Summary
There is evidence suggesting that protein kinase C (PKC) activation can prevent the enhanced network excitability associated with status epilepticus and group I metabotropic glutamate receptor (mGluR)-induced epileptogenesis. However, we observed no suppression of mGluR-induced burst prolongation in the guinea pig hippocampal slice when applied in the presence of the PKC activator phorbol-12,13-dibutyrate (PDBu). Furthermore, PDBu alone converted picrotoxin-induced interictal bursts into ictal-length discharges ranging from 2-6 s in length. This effect could not be elicited by the inactive analog 4-α-PDBu and was suppressed with the PKC inhibitor chelerythrine, indicating PKC dependence. PKC activation can enhance neurotransmitter release, and both glutamate and acetylcholine are capable of eliciting similar prolonged synchronized discharges. However, neither mGluR1 nor NMDA receptor antagonist suppressed PDBu-driven burst prolongation, suggesting that increased glutamate release alone is unlikely to account for the PKC-induced expression of ictaform discharges. Similarly, atropine, a broad-spectrum muscarinic receptor antagonist, had no effect on PKC-induced burst prolongation. By contrast, AMPA/kainate receptor antagonist abolished PKC-induced burst prolongation, and mGluR5 antagonist significantly blunted the maximum burst length induced by PKC. These data suggest that PKC-induced prolongation of epileptiform bursts is dependent on changes specific to mGluR5 and AMPA/kainate receptors and not mediated simply by a generalized increase in transmitter release.
Keywords: phorbol ester, electrophysiology, seizure discharges, ictogenesis, glutamate receptors, mGluR
1. Introduction
Activation of metabotropic receptors in the central nervous system is frequently associated with long-lasting changes in cellular excitability resulting from various second messenger cascades linked to phosphoinositide or phosphatidylcholine metabolism. Protein kinase C (PKC) activation is oftentimes a critical part of this intracellular cascade, mediating the phosphorylation of receptors, channels, and/or other enzymes and ultimately changing cellular, synaptic and network properties. Recent findings in epilepsy research suggest that PKC-driven phosphorylation may be beneficial for the prevention of group I mGluR-induced epileptogenesis (Cuellar et al., 2005) as well as the reduction of pharmacoresistant status epilepticus (Terunuma et al., 2008). Other recent studies, however, suggest that PKC activation during status epilepticus has seizure-promoting and potentially-epileptogenic effects, enhancing surface expression of NMDA receptors and reducing neuropeptide Y-mediated suppression of glutamate release (Niimura et al., 2005; Silva et al., 2007).
PKC can be activated experimentally via the use of phorbol esters, which essentially substitute for diacylglycerol (DAG) in the intracellular cascade. To examine the therapeutic potential of PKC activation in the management of epilepsy and epileptogenesis, we performed studies using the phorbol ester phorbol-12,13-dibutyrate (PDBu) and reveal for the first time its epileptogenic effect on hippocampal network activity. Portions of this work have appeared in abstract form (Faria and Merlin, 2006; Fuortes and Merlin, 2007).
2. Methods
2.1 Hippocampal slice recording techniques
Hartley guinea pigs 2 to 4 wk old (Elm Hill Laboratories) were anesthetized with halothane and decapitated in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Brains were promptly removed from the cranium and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 26 NaHCO3, 5 KCl, 1.6 MgCl2, 2.0 CaCl2, and 10 D-glucose. The hippocampus was dissected free and transverse slices 400 μm thick were prepared with a Vibratome (Technical Products International). Slices were transferred to nylon mesh in an interface recording chamber and perfused with ACSF bubbled with 95% O2-5% CO2 to pH 7.4. Temperature was maintained at 34.5-35.5°C. Slices were incubated at least 1 hr prior to recording. Intracellular recordings were obtained from CA3 stratum pyramidale using 30-80 MΩ glass capillary microfilament electrodes filled with 2 M potassium acetate; concurrent extracellular recordings were obtained from CA1 stratum pyramidale using microelectrodes with <5 MΩ tip resistance and filled with ACSF.
Data were amplified and digitized using an Axoclamp 2A and A/D converter, then stored for later analysis using pClamp 9.2 software (Axon Instruments). Hyperpolarizing current was injected through the intracellular recording electrode as needed to suppress excessive intrinsically-generated activity, allowing for better visualization of the synchronized network activity. Cells were excluded from analysis if their resting membrane potential before drug exposure was more positive than -60 mV, their action potentials were less than 50 mV in amplitude, or they failed to produce rhythmic synchronized interictal bursts of at least 250 ms duration on exposure to picrotoxin.
2.2 Materials
Pharmacological agents were bath applied. Picrotoxin was present throughout all experiments to elicit baseline interictal activity unless otherwise specified. While picrotoxin was not necessary to elicit the reported PDBu effects, it was used in most experiments (1) to provide a standardized measure of the health of the network prior to PDBu application and limit interslice variability, (2) to reduce the necessary drug application period, minimizing the likelihood of diminished slice health accounting for changes over time, and (3) to eliminate the GABAA receptor as a potential site of action to explain the PDBu-induced effects reported herein. PDBu, 4-α-PDBu, atropine and picrotoxin were obtained from Sigma-Aldrich (St. Louis, MO); (S)-3,5-dihydroxyphenylglycine (DHPG), chelerythrine, 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), D-(-)-2-amino-5-phosphonopentanoic acid (APV), (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), and 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) from Tocris Ellison (Ellisville, MO).
2.3 Data analysis
Burst duration (BD) was measured from onset of primary burst until the end of the final secondary burst and did not include postburst afterhyperpolarization. Any depolarization of at least 2 mV occurring within 50 ms of the preceding depolarization was considered part of the preceding discharge and included in BD measurement. Instantaneous burst frequencies (Hz) represent the reciprocal of the interburst intervals measured in seconds from onset of one burst to onset of the next. Significance of changes within a slice was determined using the paired Student's t-test, each slice serving as its own control. For comparisons across groups, Kruskal-Wallis non-parametric ANOVA analysis with post hoc Mann-Whitney tests was performed. P < 0.05 was deemed significant. Data are reported as means ± SEM; n, number of slices tested.
3. Results
3.1 Effect of the phorbol ester PDBu on DHPG-induced burst prolongation
Continuous bath perfusion of 50 μM picrotoxin, an antagonist of GABAA receptor-mediated inhibition, elicited rhythmically-recurring interictal-length synchronized bursts (250-450 ms each), as recorded from CA3 stratum pyramidale in hippocampal slices. Consistent with data published previously by this laboratory (Merlin and Wong, 1997), the group I mGluR-agonist DHPG (50 μM) elicited prolongation of picrotoxin-induced interictal bursts, converting bursts of 369 ± 16 ms duration into ictaform bursts 1622 ± 281 ms in duration (peak length; n=4). However, when DHPG was applied in the presence of 1 μM phorbol-12,13-dibutyrate (PDBu), burst prolongation was enhanced. Initial bursts of 328± 40 ms lengthened to a peak duration of 4754 ± 743 ms (n=3, P < 0.01). This was in conflict with expected results based on our previous data showing that PKC activation was responsible for cysteine sulfinic acid-mediated blockade of the DHPG-induced burst prolongation (Rico and Merlin, 2004; Cuellar et al., 2005). Furthermore, when slices were incubated in PDBu for 20-30 min prior to the introduction of DHPG, bursts achieved a minimum average length of 1 sec by 23 ± 5 min of DHPG application (n=3), whereas slices not exposed to PDBu required 33 ± 3 min of DHPG application before expressing bursts averaging 1 sec in length (n=4). We therefore performed additional experiments to determine whether PDBu alone had any effect on picrotoxin-induced interictal activity.
3.2 Effect of PDBu on picrotoxin-induced epileptiform discharges
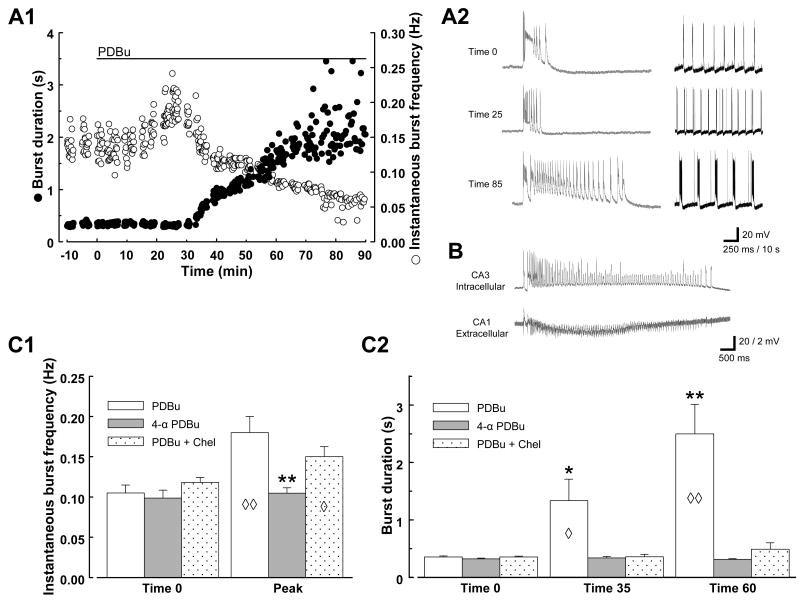
Addition of 1 μM PDBu to the perfusing solution promptly resulted in a significant increase in interictal burst frequency (Fig. 1 A1, A2, and C1; initial burst frequency 0.10 ± 0.01 Hz; increased to 0.18 ± 0.02 Hz at 20 ± 3 min, n=8; P < 0.05). This was followed by a gradual increase in the length of the discharges accompanied by a progressive decrease in the burst frequency; the bursts reached ictal length (≥1 sec) by 34 ± 4 min of exposure (range 20-45 min to reach 1 sec, n=8; Fig. 1 A1 & A2). On average, the increase in burst length reached statistical significance within 35 min (P < 0.05, n=8; Fig. C2). Peak burst length, which was achieved by 74 ± 6 min of PDBu exposure, ranged from 2.4-5.6 sec (BDcontrol 355 ± 18 ms; BD35 min, 1329 ± 374 ms; BDpeak 4289 ± 440 ms; n=8). Concurrent extracellular field recordings revealed that the discharges occurred synchronously throughout the CA3 and CA1 populations. Similar prolonged synchronized bursts could be elicited by PDBu in the absence of picrotoxin (Fig. 1B), but either a longer application period or greater concentration of drug was necessary to elicit an equivalent effect. When using the same concentration of PDBu as the experiments above (1 μM), it took approximately twice as long to elicit ictaform bursts ≥1 sec duration (68 ± 13 min, n=5).
Fig. 1. PKC activation induces ictaform activity in the hippocampal slice.
A: Example of PDBu effect on picrotoxin-induced epileptiform activity. Continuous intracellular recording from a CA3 pyramidal cell. Time course of changes in epileptiform activity is displayed in A1, with each epileptiform discharge represented by two symbols: ● representing burst duration (left y-axis) and ○ representing burst frequency (right y-axis). Time 0 represents onset of PDBu application. Representative traces from times indicated in A1 are shown in A2 on two time scales, expanded on left (250 ms calibration bar) to display burst duration and compressed on right (10 s calibration bar) to display frequency. B: Example of PDBu effect in the absence of picrotoxin. Paired recording; intracellular electrode in a CA3 pyramidal neuron (20 mV vertical calibration) and concurrent extracellular field recording from CA1 stratum pyramidale (2 mV vertical calibration). Burst displayed occurred after 100 min exposure to 1 μM PDBu. C: Summary data comparing PDBu, 4α-PDBu, and PDBu + Chel. Diamonds indicate significant changes of that group compared to itself at time 0; asterisks indicate significant difference of that group compared to the other groups. C1: Peak burst frequency was measured at the appropriate time for each slice analyzed, ranging from 13 to 25 min of drug application. n=8 for PDBu, n=5 for 4α-PDBu, n=6 for PDBu + Chel. C2: n=5 for PDBu + Chel at Time 60; all other n's unchanged from above. ◊, P < 0.05; ◊◊, P < 0.01; *, P < 0.05; **, P < 0.01.
Analogous experiments were performed in which 1 μM 4-α-PDBu (a structural analog of PDBu lacking PKC activity) was substituted for PDBu; these experiments elicited no burst acceleration or prolongation (n=5; Fig 1 C1 & C2). To further confirm the PKC-dependence of the PDBu effect, additional experiments were carried out in the presence of 10 μM chelerythrine, a PKC inhibitor. In 6 of 8 slices tested, chelerythrine significantly suppressed the induction of burst prolongation by PDBu (BDpeak 464 ± 98 ms, n=6, Fig. 1 C2), although it failed to prevent fully the increase in burst frequency (peak frequency 0.15 ± 0.01 Hz at 27 ± 3 min exposure, n=6; Fig. 1 C1). In the absence of PDBu, chelerythrine itself elicited no change in interictal burst frequency or duration (n=3, data not shown). These data suggest that PDBu-induced burst prolongation is driven by PKC activation. The mechanism for the effects of PDBu on burst frequency, however, remains unclear.
3.3 Muscarinic antagonist had no significant effect on induction of burst prolongation
Cholinergic activation in the hippocampal slice has previously been reported to produce prolonged synchronized oscillatory discharges (e.g. Bianchi and Wong, 1994). We therefore examined whether cholinergic activation was necessary for the production of prolonged ictaform bursts with PDBu. PDBu application in the presence of the muscarinic blocker atropine (10 μM) elicited an increase in burst frequency which peaked at 23 ± 4 min (peak frequency 0.15 ± 0.02 Hz, n=5) and was followed by a progressive increase in burst duration. A peak burst length of 3190 ± 500 ms was achieved at 63 ± 7 min (n=5). These results were not significantly different from those elicited by PDBu alone (P > 0.05).
3.4 Differential effects of ionotropic glutamate receptor (iGluR) antagonists on PDBu-induced burst prolongation
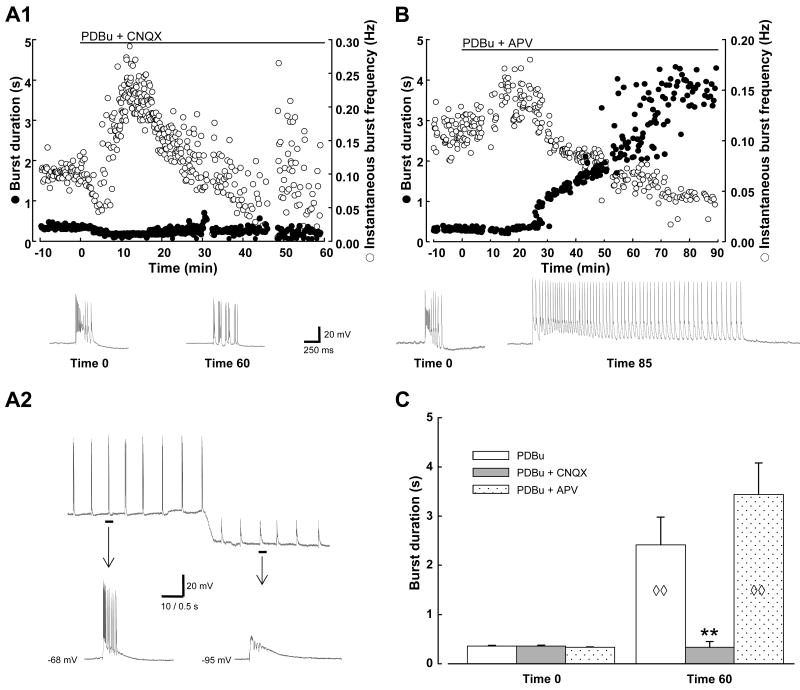
Additional experiments examined the role of glutamatergic transmission in the expression of the PDBu-induced ictaform bursts. The AMPA/kainate receptor antagonist CNQX had a striking effect on the PDBu-induced activity. Co-application of 20 μM CNQX and 1 μM PDBu (Fig. 2 A1) resulted in a very transient initial slowing of picrotoxin-induced bursting, rapidly followed by the expected increase in burst frequency (from 0.09 ± 0.005 to 0.17 ± 0.02 Hz, n=7) but no burst prolongation ensued. The increase in burst frequency was not sustained; bursts gradually returned to initial frequency within 10-20 min, despite the lack of burst lengthening. At 60 min of co-application of PDBu and CNQX, bursts remained unchanged from their initial length (initial BD 390 ± 13 ms; BD60 min 334 ± 114 ms, n=6; Fig. 2C). However, as time went on there was increasing variability of burst frequency, suggesting AMPA receptor activation may contribute to burst initiation and the maintenance of rhythmic network activity. In addition, the interictal-length bursts that continued to be expressed in the presence of CNQX had a somewhat altered conformation, with marked shortening or absence of the classic paroxysmal depolarizing shift (PDS), resulting in a burst essentially comprised of a cluster of secondary bursts. Nevertheless, the remaining bursts were confirmed to represent synaptically-driven synchronized network activity by the following features: (1) Similar to the initial picrotoxin-induced activity, these bursts were rhythmically recurring and were neither suppressed nor changed in frequency or length when hyperpolarizing current was injected in the intracellular recording electrode (Fig. 2 A2). (2) In experiments in which field recordings were concurrently performed, the bursts were observed simultaneously on both the intracellular and field recordings. Additional experiments in which PDBu was applied in the presence of complete block of iGluRs (via co-application of CNQX and APV) failed to produce any synchronized bursting (n=4), further suggesting that synaptic activation of iGluRs is necessary to allow for expression of PDBu-induced bursting.
Fig. 2. Differential effects of AMPA/kainate and NMDA receptor antagonists on PKC-induced burst prolongation.
Examples of PDBu application in the presence of iGluR antagonists CNQX (A1) and APV (B), with representative traces displayed below each graph. Calibration bars in A1 also apply to traces shown in B. Note the increased variability of burst frequency in the presence of CNQX when compared with other experimental protocols. A2: Another example of an experiment in which PDBu was applied in the presence of CNQX; intracellular recording from CA3 is shown at 20 min of co-application. C: Summary data comparing changes in burst duration elicited in the presence of the iGluR antagonist indicated vs. PDBu alone (control data from Fig. 1). Diamonds indicate significant change for that group compared to itself at time 0; asterisks indicate significant difference from all other groups. Sample sizes: PDBu, n=8; PDBu + CNQX, n=8 at Time 0, n=6 at Time 60; PDBu + APV, n=9. ◊◊, P < 0.01; **, P < 0.01.
In marked contradistinction to the significant impact of AMPA/kainate antagonist on PDBu-induced burst prolongation, 50 μM APV, an NMDA receptor antagonist, when applied alone, had no significant effect on the expected PDBu-induced changes in burst frequency and duration. In the presence of APV, a maximal increase in burst frequency was seen at 17 ± 2 min (0.17 ± 0.01 Hz, n=9), followed by an increase in burst length. Although there seemed to be a blunting of the depolarizing plateau underlying each burst, this appeared to have no impact on the development of burst prolongation. Bursts reached 1 sec by 27 ± 3 min, and achieved a peak duration of 4692 ± 521 ms by 66 ± 8 min (n=9; Fig. 2B and C).
3.5 Differential effects of group I metabotropic glutamate receptor (mGluR) antagonists on PDBu-induced burst prolongation
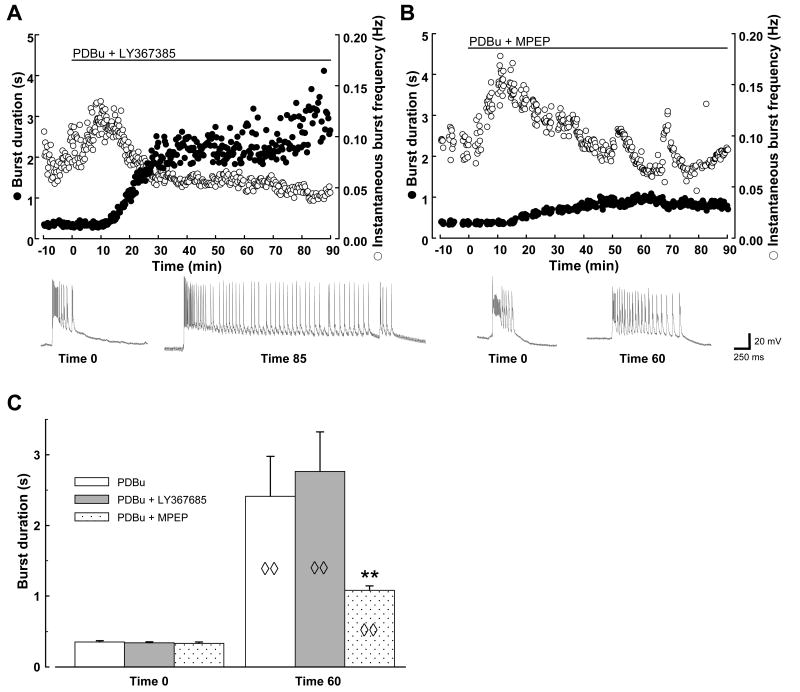
Neither the mGluR1 antagonist LY367385 (25 μM) nor the mGluR5 antagonist MPEP (25 μM) could fully prevent the induction of prolonged synchronized bursts by PDBu. Nevertheless, there was a significant difference between the efficacy of the two antagonists. In the presence of LY367385, PDBu elicited bursts that were not significantly different from those induced in the absence of the mGluR1 blocker: initial bursts of 340 ± 16 ms first accelerated to 0.17 ± 0.02 Hz (n=8), then significantly lengthened to 1898 ± 495 ms within 35 min of PDBu application, achieving a peak length of 3556 ± 529 ms by 83 ± 9 min (n=8; Fig. 3A and C). Bursts accelerated in the presence of the mGluR5 antagonist MPEP as well (to 0.23 ± 0.02 Hz, n=9), but the induced burst prolongation at 60 min was significantly attenuated when compared with control or with responses in the presence of mGluR1 antagonist (1083 ± 64 ms, n=9; Fig. 3B and C). It was noted that the mGluR5 antagonist was significantly less effective at preventing PDBu-induced burst prolongation than the AMPA/kainate receptor antagonist (c.f. Fig. 2).
Fig. 3. Differential effects of mGluR1 and mGluR5 antagonists on PKC-induced burst prolongation.
Examples of PDBu application in the presence of group I mGluR antagonists LY367385 (A) and MPEP (B), with representative traces displayed below each graph. Calibration bars apply to all traces. Episodic irregularities in burst frequency in the presence of MPEP were unique to this experiment and not representative of all slices tested. C: Summary data, n=8 for PDBu and for PDBu + LY367385; n=9 for PDBu + MPEP. Diamonds indicate significant changes of group indicated compared to itself at time 0; asterisks indicate significant difference from other groups shown. ◊◊, P < 0.01; **, P < 0.01.
In the presence of mGluR5 antagonist, one might expect PKC-induced changes at mGluR1 to become unmasked as a result of blocking mGluR5-dependent desensitization of the mGlu1 receptor (Poisik et al., 2003). To clarify the role of the two receptor subtypes, additional experiments were performed in which MPEP and LY367385 were co-applied. The data revealed no significant added suppression of burst prolongation at 35 min in the presence of LY367385 + MPEP vs. MPEP alone (BDMPEP 881 ± 154 ms vs. BDLY/MPEP 642 ± 125 ms, n=9 for each), with both groups displaying significant burst prolongation (P < 0.05). Furthermore, although at 60 min there appeared to be some added benefit of mGluR1 suppression (BDLY/MPEP 766 ± 123 ms, n=9), there was no difference in the peak burst length achieved in the two groups (MPEP alone, 1233 ± 92 ms; LY/MPEP, 1312 ± 148 ms; n=9 for both).
4. Discussion
4.1 The excitatory and inhibitory cellular and synaptic effects of PKC activation
There are numerous reported effects of PKC activation with PDBu, both on intrinsic cellular properties and synaptic responses. Although many of these effects are seen with muscarinic and mGluR activation as well, they do not always share a common PKC-relevant mechanism (e.g. Sim et al., 1992). Cellular effects include reduced spike accommodation (Malenka et al., 1986a), antagonism of a voltage-sensitive chloride current (Madison et al., 1986), and suppression of the potassium current IAHP (Baraban et al., 1985; Malenka et al., 1986a; Sim et al., 1992; Grabauskas et al., 2006). Accelerated calcium clearance from the cytoplasm has also been reported; this occurs via both increased calcium efflux and increased sequestration in intracellular stores (Usachev et al. 2006). Most of these effects would be expected to enhance intrinsic cellular excitability.
Effects of phorbol esters at the synaptic junction have less clear implications for network excitability. Phorbol esters have been reported to enhance both spontaneous and evoked release of neurotransmitters (Malenka et al., 1986b; Terrian et al., 1993; Hori et al., 1999; Waters and Smith, 2000; Brager et al., 2003; Stocca and Lovinger, 2003). However, the excitatory impact of this enhanced glutamate release may be counterbalanced by increased glutamate transport in glial cells (Daniels and Vickroy, 1999). Furthermore, PDBu increases both the frequency and amplitude of miniature excitatory postsynaptic currents, implicating both pre- and postsynaptic sites of action (Carroll et al., 1998); phosphorylation of the AMPA receptor has been a proposed mechanism for these effects.
One would predict that the multiple cellular and synaptic sites of PKC action listed above work in concert to determine the impact of PKC activation on network activity, but one cannot readily predict what that impact will be from these studies on cellular and synaptic responses. Our data reveal that the excitatory effects overcome the suppressive effects to result in the expression of robust ictal-length oscillatory discharges. With each burst lasting over 1s, these synchronized discharges may be considered seizure-length, or ictaform, as their occurrence in vivo would likely be accompanied by a clinical seizure.
4.2 Generalized enhancement of transmitter release does not alone account for the PKC-induced production of ictal discharges in the hippocampal slice
The ictal discharge is a synchronized event resulting from an imbalance of excitation vs. inhibition in the neuronal network. In the guinea pig acute hippocampal slice preparation, complete disinhibition via concurrent blockade of GABAA and GABAB receptors is insufficient to produce ictal-length discharges (see Huszár and Merlin, 2004), so modifications induced in interneurons or GABA receptors seem unlikely to account for the PKC-driven network effects reported herein. The prolonged synchronized bursts induced in our studies resemble those previously elicited via activation of cholinergic or glutamatergic receptors. Specifically, muscarinic (Bianchi and Wong, 1994, D'Antuono et al., 2001; Cobb et al., 2003), NMDA (Traub et al., 1996), and group I metabotropic glutamate (Taylor et al., 1995; Merlin and Wong, 1997; Merlin 1999) receptor activation can all result in the expression of prolonged synchronized discharges in vitro as well as seizures in vivo. As PKC activation can enhance the release of neurotransmitters, a simple explanation for the effects seen here could have been potentiated cholinergic and/or glutamatergic transmission. However, the failure to reduce the induced burst prolongation with muscarinic, NMDA, or mGluR1 receptor antagonist suggests that a strictly presynaptic mechanism of enhanced transmitter release cannot solely account for the bursts seen here. The negligible role for NMDA receptor activation in the burst prolongation is additionally surprising in light of previous studies indicating that PKC activation can increase membrane expression of NMDA receptors (Niimura et al., 2005). These negative results suggest that changes at NMDA or mGlu1 receptors or presynaptic targets, assuming such changes occur in our experiments, have a minimal role when compared with the modifications induced elsewhere as discussed in the next section. One should keep in mind, however, that the data merely indicate modification at any one of these alone is not sufficient to elicit our observed effects; they do not rule out the possibility that such changes may still be necessary co-contributors to allow for the robust effects reported herein.
4.3 Receptor-specific PKC-induced effects underlie burst prolongation: roles of AMPA/kainate receptors and mGluR5
We found it surprising that the AMPA/kainate receptor antagonist CNQX failed to abolish the production of interictal-length bursts in the presence of PDBu, as AMPA/kainate receptor activation is traditionally believed to be critical for initiation of synchronized discharges. However, one should note that PKC activation can relieve the magnesium block of the NMDA receptor channel (Chen and Huang, 1992), which could provide an explanation for the continued interictal bursting in the presence of CNQX. In experiments performed in the presence of both CNQX and APV, no synchronized bursting could be elicited by PDBu, suggesting that NMDA receptor activation sustains the persistent PDBu-induced interictal bursting in the presence of AMPA/kainate antagonism.
The dramatic suppression of PKC-induced burst prolongation by CNQX suggests that the burst prolongation is largely dependent on potentiation of AMPA/kainate responses. And indeed, PKC activation can potentiate AMPA and kainate responses via phosphorylation (Carroll et al., 1998; Cho et al., 2003) and increased receptor incorporation in the membrane (Boehm et al., 2006).
Our data also revealed that mGluR5 antagonist, while effective at suppressing PKC-induced burst prolongation, is significantly less effective than antagonist of AMPA/kainate receptors. One can elicit a significant burst prolongation at 35 min of PDBu application in the presence of mGluR5 antagonist, but the response is dampened as the prolongation progresses over time, as if mGluR5 is recruited into the picture at a later time point than AMPA/kainate or only after some minimum AMPA-mediated prolongation first occurs. The underlying mechanism for this mGluR5-dependent component of burst prolongation may involve PKC-induced aggregation of the anchoring protein Homer 1a (Kato et al., 2001), thereby enhancing the role of mGluR5 activation in the expression of prolonged synchronized bursts. The perisynaptic localization of group I mGluRs (Luján et al., 1996) suggests that their role in the production of bursts only becomes significant when the glutamate released to produce a burst is sufficient to spill over to perisynaptic zones, making it more likely to recruit activity of group I mGluRs during the production of longer bursts. The source of the enhanced glutamate release may be nothing more than enhanced network firing due to AMPA/kainate potentiation, but it may additionally involve contributions from some of the presynaptic or intrinsic cellular effects of PKC activation enumerated earlier.
In the face of this enhanced excitation and the significant effect of mGluR5 antagonist, the negligible role of mGluR1 is somewhat surprising, and may be due to receptor desensitization or internalization, generally believed to be phosphorylation-independent phenomena (Dhami and Furguson, 2006).
4.4 Effect of PKC activation on network activity associated with seizures and epileptogenesis
In the presence of enhanced excitatory activity accompanying status epilepticus (SE), contradictory data have recently been presented. Increased surface expression of NMDA receptors (Niimura et al., 2005) and reduced sensitivity to neuropeptide Y-mediated suppression of glutamate release (Silva et al., 2007) have both been reported to accompany SE and are both driven by excessive PKC activation. On the other hand, deficient PKC activation during SE has been blamed for the GABAA receptor internalization held responsible for pharmacoresistance of prolonged SE (Terunuma et al., 2008). Furthermore, experiments in which the group I mGluR agonist DHPG is used to induce persistent ictal-length discharges in vitro reveal that cysteine sulfinic acid, an agonist of the PLD-coupled mGluR, can be used to prevent this group I mGluR-induced epileptogenesis (Rico and Merlin, 2004), and this antiepileptogenic effect appears to be mediated by PKC activation (Cuellar et al., 2005).
Our current data reveal that generalized PKC activation is not of therapeutic value, as it will itself induce seizure discharges. There are numerous PKC isoforms, activation of each likely to have different effects. In addition, the timing and precise location of the PKC activation will surely have an impact on the cellular effect of this activation. Hence, one must find ways to activate PKC in a targeted and isoform-specific manner if one is to achieve therapeutic effects in the management of seizures and epilepsy.
Acknowledgments
This work was funded by NIH grant NS40387 to L.R.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baraban JM, Snyder SH, Alger BE. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci USA. 1985;82:2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Wong RKS. Carbachol-induced synchronized rhythmic bursts in CA3 neurons of guinea-pig hippocampus in vitro. J Neurophysiol. 1994;72:131–138. doi: 10.1152/jn.1994.72.1.131. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003;6:551–552. doi: 10.1038/nn1067. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol. 1998;80:2797–2800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Cho K, Francis JC, Hirbec H, Dev K, Brown MW, Henley JM, Bashir ZI. Regulation of kainate receptors by protein kinase C and metabotropic glutamate receptors. J Physiol. 2003;548(Pt 3):723–730. doi: 10.1113/jphysiol.2003.040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Larkman PM, Bulters DO, Oliver L, Gill CH, Davies CH. Activation of Ih is necessary for patterning of mGluR and mAChR induced network activity in the hippocampal CA3 region. Neuropharmacology. 2003;44:293–303. doi: 10.1016/s0028-3908(02)00405-7. [DOI] [PubMed] [Google Scholar]
- Cuellar JC, Griffith EL, Merlin LR. Contrasting roles of protein kinase C in induction versus suppression of group I mGluR-mediated epileptogenesis in vitro. J Neurophysiol. 2005;94:3643–3647. doi: 10.1152/jn.00548.2005. [DOI] [PubMed] [Google Scholar]
- D'Antuono M, Kawasaki H, Palmieri C, Avoli M. Network and intrinsic contributions to carbachol-induced oscillations in the rat subiculum. J Neurophysiol. 2001;86:1164–1178. doi: 10.1152/jn.2001.86.3.1164. [DOI] [PubMed] [Google Scholar]
- Daniels KK, Vickroy TW. Reversible activation of glutamate transport in rat brain glia by protein kinase C and an okadaic acid-sensitive phosphoprotein phosphatase. Neurochem Res. 1999;24:1017–1025. doi: 10.1023/a:1021004809991. [DOI] [PubMed] [Google Scholar]
- Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111:260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Faria LC, Merlin LR. PKC activation induces ictaform bursts in hippocampal slices. San Diego, CA: American Epilepsy Society; 2006. Program #3.024. 2006 Abstract Viewer. [Google Scholar]
- Fuortes M, Merlin LR. PKC-induced ictaform bursts in the hippocampal slice are partially mediated by mGluR5. Philadelphia, PA: American Epilepsy Society; 2007. Program #IW.17. 2007 Abstract Viewer. [Google Scholar]
- Grabauskas G, Chapman H, Wheal HV. Role of protein kinase C in modulation of excitability of CA1 pyramidal neurons in the rat. Neuroscience. 2006;139:1301–1313. doi: 10.1016/j.neuroscience.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Hori T, Takai Y, Takahashi T. Presynaptic mechanism for phorbol ester-induced synaptic potentiation. J Neurosci. 1999;19:7262–7267. doi: 10.1523/JNEUROSCI.19-17-07262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszár P, Merlin LR. Contribution of GABAB receptor-mediated inhibition to the expression and termination of group I mGluR-induced ictaform bursts. Epilepsy Res. 2004;61:161–165. doi: 10.1016/j.eplepsyres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Kato A, Fukuda T, Fukazawa Y, Isojima Y, Fujitani K, Inokuchi K, Sugiyama H. Phorbol esters promote postsynaptic accumulation of Vesl-1S/Homer-1a protein. Eur J Neurosci. 2001;13:1292–1302. doi: 10.1046/j.0953-816x.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Madison DV, Malenka RC, Nicoll RA. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986;321:695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Andrade R, Nicoll RA. Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J Neurosci. 1986a;6:475–480. doi: 10.1523/JNEUROSCI.06-02-00475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986b;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Wong RKS. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Merlin LR. Group I mGluR-mediated silent induction of long-lasting epileptiform discharges. J Neurophysiol. 1999;82:1078–1081. doi: 10.1152/jn.1999.82.2.1078. [DOI] [PubMed] [Google Scholar]
- Niimura M, Moussa R, Bissoon N, Ikeda-Douglas C, Milgram NW, Gurd JW. Changes in phosphorylation of the NMDA receptor in the rat hippocampus induced by status epilepticus. J Neurochem. 2005;92:1377–1385. doi: 10.1111/j.1471-4159.2005.02977.x. [DOI] [PubMed] [Google Scholar]
- Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23:122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico MJ, Merlin LR. Evidence that phospholipase D activation prevents group I mGluR-induced persistent prolongation of epileptiform bursts. J Neurophysiol. 2004;91:2385–2388. doi: 10.1152/jn.01140.2003. [DOI] [PubMed] [Google Scholar]
- Silva AP, Lourenco J, Xapelli S, Ferreira R, Kristiansen H, Woldbye DPD, Oliveira CR, Malva JO. Protein kinase C activity blocks neuropeptide Y-mediated inhibition of glutamate release and contributes to excitability of the hippocampus in status epilepticus. FASEB J. 2007;21:671–681. doi: 10.1096/fj.06-6163com. [DOI] [PubMed] [Google Scholar]
- Sim JA, Gerber U, Knöpfel T, Brown DA. Evidence against a role for protein kinase C in the inhibition of the calcium-activation potassium current IAHP by muscarinic stimulants in rat hippocampal neurons. Eur J Neurosci. 1992;4:785–791. doi: 10.1111/j.1460-9568.1992.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Stocca G, Lovinger DM. Phorbol ester uncouples adenosine inhibition of presynaptic Ca2+ transients at hippocampal synapses. Hippocampus. 2003;13:355–360. doi: 10.1002/hipo.10088. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Merlin LR, Wong RKS. Synchronized oscillations in hippocampal CA3 neurons induced by metabotropic glutamate receptor activation. J Neurosci. 1995;15:8039–8052. doi: 10.1523/JNEUROSCI.15-12-08039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrian DM, Ways DK, Gannon RL, Zetts DA. Transduction of a protein kinase C-generated signal into the long-lasting facilitation of glutamate release. Hippocampus. 1993;3:205–220. doi: 10.1002/hipo.450030212. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Borck C, Colling SB, Jefferys JGR. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Usachev YM, Marsh AJ, Johanns TM, Lemke MM, Thayer SA. Activation of protein kinase C in sensory neurons accelerates Ca2+ uptake into the endoplasmic reticulum. J Neurosci. 2006;26:311–318. doi: 10.1523/JNEUROSCI.2920-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J Neurosci. 2000;20:7863–7870. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]