while in the MRI scanner and was not included in analyses.
To isolate regions of interest, we performed a voxel-wise mixed-model analysis of variance (ANOVA) with instruction set (Instructed-Yes vs. Instructed-No) as a between-subjects variable and cue (neutral vs. cigarette) as a repeated-measures variable. One objective of this analysis was the localization of regions that exhibited preferential activation by the cigarette cue (main effect of cue). For cue main effects, the voxel-wise significance threshold was set at a p value of less than .005 (uncorrected for multiple comparisons). Main effect regions of activation comprising fewer than five contiguous voxels were not considered significant, to reduce the risk of false positives (Forman et al., 1995).
In addition to an examination of cue main effects, the primary analytic objective was the identification of regions that demonstrated differential activation during cue exposure as a function of perceived drug use opportunity (instruction set×cue interaction). Given the exploratory nature of this study and the findings from our review pointing to DLPFC and OFC as regions most influenced by perceived drug use opportunity (Wilson et al., 2004), we chose a liberal voxel-wise alpha of p less than .01 (uncorrected for multiple comparisons) and spatial extent threshold of three or more contiguous voxels for detecting an interaction between instruction set and cue. To determine the nature of the interaction for regions meeting these criteria, we examined the effects of cue (neutral vs. cigarette) separately for each instructional group. We did not have specific a priori hypotheses regarding interaction effects occurring outside of DLPFC and OFC and, because our threshold does not provide adequate protection against Type I errors across the whole brain, activations falling beyond these regions are reported for completeness but are not a focus of discussion.
Results
Self-reported urge
A 2 (instruction)×4 (time) repeated-measures ANOVA, with the four urge ratings as a repeated-measures variable, revealed a main effect of time, F(3, 54)=24.9, p<.001. Urge ratings rose over time for both groups (mean urge ratings collapsed across groups: urge #1=60.5 [SD=21.8]; urge #2=61.4 [SD=22.9]; urge #3=71.2 [SD=25.4]; urge #4=74.4 [SD=23.3]). The instruction set main effect and the instruction set×time interaction were not significant. Though in the expected direction, the increase in urge during cigarette cue presentation for Instructed-Yes participants was not significantly higher than it was for Instructed-No participants.
Imaging data
Main effect of cue
Regions exhibiting a main effect of cue are summarized in Table 2. Several brain regions exhibited differential activation during cigarette versus neutral cue exposure, including multiple sites in the occipital, temporal, and parietal cortices; the posterior cingulate gyrus; thalamus; lentiform; and insula. Of particular relevance, significantly greater BOLD signal during cigarette relative to neutral cue exposure was detected in a large cluster in the anterior cingulate extending to medial frontal gyrus (BA 32/8).
Table 2.
Anatomical regions exhibiting a main effect of cue.
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Anatomical region | Broadmann's area | x | y | z | Average F ratio | |
| Cigarette>neutral | ||||||
| L ACC/superior frontal gyrus | 32 | −4 | 43 | 8 | 15.02 | |
| R posterior cingulate gyrus | 23 | 4 | −43 | 29 | 16.36 | |
| L superior/middle temporal gyrus | 21 | −50 | −23 | −1 | 23.81 | |
| R superior/middle temporal gyrus | 21 | 56 | −28 | −3 | 20.99 | |
| L inferior parietal lobule | 39 | −54 | −68 | 24 | 12.59 | |
| L superior occipital gyrus | 19 | −41 | −77 | 29 | 14.06 | |
| L middle occipital gyrus | 18 | −26 | −96 | 5 | 16.85 | |
| R cuneus | 18 | 23 | −100 | −1 | 12.91 | |
| R fusiform gyrus | 23 | −96 | −12 | 11.24 | ||
| Neutral>cigarette | ||||||
| L cingulate gyrus | 24 | −14 | −14 | 39 | 11.33 | |
| R cingulate gyrus | 24 | 20 | −17 | 38 | 13.03 | |
| L cingulate gyrus | 31 | −19 | −34 | 39 | 11.4 | |
| R middle occipital gyrus | 19 | 31 | −65 | 6 | 11.6 | |
| L precuneus | 7 | −14 | −74 | 35 | 11.77 | |
| L lingual gyrus/cuneus | 17/18/19 | −20 | −78 | 5 | 15.46 | |
| L insula | 13 | −40 | −7 | −2 | 12.9 | |
| L thalamus | −7 | −34 | 12 | 13.07 | ||
| R thalamus | 4 | −10 | 0 | 13.05 | ||
| L lentiform nucleus | −19 | −13 | 5 | 15.54 | ||
| R lentiform nucleus | 21 | −9 | 7 | 20.09 | ||
Note. Brodmann's areas and stereotaxic coordinates are given for local maxima of activation cluster in Talairach and Tournoux (1988) atlas space. ACC, anterior cingulate cortex; L, left; R, right.
Instruction set×cue interaction
We were interested principally in identifying regions exhibiting an instruction set×cue interaction. Significant effects were observed in multiple areas in prefrontal, temporal, and occipital cortices, as well as in the parahippocampus. Several patterns underlying these interactions were found (summarized in Table 3). As predicted, significant interaction effects were found in bilateral DLPFC (middle/inferior frontal gyri, BA 9/46), ventromedial prefrontal cortex (VMPFC; medial frontal gyrus, BA 10), and left ventrolateral prefrontal cortex (VLPFC; inferior frontal gyrus, BA 47) (Figure 2). VMPFC and VLPFC are closely related to the medial and lateral sectors of OFC, respectively (Krawczyk, 2002).
Table 3.
Anatomical regions exhibiting a significant instruction set×cue interaction.
| Talairach coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Anatomical region | Brodmann's area |
Y | N | x | y | z | Average F ratio |
| L inferior frontal gyrus (DLPFC) | 9 | ⇩ | ns | −48 | 6 | 25 | 12.6 |
| R middle frontal gyrus (DLPFC) | 9 | ⇩ | ns | 30 | 35 | 37 | 12.05 |
| R middle frontal gyrus (DLPFC) | 46 | ⇩ | ns | 46 | 18 | 21 | 9.31 |
| R superior frontal gyrus | 10 | ns | ⇧ | 35 | 59 | 14 | 10.39 |
| R middle frontal gyrus | 10 | ⇩ | ⇧ | 37 | 52 | −2 | 9.88 |
| L inferior frontal gyrus (VLPFC) | 47 | ⇩ | ns | −47 | 27 | −5 | 9.88 |
| L superior frontal gyrus (VMPFC) | 10 | ⇧ | ns | −8 | 57 | −7 | 9.22 |
| R precentral gyrus | 6 | ⇧ | ns | 70 | 4 | 16 | 10.64 |
| L middle temporal gyrus | 21 | ⇧ | ns | −67 | −7 | −1 | 12.41 |
| R superior temporal gyrus | 22 | ⇩ | ns | 48 | 7 | −6 | 10.32 |
| L parahippocampal gyrus | ⇩ | ns | −23 | −16 | −10 | 10.31 | |
| L cuneus | 19 | ns | ⇧ | −8 | −96 | 31 | 16.27 |
| L inferior/middle occipital gyrus | 19/18 | ⇩ | ns | −36 | −68 | −6 | 11.44 |
Note. Brodmann's areas and stereotaxic coordinates are given for local maxima of activation cluster in Talairach and Tournoux (1988) atlas space. DLPFC, dorsolateral prefrontal cortex; L, left; N, Instructed-No group; ns, not significant; R, right; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex; Y, Instructed-Yes group. ⇧, significantly greater activation during cigarette relative to neutral cue exposure; ⇩, significantly greater activation during neutral relative to cigarette cue exposure.
Figure 2.
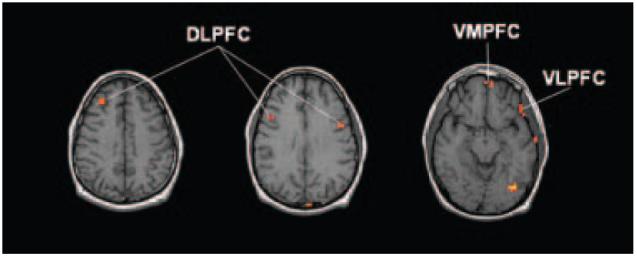
A priori regions of interest exhibiting a significant instruction set×cue interaction. DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex. Images are right–left reversed.
Discussion
The present study examined neural activity elicited by cigarette cue exposure in male cigarette smokers. Several brain regions exhibited differential activation during cigarette relative to neutral stimulus presentation independent of whether or not participants expected to smoke during the study. Activation patterns in visual (lingual gyrus, cuneus), posterior parietal, and auditory (temporal) cortices (Mersulam, 1998) suggest that visuospatial and auditory processing resources were recruited to a greater extent by the cigarette cue than by the neutral cue. In contrast, we observed comparatively greater activation of regions associated with memory-related processing (parahippocampus, posterior cingulate; Duzel et al., 2003; Maddock, Garrett, & Buonocore, 2001) and control of movement (globus pallidus; DeLong, Crutcher, & Georgopoulos, 1985) during neutral cue exposure than during cigarette cue exposure. Such “negative activations” have been reported in numerous studies (e.g., Bonson et al., 2002; Childress et al., 1999; Daglish et al., 2001; Due, Huettel, Hall, & Rubin, 2002; Kilts et al., 2001, 2004; Tapert, Brown, Baratta, & Brown, 2004) and may reflect more unconstrained mental processing involving retrieval (e.g., daydreaming; Binder et al., 1999) during neutral cue presentation than during cigarette cue presentation. Alternatively the neutral objects may have engaged greater memory resources and elicited more extensive physical manipulation than the cigarette cue because they were more novel to smokers than was the cigarette.
Of particular interest, significantly greater activation of the ACC occurred during cigarette cue exposure than during neutral cue exposure, regardless of perceived drug availability. As mentioned earlier, the ACC is the most frequently reported region of activation in studies of human drug craving that have, thus far, not directly manipulated perceived drug use opportunity. Thus the ACC appears to contribute to aspects of cue-elicited craving that are not robustly affected by perceived opportunity to consume, such as assessment of the motivational value associated with drug cues based on an extensive drug use history (Bush, Luu, & Posner, 2000; See, 2002). A recent study by Brody and colleagues (2004) found that cigarette smokers treated with bupropion exhibited less cue-elicited activation of the ACC than did untreated smokers. However, the majority of treated participants did not achieve abstinence (i.e., they had “diminished usage”) and were not required to abstain before participation. Thus the extent to which this group anticipated the opportunity to smoke after the study is unclear, making it difficult to ascertain the impact of drug use expectancy versus other treatment-related factors (e.g., direct pharmacological actions of bupropion) on cue-evoked ACC activation in this study.
The primary aim of this preliminary study was to identify regions exhibiting cue-elicited activation that was modulated by instructed smoking expectancy. Consistent with hypotheses, we found that activation of regions within OFC and DLPFC was sensitive to smoking expectancy. Specifically, cigarette cue exposure was associated with increased activation of VMPFC (i.e., medial OFC; Krawczyk, 2002) relative to neutral cue exposure, only when smoking was imminent. In contrast, we observed less activation of VLPFC (i.e., lateral OFC) during cigarette cue presentation than during neutral cue presentation among participants who were expecting to smoke. Similarly, we observed significantly less cigarette-elicited activation of DLPFC in participants expecting to smoke during the study.
These findings suggest that OFC and DLPFC are sensitive to perceived drug use opportunity (Wilson et al., 2004). However, the precise manner in which responses in these regions are affected by drug use expectancy are complex and appear to depend on several factors. One potential factor is the time delay before smokers can satisfy their craving by smoking. In the present study, abstinence-avoidant smokers anticipating either a relatively short (40 s) or long (over 1 hr) delay were presented with one of their cigarettes. Relative to neutral cue presentation, this paradigm resulted in cigarette-elicited increases in medial OFC and decreases in lateral OFC only among participants expecting to smoke soon. This finding may reflect explicit representation of drug use expectancy or the processing of drug cues as predictors of reward by medial OFC, with a concomitant decrease in the need for lateral OFC-mediated inhibitory control, given that smoking was expected to occur almost immediately after cigarette exposure (Elliott, Dolan, & Frith, 2000; London, Ernst, Grant, Bonson, & Weinstein, 2000; Volkow & Fowler, 2000). Consistent with this notion, the majority of previous studies reporting drug cue-elicited activation of OFC found increases falling within more lateral portions of OFC in actively using participants who presumably anticipated waiting until leaving the experiment before having the opportunity to consume drugs (i.e., participants presumably would try to inhibit responses typically elicited by cue exposure; Bonson et al., 2002; Brody et al., 2002; Myrick et al., 2004; but see Wrase et al., 2002). (Wang et al. [1999] found significant cue effects in OFC using a region-of-interest analysis but did not distinguish between medial OFC and lateral OFC in their report. Tapert et al. [2003] found cue-elicited activation of both medial and lateral OFC in a study recruiting adolescents [aged 14–17 years]. This finding is consistent with other data suggesting that reward-related activation of medial and lateral OFC may behave differently in younger versus older populations [May et al., 2004].) Moreover, Grant and colleagues (1996) found significant cue-evoked activation of medial OFC in active cocaine abusers who were told they would be allowed to self-administer the cocaine presented to them following completion of experimental procedures.
We found that DLPFC, like OFC, responded differentially to the smoking-related and neutral cues only in smokers expecting an opportunity to smoke almost immediately, exhibiting less activation to the cigarette than to the neutral stimulus. As noted, the majority of studies recruiting actively using addicts have reported activation of DLPFC to be increased by drug cues, whereas studies involving treatment-seeking addicts generally fail to find cue effects in DLPFC. Thus we speculate that processes mediated by DLPFC are recruited particularly in abstinence-avoidant addicts who anticipate a delay between cue exposure and drug use. In contrast, DLPFC resources appear not to be called upon (or are actively suppressed) in two distinct conditions: When abstinence-avoidant users anticipate almost no delay between cue presentation and drug consumption (as in the present study) or when those undergoing cue exposure are abstinence seeking.
The primary aim of the present study was to examine the effects of smoking expectancy on the neural response to a cigarette cue. We reported previously that treatment status, a proxy for drug use opportunity, appears to significantly influence responses to drug-related stimuli in the prefrontal cortex (Wilson et al., 2004). The present study, which found significant expectancy effects in OFC and DLPFC, suggests that drug use expectancy can affect the way in which smoking-related information is processed in these regions.
While promising, these initial findings should be interpreted cautiously because of several study limitations. First, interaction effects were obtained at a fairly liberal statistical threshold, which did not correct for multiple comparisons. Thus, although our confidence in these results is strengthened by the identification of a priori regions of interest, these data must be considered preliminary. Future research with larger samples would be useful to increase our confidence in this pattern of findings. Second, although we successfully identified OFC and DLPFC as regions modulated by smoking expectancy, the observed patterns of effects were fairly complex. We have attempted to account for both points of convergence and points of discrepancy between the current dataset and data from our prior review (Wilson et al., 2004) through a consideration of factors (e.g., delay, motivation regarding future drug use) that may affect how perceived opportunity influences cue reactivity. Nevertheless, these interpretations await direct empirical investigation. Third, despite being in the expected direction, predicted effects of expectancy on self-reported urge failed to reach significance. Consequently, the relationship between the observed effects of the smoking expectancy on neural activity and the subjective experience of craving is unclear. Fourth, aspects of the cue exposure procedure used in the present study may have influenced the observed pattern of cue-elicited neural responses. Specifically, participants were asked to hold a cigarette while they lay supine in an MRI machine. Because we did not assess the affective state of participants during cue exposure, we cannot determine the extent to which anxiety or arousal associated with this procedure may have influenced the study results. Fifth, the objects were presented in a fixed order across participants; as such, we cannot rule out effects specific to the order of cue presentation. Finally, we restricted the sample to males based on research demonstrating that male and female smokers differ in their responses to smoking-related stimuli and nicotine administration (e.g., Perkins et al., 2001). We sought to reduce the possibility of introducing such variance given the relatively small sample size. Whether our findings generalize to female smokers awaits direct investigation.
Despite these limitations, the present findings highlight the importance of perceived drug use opportunity as an area of investigation for addiction researchers using functional neuroimaging to study cue reactivity. Further, our data generate intriguing and testable hypotheses regarding the complex and context-dependent contributions of subregions of PFC to drug craving and addiction. Future research examining the influence of perceived drug use opportunity and other contextual variables on cue-elicited neural activity has the potential to refine and extend contemporary neurobiological models of addiction and craving in which the PFC is featured prominently (e.g., Goldstein & Volkow, 2002; Jentsch & Taylor, 1999; London et al., 2000).
Acknowledgments
This research was supported by grants from the University of Pittsburgh F.I.R.P. and from the National Institute on Drug Abuse to M.A.S. (DA10605) and J.A.F. (DA14103), and by a Ford Foundation Predoctoral Fellowship to S.J.W. The authors thank Erik Reichle, Jason Chien, Susan Ravizza, Elizabeth Tricomi, Kate Fissel, and Walter Schneider for helpful discussion. This paper is based on a master's thesis completed by S.J.W. Portions of these data were presented at the annual meeting of the Cognitive Neuroscience Society (San Francisco, April 2004).
References
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee GP, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: A preliminary study. Psychiatry Research. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr., Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional resonance neuroimages. Computational and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. American Journal of Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos P. Primate globus pallidus and subthalamic nucleus: Functional organization. Journal of Neurophysiology. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O'Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze H. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configuration. Journal of Neuroscience. 2003;23:9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Fissell C, Tseytlin E, Cunningham D, Iyer K, Carter CS, Schneider W, Cohen JD. A graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–125. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. doi: 10.1007/s00213-004-1828-4. in press. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Functional imaging of craving. Alcohol Research & Health. 1999;23:187–196. [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: Evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler K. The neural correlates of cue-induced craving in cocaine-dependent women. American Journal of Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and Biobehavioral Reviews. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Dietary caffeine consumption modulates fMRI measures. Neuroimage. 2002;17:751–757. [PubMed] [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditions of environmental cues to cigarette smoking. Experimental and Clinical Psychopharmacology. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: Functional imaging. Cerebral Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mersulam M-M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes DJ, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: A facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacology, Biochemistry, and Behavior. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addictive Behaviors. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sciences. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Cherry S, Mazziotta J. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Woods R, Mazziotta J, Cherry S. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Wrase J, Gruesser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. European Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: Impact on an electrophysio-logical index of approach motivation. Journal of Abnormal Psychology. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]


