Introduction
The business of defending the body against invading microorganisms relies heavily on the action of monocytes/macrophages and granulocytes (also called neutrophils). These differentiated cell types are derived from myeloid stem and progenitor cells present in the bone marrow. In response to foreign microorganisms, the production of monocytes/macrophages and granulocytes is dramatically increased, allowing the host to fight infection. Once the infection has cleared, the production of these cells is curtailed and their circulating and tissue levels quickly return to normal. In particular, the levels of granulocytes drop quickly once they are no longer needed, due to the inherent short half-lives of these differentiated cells and their rapid death via apoptosis.
The physiological production of mature myeloid lineage cells from bone marrow progenitors has taken on special significance in the field of myeloid leukemias. Myeloid leukemias are characterized by genetic defects which serve to block the process of myeloid differentiation. The consequences of these differentiation blockades are twofold. First, the normal production of monocytes/macrophages and granulocytes is impaired. In cases of acute myeloid leukemias this can lead to high vulnerability to infections. Second, blockade of myeloid differentiation leads to aberrant accumulation of proliferative blast cells in the bone marrow. Eventually these proliferating and malignant blasts begin to crowd out normal marrow progenitor cells, further impairing effective immune responses, and manifesting full-blown leukemia (Sachs, 1980).
Molecular studies have identified a number of genetic defects that contribute to differentiation blockades in myeloid leukemias. Chromosomal translocations that give rise to fusion oncoproteins such as PML/RARα(de The et al., 1990; Kakizuka et al., 1991; Pandolfi et al., 1991), AML1/ETO (Erickson et al., 1992; Yuan et al., 2001) and CBFβ/MYH11 (Kogan et al., 1998), occur with high frequency in acute myeloid leukemia (AML). Often these fusion proteins incorporate portions of important transcription factors, such as RARα, and the resulting oncoprotein exhibits altered transcriptional activity relative to its wild-type counterpart (Melnick and Licht, 1999; Redner, 2002). Other types of AML express mutant forms of cytokine receptors, including FLT3/ITD (Gilliland and Griffin, 2002) or truncated G-CSF receptor (Dong et al., 1995). In addition, mutant forms of PU.1 and C/EBPα(Mueller et al., 2002; Snaddon et al., 2003), transcription factors that are known to be important for myeloid differentiation, are commonly observed in AML.
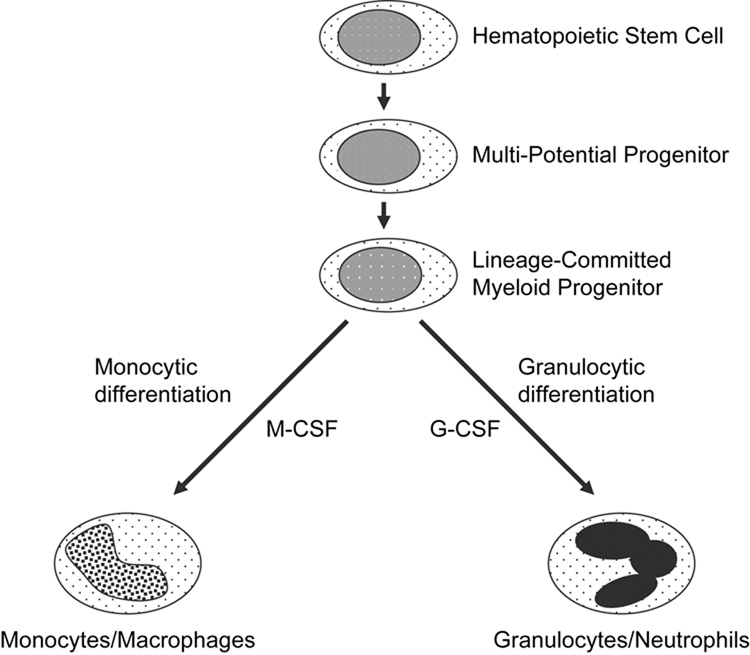
While the biological impact of many AML-associated genetic defects have become apparent, namely, blockade of myeloid differentiation, the molecular mechanisms of these blockades remain incompletely understood. Attempts to understand the mechanisms of differentiation blockades in AML have been slowed due to only modest understanding of the molecular mechanisms that are responsible for normal myeloid differentiation. What has been established is that normal differentiation of myeloid progenitors into monocytes/macrophages or granulocytes is governed by the action of hematopoietic cytokines. For example, monocytic differentiation is driven, in part, by M-CSF, while granulocytic differentiation is driven, in part, by the action of G-CSF (Figure 1). These differentiation-inducing cytokines bind to cognate receptors on the cell surface, leading to the activation of a number of intracellular signaling pathways and molecules, including tyrosine and serine/threonine kinases. Eventually, these signaling pathways induce or activate key transcription factors, such as PU.1 and C/EBPα, that serve to drive the process of myeloid differentiation. Recently, the development of unique animal models and application of highly specific pharmacologic inhibitors has begun to shed light on the importance and role of intracellular kinases in the regulation of myeloid differentiation. This review will focus on the involvement and role of Src family kinases (SFKs) and the MEK/ERK pathway in mediating normal myeloid differentiation. In addition, the consequences of aberrant SFK or MEK/ERK expression or activation towards the development and progression of leukemia will be discussed.
Fig. 1.
Schematic of myeloid differentiation. G-CSF promotes granulocytic differentiation, while M-CSF promotes monocytic differentiation.
Structure, function, and activation of Src family kinases
Src family kinases (SFKs) are intracellular tyrosine kinases that are activated upon engagement of growth factor or cytokine receptors, cellular attachment, or binding of antigen to antigen receptors. Nine different SFKs are known to exist, and include Src, Lyn, Fgr, Hck, Lck, Blk, Fyn, Yes, and Yrk (Thomas and Brugge, 1997). While Src, Fyn, and Yes are ubiquitously expressed, the expression of Lyn, Fgr, Hck, Blk, and Lck appears to be largely restricted to hematopoietic cells (Abram and Courtneidge, 2000; Corey and Anderson, 1999). Myeloid lineage cells predominantly express Lyn, Fgr, and Hck. Src was the first member of this family to be discovered and has represented the prototype for molecular and functional studies (Boggon and Eck, 2004; Martin, 1970). Additionally, Src is the SFK that has most frequently been associated with human cancers.
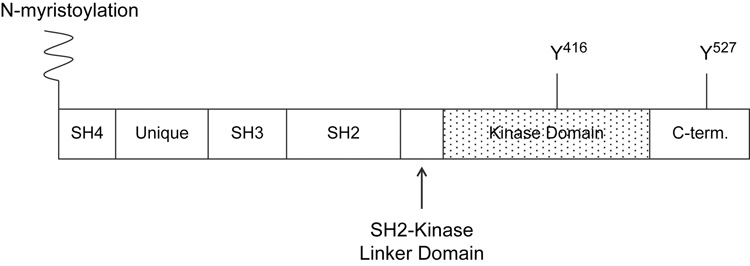
The overall structural organization of SFKs is conserved among the different family members and is reasonably represented by that of Src. Src consists of at least seven distinct functional domains (Figure 2). The N-terminus of Src begins with an SH4 domain, which contains sites for myristoylation and palmitoylation. SFKs are consistently modified by myristolyation, and occasionally by palmitoylation (Koegl et al. 1994; Resh, 1999). These modifications serve to anchor SFKs on the inners surface of the plasma membrane, bringing them into close proximity with activators such as cytokine receptors and substrates such as FAK. Following the SH4 domain is a unique region that differs among the different SFKs. The unique region is followed by an SH3 domain. The SH3 domain binds to proline-rich regions in substrate proteins, and, as described below, participates in intramolecular interactions that are important for autoinhibition of the enzyme (Gonfloni et al., 1997; Moarefi et al., 1997).The SH3 domain is followed by an SH2 domain which, like the SH2 domains in numerous other signaling proteins, recognizes and binds to phophorylated tyrosine residues. The Src SH2 domain also participates in autoinhibition of the enzyme through binding to phosphoTyr527 present in the C-terminal region of the protein (Sicheri et al., 1997; Williams et al., 1997; Xu et al., 1997). Between the SH2 domain and the catalytic domain lies a region termed the SH2-kinase linker domain. The catalytic domain (also called the SH1 domain) of Src is a bi-lobed structure, where ATP nestles into the hinge region connecting the two lobes (Boggon and Eck, 2004). The C-terminal lobe of the catalytic domain contains an activation loop harboring Tyr416; phosphorylation of this residue is important for activation of the enzyme (Roskoski, 2005; Smart et al., 1981). The catalytic domain is followed by the C-terminal region containing the phosphoTyr527 regulatory residue (Cooper et al., 1986; Roskoski, 2005).
Fig. 2.
Schematic of Src structure.
In an unstimulated state, the activity of Src is autoinhibited via intramolecular interactions. Inactive Src lacks phosphorylation at Tyr416, but is phosphorylated on Tyr527 by c-Src kinase (CSK) or the related enzyme, CSK homologous kinase (CHK) (Davidson et al., 1997; Hamaguchi et al., 1996; Nada et al., 1991). The phosphoTyr527 residue is bound via an intramolecular interaction with the SH2 domain of Src (Sicheri et al., 1997; Williams et al., 1997; Xu et al., 1997). Similarly, in the inactive Src enzyme, the SH3 domains binds to a proline-containing sequence in the SH2-kinase linker domain (Gonfloni et al., 1997; Moarefi et al., 1997). Together, these two intramolecular interactions serve to prevent phosphorylation of Tyr416 in the enzyme activation loop (Xu et al., 1999). The importance of these intramolecular interactions has been clearly delineated by the determination of the crystal structures of Src and Hck in the autoinhibited state (Schindler et al., 1999; Sicheri et al., 1997; Xu et al., 1997). In addition, viral Src (v-Src) is known to be constitutively active and is missing the corresponding Tyr527 regulatory residue (Cooper et al., 1986; Takeya and Hanafusa, 1983). Moreover, mutations in the SH3 or SH2 domains of Src that abrogate the key intramolecular interactions have been shown to result in enzyme activation (Boggon and Eck, 2004).
Activation of Src requires dephosphorylation of Tyr527 and phosphorylation of Tyr416 in the activation loop (Roskoski, 2005). Both of these events are dependent on disruption of the interaction between the SH2 domain and phosphoTyr527, as well as the interaction between the SH3 domain and the SH2-kinase linker domain. Disruption of the SH2/phosphoTyr527 interaction can occur when the SH2 domain binds instead to phosphotyrosine residues on a different protein. It is interesting that the preferred binding substrate of the Src SH2 domain is phosphoTyr-Glu-Glu-Ile (Songyang et al., 1993), though the site surrounding Tyr527 in Src contains a glycine residue instead of an isoleucine residue. This has suggested that proteins bearing the preferred sequence may be able to easily disrupt the Src SH2/phosphoTyr527 intramolecular interaction. Similarly, the SH2-kinase linker region lacks the Pro-Xxx-Xxx-Pro sequence preferred by SH3 domains, indicating that proteins bearing this sequence may be likely to displace the intramolecular inhibitory interaction between the Src SH3 domain and the SH2- kinase linker region.
Src family kinases in myeloid malignancies
Members of the SFK family have been implicated in both chronic and acute myeloid leukemias. In the case of CML, SFKs likely mediate survival and proliferative signals emanating from the BCR/ABL tyrosine kinase. Hck and Lyn physically associate with BCR/ABL, leading to their activation (Danhauser-Riedl et al., 1996; Klejman et al., 2002). Activation of Hck by BCR/ABL has been shown to lead to activation of STAT5, an important downstream mediator of BCR/ABL (Klejman et al., 2002). Experiments utilizing pharmacologic or dominant-negative inhibitors of SFKs have demonstrated important roles for these enzymes in facilitating BCR/ABL-dependent proliferation and cytokine independence (Klejman et al., 2002; Lionberger et al., 2000; Wilson et al., 2002). Moreover, overexpression or hyperactivation of SFKs has been observed in cases of CML that are resistant to the BCR/ABL inhibitor STI-571 (Kantarjian et al., 2007). These findings have suggested that targeting of the SFKs may have therapeutic benefit in the treatment of STI-571-resistant CML. Interestingly, structural studies have shown that Src and ABL bear striking similarities (Nagar et al., 2002; Nagar et al., 2003). It is not surprising then, that several small molecule inhibitors of SFKs also potently inhibit ABL and BCR/ABL (Kantarjian et al., 2007). In particular, dasatinib (BMS-354825; (Lombardo et al., 2004)) inhibits SFKs and BCR/ABL with IC50 values in the low nanomolar range. Notably, dasatinib inhibits BCR/ABL-induced cell growth in STI-571 resistant cells, and exhibits anti-tumor effects in murine models of STI-571-resistant disease (O'Hare et al., 2005; Shah et al., 2004). Treatment with dasatinib has demonstrated significant clinical benefit in several human trials of STI-571-resistant CML (Cortes et al., 2007; Guilhot et al., 2007; Hochhaus et al., 2007; Talpaz et al., 2006)
Overexpression and/or hyperactivation of SFKs have also been observed in acute leukemias. Lyn is overexpressed and hyperactivated in a majority of primary AML blasts, and pharmacologic inhibition of SFKs inhibits the growth of human leukemia cell lines and patient blasts (Roginskaya et al., 1999; Sakhinia et al., 2005). Recent evidence indicates that SFKs, particularly Lyn, may mediate the effects of FLT3/ITD in AML (Okamoto et al., 2007; Robinson et al., 2005). The FLT3/ITD mutation is found in approximately 30 percent of AML patients (Small, 2006). While stimulation of wild-type FLT3 receptor results in binding and activation of Lyn, the FLT3/ITD constitutively-active mutant binds Lyn with even greater affinity (Okamoto et al., 2007). Moreover, pharmacologic inhibition of SFKs, or siRNA-mediated downregulation of Lyn, suppresses cytokine-independent growth of FLT3/ITD-expressing cells (Okamoto et al., 2007; Robinson et al., 2005). Thus, while SFKs have been shown to play an important role in mediating the effects of BCR/ABL in CML, they also appear to play a critical role in mediating the effects of FLT3/ITD expression in AML.
Src family kinases in myeloid differentiation and myelopoiesis
Evidence suggesting a role for SFKs in myeloid differentiation and myelopoiesis in vivo has come from several different angles. Early correlative studies noted an increase in the expression and activities of SFKs during myeloid differentiation. Specifically, Src was noted to be induced and activated during monocytic differentiation of HL60 and U937 cells (Barnekow and Gessler, 1986; Gee et al., 1986; Meier et al., 1992; Yu and Glazer, 1987). Lyn and Fgr were found to be induced and activated in HL60 cells undergoing either monocytic or granulocytic differentiation (Katagiri et al., 1991; Katagiri et al., 1996; Miyazaki et al., 1993; Notario et al., 1989). Induced expression of Fgr and Hck was also observed during myeloid differentiation of leukemic blasts (Willman et al., 1991), while Fgr was found to be upregulated during myeloid differentiation of normal hematopoietic progenitors (Link and Zutter, 1995; Willman et al., 1987). The induction of SFK activities during myeloid differentiation is consistent with their activation by differentiation-inducing cytokines. G-CSF promotes granulocytic differentiation, and ligand-activated G-CSF receptors bind and activate both Lyn and Hck (Corey et al., 1994; Ward et al., 1998).
The precise role that SFKs play during myeloid differentiation remains unclear. However, evidence has accumulated that SFKs play an important role in promoting cell survival during the differentiation process. Antisense-mediated downregulation of Fgr or Lyn stimulates cell death during retinoic acid-induced differentiation of HL60 cells (Katagiri et al., 1996). Lyn also appears to mediate G-CSF-induced activation of PI3-K and Akt (Zhu et al., 2006), cellular kinases that inhibit apoptotic cell death. As both monocytic and granulocytic differentiation require days to complete, mechanisms, possibly involving SFKs, clearly must be in place to sustain the survival of the nonproliferating, differentiating cells.
Insights regarding the roles of SFKs during myelopoiesis in vivo have come from gene knockout mice. Src knockout mice exhibit normal myelopoiesis, but are deficient in bone remodeling and develop osteopetrosis (Soriano et al., 1991). Mice that are deficient in Lyn, Fgr, and Hck display normal resting granulopoiesis (Fitzer-Attas et al., 2000; Meng and Lowell, 1997). However, lyn−/− mice have increased numbers of granulocytic progenitors, granulocytic-monocytic progenitors, and mutipotential progenitors (Harder et al., 2001; Mermel et al., 2006). In addition, Lyn-deficient mice develop monocyte/macrophage tumors (Harder et al., 2001). Collectively, these findings suggest an important role for Lyn in negatively regulating progenitor pool expansion. A hyper-responsiveness to G-CSF is seen in myeloid progenitors from hck−/− mice, indicating a role for Hck in suppressing G-CSF-induced proliferation of granulocyte progenitors (Mermel et al., 2006). hck−/−fgr−/− double knockout mice manifest impaired adhesion-dependent neutrophil functions, suggesting a role for SFKs in the cellular function of these highly differentiated cells (Lowell et al., 1996; Mocsai et al., 1999). Triple knockout mice that are deficient in Hck, Lyn, and Fgr exhibit elevated levels of bone marrow progenitors and enhanced neutrophil responsiveness to G-CSF (Mermel et al., 2006). Although many of the abnormalities observed in SFK knockout mice are subtle in nature, it should be appreciated that redundancy among the different SFKs may serve to mask additional roles in myeloid differentiation and myelopoiesis. None-the-less, clear evidence exists that certain SFKs act to inhibit progenitor pool expansion, and are important for the specialized function of fully differentiated myeloid cells.
The MEK/ERK signaling pathway
The induction of myeloid differentiation by hematopoietic cytokines such as G-CSF (granulocytic differentiation) or M-CSF (monocytic differentiation) leads to the activation of corresponding cell surface receptors for these cytokines. Activation of the G-CSF or M-CSF receptors (G-CSFR or M-CSFR) results in the activation of a number of intracellular signaling pathways and molecules, including kinases and nonkinases, and ultimately results in the activation of specific transcription factors. It is currently understood that the activation of these transcription factors, and the subsequent modulation of target gene expression, drives the differentiation process.
While the M-CSFR is a ligand activated tyrosine kinase (Sherr and Rettenmier, 1986; Sherr et al., 1988), the G-CSFR does not contain intrinsic kinase activity. Rather, ligand binding induces G-CSFR dimerization, followed by activation of receptor-associated kinases, including members of the JAK kinase family (Ihle and Kerr, 1995). In either case, activation of the G-CSFR or the M-CSFR has been shown to result in the rapid phosphorylation and activation STAT3, STAT5, PI-3K, and PLC-γ(de Koning et al., 1998; Hunter and Avalos, 1998; Liu et al., 1998; Tian et al., 1996). Ras also becomes activated, and this leads to the recruitment and activation of the Raf kinase family, comprised of Raf-1, A-Raf, and B-Raf (de Koning et al., 1998; McCubrey et al., 2006). Raf enzymes phosphorylate a number of proteins, but key among these substrates are the kinases MEK-1 and MEK-2. All three Raf enzymes phosphorylate MEK-1 and MEK-2 on two closely spaced serine residues (Ser217 and Ser221 in human MEK-1), which results in activation of the MEK-1/-2 enzymes (Alessi et al., 1994; Dent et al., 1992; Kyriakis et al., 1992; Papin et al., 1995). MEK-1 and -2 are dual specificity kinases, and are somewhat remarkable in that their only known targets are the kinases ERK-1 and ERK-2 (also known at MAP kinase-1 and MAP kinase-2). MEK-1/-2 phosphorylate ERK-1 and -2 on threonine and tyrosine residues (Thr202 and Tyr204 in ERK-1; Thr185 and Tyr187 in ERK-2), leading to the activation of the ERK-1/-2 enzymes (Chang and Karin, 2001; Crews et al., 1992; Payne et al., 1991). Activated ERK-1 and -2 have been shown to phosphorylate a number of different substrates, including p90RSK and the transcription factors Elk-1, AP-1, and c-Myc (Deng et al., 2000; Hill et al., 1993; Karin, 1995; Lazar et al., 1995; Seth et al., 1992).
The ability of the MEK/ERK signaling pathway to promote cellular proliferation has been well-established (Cowley et al., 1994; Hoshino et al., 1999; Mansour et al., 1994; McCubrey et al., 2007a; Pages et al., 1993). The impact of MEK/ERK activation on proliferation appears to stem, at least in part, from ERK-mediated phosphorylation of AP-1, a transcription factor that drives expression of the cyclin D1 gene (Treinies et al., 1999). ERK-1 and -2 also phosphorylate carbamoyl phosphate synthetase II, a key enzyme in pyrimidine biosynthesis (Graves et al., 2000).
Activation of the MEK/ERK pathway also supports cellular survival, by inhibiting apoptosis (Ballif and Blenis, 2001; Bonni et al., 1999; Deng et al., 2000; Hoshino et al., 1999). Indeed, the anti-apoptotic action of BCR/ABL has been shown to be dependent on downstream activation of the MEK/ERK pathway (Jin et al., 2006; Kang et al., 2000; Nawata et al., 2003; Yu et al., 2002b). Moreover, forced expression of constitutively-active MEK-1 enzyme potently inhibits cytokine withdrawal-induced apoptosis (Blalock et al., 2000). These effects on cell survival may be due to ERK-1/-2- mediated phosphorylation of the apoptosis regulatory proteins Bcl-2 and Bim (Deng et al., 2000; Ley et al., 2003). In addition, ERK-1/-2 also influence the cellular activities of caspase-9, Bad, Mcl-1, and survivin (McCubrey et al., 2007b).
In addition to effects on cell survival and proliferation, recent evidence indicates that MEK/ERK activation can promote cellular differentiation (Miranda and Johnson, 2007). These findings, and the role that cellular context plays in the ability of the MEK/ERK pathway to stimulate differentiation, are discussed below.
Activation and importance of the MEK/ERK pathway in myeloid malignancies
The importance of the MEK/ERK pathway in promoting proliferation and survival is underscored by the prevalence of MEK/ERK hyperactivation in myeloid malignancies. Sustained hyperactivation of the MEK/ERK pathway has been reported in a majority of acute and chronic myeloid leukemias (Kim et al., 1999; Kornblau et al., 2006; Milella et al., 2001; Platanias, 2003; Ricciardi et al., 2005; Staber et al., 2004; Towatari et al., 1997). Pharmacologic inhibition of the MEK/ERK pathway inhibits proliferation and induces apoptosis in primary AML blasts (Lunghi et al., 2003). Moreover, in AML cells undergoing retinoid-induced differentiation, inhibition of MEK/ERK signaling converts the differentiation response to an apoptotic response (Milella et al., 2007). In cell line models, forced expression of a constitutively-active MEK-1 enzyme protects against cytokine withdrawal- and chemotherapy-induced apoptosis (Blalock et al., 2000; McCubrey et al., 2007b).
The MEK/ERK pathway likely plays an important role in mediating the effects of BCR/ABL in CML and FLT3/ITD in AML. The BCR/ABL tyrosine kinase activates the MEK/ERK pathway, leading to induction of proliferation and suppression of apoptosis (Cortez et al., 1997; Jin et al., 2006). The anti-apoptotic effects of BCR/ABL have been shown to mediated, in part, via MEK-1-mediated activation of NF-kB (Nawata et al., 2003). The FLT3/ITD mutant cytokine receptor commonly expressed in AML also activates the MEK/ERK signaling pathway (Stirewalt and Radich, 2003). In this case, activated ERKs may phosphorylate C/EBPαon serine 21, inhibiting the activity of this key transcription factor and, thereby, contributing to the differentiation blockade in FLT3/ITD-expressing AML (Radomska et al., 2006; Ross et al., 2004).
The central importance of the MEK/ERK pathway in sustaining cellular proliferation and survival in a large percentage of myeloid leukemias suggests that molecular targeting of this pathway may enhance the efficacy of anti-leukemic agents. Indeed, pharmacologic inhibition of the MEK/ERK pathway synergizes or enhances the anti-leukemic activities of a variety of agents. Specifically, MEK/ERK inhibition in AML cells has manifested synergistic (or enhancement) induction of apoptosis when combined with paclitaxel (Yu et al., 2001), TNFα(Nakada et al., 2001), the Bcl-2 inhibitor HA14-1 (Milella et al., 2002), UCN-01 (Yu et al., 2002a), radiation (Shonai et al., 2002), STI-571 (Yu et al., 2002b), lovastatin (Wu et al., 2004), and histone deacetylase inhibitors (Yu et al., 2005). The remarkable successes of these in vitro studies has heightened enthusiasm for clinical trials evaluating MEK/ERK inhibitors in combination with standard anti-leukemia agents
Role of the MEK/ERK pathway in myeloid differentiation
As described above, an extensive literature supports an important role for the MEK/ERK pathway in promoting the proliferation and survival of myeloid leukemia cells. The sheer volume and clarity of this data has somewhat overshadowed recent findings linking MEK/ERK activation to induction of myeloid differentiation. Effects on differentiation have largely gone unnoticed since most studies investigating this pathway in myeloid lineage cells have been performed in differentiation-incompetent leukemic cells (Miranda and Johnson, 2007). However, studies using differentiation-competent myeloid cell lines, as well as normal myeloid progenitors, have now clearly demonstrated an important role for MEK/ERK activation in myeloid differentiation (Miranda and Johnson, 2007).
Experiments employing pharmacologic inhibitors of the MEK enzymes have shown that the MEK/ERK pathway is important for phorbol 12-myristate 13-acetate (PMA)-induced differentiation of K562 (Herrera et al., 1998; Shelly et al., 1998), TF1a (Hu et al., 2000), and U937 cells (Miranda et al., 2002). Similarly, in HL60 cells, activation of the MEK/ERK pathway is required for PMA- or 1,25-dihydroxyvitamin D3- induced monocytic differentiation and ATRA-induced granulocytic differentiation (Miranda et al., 2003; Miranda et al., 2002; Wang and Studzinski, 2001; Yen et al., 1998). Induction of monocytic or granulocytic differentiation by chemical stimuli results in rapid and prolonged activation of the MEK/ERK pathway, and prolonged, versus transient, activation appears necessary for fostering differentiation (Hu et al., 2000; Miranda et al., 2002). The induction of monocytic differentiation by IL-6 or M-CSF, and the induction of granulocytic differentiation by G-CSF, also is accompanied by rapid and sustained activation of the MEK/ERK pathway (Gobert Gosse et al., 2005; Miranda et al., 2005). Moreover, the requirement for sustained activation of this pathway during cytokine-induced myeloid differentiation has been demonstrated using both cell line models and bone marrow-derived normal myeloid progenitors (Miranda et al., 2005). Inhibition of cytokine-induced differentiation following pharmacologic inhibition of the MEK/ERK pathway is associated with inhibition of the STAT3 and PU.1 transcription factors, which may account for the blockade in differentiation (Miranda et al., 2005). Additional studies have shown that the MEK/ERK pathway may regulate the phosphorylation and/or localization of C/EBPα(Radomska et al., 2006; Ross et al., 2004), C/EBPβ(Marcinkowska et al., 2006), and AML1 (Tanaka et al., 1996), transcription factors that are known to be important for myeloid differentiation. Lastly, recent studies indicate significant crosstalk between cytokine-induced differentiation pathways and ATRA-induced differentiation pathways. The abilities of G-CSF and GM-CSF to potentiate ATRA-induced differentiation of AML cell lines and primary AML cells is strictly dependent on the MEK/ERK pathway (Glasow et al., 2005).
Summary
The production of mature monocytes/macrophages and granulocytes via myeloid differentiation is a central component of the host defense mechanism against invading microorganisms. However, in myeloid leukemias genetic changes lead to blockade of myeloid differentiation. When this happens, immune responses can become severely impaired and the accumulation of proliferative blasts causes bone marrow crowding and onset of symptomatic leukemia. There is considerable hope that molecular targeting of specific signaling pathways and proteins will prove to be a viable strategy for restoring differentiation potential in myeloid leukemias. Indeed, in acute promyelocytic leukemia, treatment with ATRA can overcome the differentiation blockade and is an effective curative approach. To devise strategies and reagents that can be used to induce differentiation in other myeloid leukemias, it is important to gain an understanding of the molecular pathways that drive the normal differentiation process. Emerging evidence implicates both Src family kinases and the MEK/ERK pathway in regulating myeloid differentiation.
It is interesting that Src family kinases appear to be negative regulators of myelopoiesis, while the MEK/ERK pathway is an important positive regulator of both monocytic and granulocytic differentiation. This suggests that pharmacologic inhibitors of SFKs may be of value in restoring or enhancing myeloid differentiation. In this regard, the SFK inhibitor dasatinib has recently been approved by the FDA for use in imatinib-resistant CML. Evaluation of dasatinib, alone or in combination with differentiation inducers, in the treatment of differentiation-defective AML seems warranted.
The important role that the MEK/ERK pathway plays in promoting myeloid differentiation appears to conflict with observations that the MEK/ERK pathway is hyperactivated or overexpressed in a majority of primary AMLs. Moreover, pharmacologic inhibition of the MEK/ERK pathway in AML results in the induction of apoptosis, indicating that MEK/ERK activation is important for the survival of AML cells. Collectively, these data suggest that the MEK/ERK pathway may play more than one role in myeloid lineage cells, depending on the cellular context. In normal myeloid cells, activation of the MEK/ERK pathway is important for promoting differentiation. However, when differentiation becomes blocked, as is the case in most AMLs, MEK/ERK activation can no longer drive differentiation, and instead begins to support cellular survival or proliferation. Thus, therapeutic strategies aimed at provoking myeloid differentiation in AML by stimulating the MEK/ERK pathway are unlikely to be successful unless the differentiation blockade is simultaneously relieved.
Acknowledgments
This work was supported by National Institutes of Health grant R01 CA108904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abram CL, Courtneidge SA. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. Embo J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- Barnekow A, Gessler M. Activation of the pp60c-src kinase during differentiation of monomyelocytic cells in vitro. Embo J. 1986;5:701–705. doi: 10.1002/j.1460-2075.1986.tb04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock WL, Pearce M, Steelman LS, Franklin RA, McCarthy SA, Cherwinski H, McMahon M, McCubrey JA. A conditionally-active form of MEK1 results in autocrine tranformation of human and mouse hematopoietic cells. Oncogene. 2000;19:526–536. doi: 10.1038/sj.onc.1203337. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Corey SJ, Anderson SM. Src-related protein tyrosine kinases in hematopoiesis. Blood. 1999;93:1–14. [PubMed] [Google Scholar]
- Corey SJ, Burkhardt AL, Bolen JB, Geahlen RL, Tkatch LS, Tweardy DJ. Granulocyte colony-stimulating factor receptor signaling involves the formation of a three-component complex with Lyn and Syk protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1994;91:4683–4687. doi: 10.1073/pnas.91.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, Rousselot P, Kim DW, Ritchie E, Hamerschlak N, Coutre S, Hochhaus A, Guilhot F, Saglio G, Apperley J, Ottmann O, Shah N, Erben P, Branford S, Agarwal P, Gollerkeri A, Baccarani M. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- Cortez D, Reuther G, Pendergast AM. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Crews CM, Alessandrini A, Erikson RL. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Danhauser-Riedl S, Warmuth M, Druker BJ, Emmerich B, Hallek M. Activation of Src kinases p53/56lyn and p59hck by p210bcr/abl in myeloid cells. Cancer Res. 1996;56:3589–3596. [PubMed] [Google Scholar]
- Davidson D, Chow LM, Veillette A. Chk, a Csk family tyrosine protein kinase, exhibits Csk-like activity in fibroblasts, but not in an antigen-specific T-cell line. J Biol Chem. 1997;272:1355–1362. doi: 10.1074/jbc.272.2.1355. [DOI] [PubMed] [Google Scholar]
- de Koning JP, Soede-Bobok AA, Schelen AM, Smith L, van Leeuwen D, Santini V, Burgering BMT, Bos JL, Lowenberg B, Touw IP. Proliferation signaling and activation of Shc, p21Ras, and Myc via tyrosine 764 of human granulocyte colony-stimulating factor receptor. Blood. 1998;91:1924–1933. [PubMed] [Google Scholar]
- de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci U S A. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Haser W, Haystead TA, Vincent LA, Roberts TM, Sturgill TW. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Dong F, Brynes RK, Tidow N, Welte K, Lowenberg B, Touw IP. Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med. 1995;333:487–493. doi: 10.1056/NEJM199508243330804. [DOI] [PubMed] [Google Scholar]
- Erickson P, Gao J, Chang KS, Look T, Whisenant E, Raimondi S, Lasher R, Trujillo J, Rowley J, Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992;80:1825–1831. [PubMed] [Google Scholar]
- Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–682. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee CE, Griffin J, Sastre L, Miller LJ, Springer TA, Piwnica-Worms H, Roberts TM. Differentiation of myeloid cells is accompanied by increased levels of pp60c-src protein and kinase activity. Proc Natl Acad Sci U S A. 1986;83:5131–5135. doi: 10.1073/pnas.83.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9:274–281. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Glasow A, Prodromou N, Xu K, von Lindern M, Zelent A. Retinoids and myelomonocytic growth factors cooperatively activate RARA and induce human myeloid leukemia cell differentiation via MAP kinase pathways. Blood. 2005;105:341–349. doi: 10.1182/blood-2004-03-1074. [DOI] [PubMed] [Google Scholar]
- Gobert Gosse S, Bourgin C, Liu WQ, Garbay C, Mouchiroud G. M-CSF stimulated differentiation requires persistent MEK activity and MAPK phosphorylation independent of Grb2-Sos association and phosphatidylinositol 3-kinase activity. Cell Signal. 2005;17:1352–1362. doi: 10.1016/j.cellsig.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Gonfloni S, Williams JC, Hattula K, Weijland A, Wierenga RK, Superti-Furga The role of the linker between the SH2 domain and catalytic domain in the regulation and function of Src. Embo J. 1997;16:7261–7271. doi: 10.1093/emboj/16.24.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LM, Guy HI, Kozlowski P, Huang M, Lazarowski E, Pope RM, Collins MA, Dahlstrand EN, Earp HS, Evans DR. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature. 2000;403:328–332. doi: 10.1038/35002111. [DOI] [PubMed] [Google Scholar]
- Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, Heim D, Larson RA, Branford S, Muller MC, Agarwal P, Gollerkeri A, Talpaz M. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- Hamaguchi I, Yamaguchi N, Suda J, Iwama A, Hirao A, Hashiyama M, Aizawa S-I, Suda T. Analysis of CSK homologous kinase (CHK/HYL) in hematopoiesis by utilizing gene knockout mice. Biochem Biophys Res Commun. 1996;224:172–179. doi: 10.1006/bbrc.1996.1003. [DOI] [PubMed] [Google Scholar]
- Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- Herrera R, Hubbell S, Decker S, Petruzzelli L. A role for the MEK/MAPK pathway in PMA-induced cell cycle arrest: modulation of megakaryocytic differentiation of K562 cells. Exp Cell Res. 1998;238:407–414. doi: 10.1006/excr.1997.3847. [DOI] [PubMed] [Google Scholar]
- Hill CS, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Kantarjian HM, Baccarani Mq, Lipton JH, Apperley JF, Druker BJ, Facon T, Goldberg SL, Cervantes F, Niederwieser D, Silver RT, Stone RM, Hughes TP, Muller MC, Ezzeddine R, Countouriotis AM, Shah NP. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- Hu X, Moscinski LC, Valkov NI, Fisher AB, Hill BJ, Zuckerman KS. Prolonged activation of the mitogen-activated protein kinase pathway is required for macrophage-like differentiation of a human myeloid leukemic cell line. Cell Growth Differ. 2000;11:191–200. [PubMed] [Google Scholar]
- Hunter MG, Avalos BR. Phosphatidylinositol 3'-kinase and SH2-containing inositol phosphatase (SHIP) are recruited by distinct positive and negative growth-regulatory domains in the granulocyte colony-stimulating factor receptor. J Immunol. 1998;160:4979–4987. [PubMed] [Google Scholar]
- Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Jin A, Kurosu T, Tsuji K, Mizuchi D, Arai A, Fujita H, Hattori M, Minato N, Miura O. BCR/ABL and IL-3 activate Rap1 to stimulate the B-Raf/MEK/Erk and Akt signaling pathways and to regulate proliferation, apoptosis, and adhesion. Oncogene. 2006;25:4332–4340. doi: 10.1038/sj.onc.1209459. [DOI] [PubMed] [Google Scholar]
- Kakizuka A, Miller WH, Jr, Umesono K, Warrell RP, Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Kang CD, Yoo SD, Hwang BW, Kim KW, Kim DW, Kim CM, Kim SH, Chung BS. The inhibition of ERK/MAPK not the activation of JNK/SAPK is primarily required to induce apoptosis in chronic myelogenous leukemic K562 cells. Leuk Res. 2000;24:527–534. doi: 10.1016/s0145-2126(00)00010-2. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Giles F, Quintas-Cardama A, Cortes J. Important therapeutic targets in chronic myelogenous leukemia. Clin Cancer Res. 2007;13:1089–1097. doi: 10.1158/1078-0432.CCR-06-2147. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Katagiri K, Katagiri T, Koyama Y, Morikawa M, Yamamoto T, Yoshida T. Expression of src family genes during monocytic differentiation of HL-60 cells. J Immunol. 1991;146:701–707. [PubMed] [Google Scholar]
- Katagiri K, Yokoyama KK, Yamamoto T, Omura S, Irie S, Katagiri T. Lyn and Fgr protein-tyrosine kinases prevent apoptosis during retinoic acid-induced granulocytic differentiation of HL-60 cells. J Biol Chem. 1996;271:11557–11562. doi: 10.1074/jbc.271.19.11557. [DOI] [PubMed] [Google Scholar]
- Kim SC, Hahn JS, Min YH, Yoo NC, Ko YW, Lee WJ. Constitutive activation of extracellular signal-regulated kinase in human acute leukemias: combined role of activation of MEK, hyperexpression of extracellular signal-regulated kinase, and downregulation of a phosphatase, PAC1. Blood. 1999;93:3893–3899. [PubMed] [Google Scholar]
- Klejman A, Schreiner SJ, Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE, Skorski T. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. Embo J. 2002;21:5766–5774. doi: 10.1093/emboj/cdf562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Zlatkine P, Ley SC, Courtneidge SA, Magee AI. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem J. 1994;303(Pt 3):749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan SC, Lagasse E, Atwater S, Bae SC, Weissman I, Ito Y, Bishop JM. The PEBP2betaMYH11 fusion created by Inv(16)(p13;q22) in myeloid leukemia impairs neutrophil maturation and contributes to granulocytic dysplasia. Proc Natl Acad Sci U S A. 1998;95:11863–11868. doi: 10.1073/pnas.95.20.11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Link DC, Zutter M. The proto-oncogene c-fgr is expressed in normal mantle zone B lymphocytes and is developmentally regulated during myelomonocytic differentiation in vivo. Blood. 1995;85:472–479. [PubMed] [Google Scholar]
- Lionberger JM, Wilson MB, Smithgall TE. Transformation of myeloid leukemia cells to cytokine independence by Bcr-Abl is suppressed by kinase-defective Hck. J Biol Chem. 2000;275:18581–18585. doi: 10.1074/jbc.C000126200. [DOI] [PubMed] [Google Scholar]
- Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi P, Tabilio A, Dall'Aglio PP, Ridolo E, Carlo-Stella C, Pelicci PG, Bonati A. Downmodulation of ERK activity inhibits the proliferation and induces the apoptosis of primary acute myelogenous leukemia blasts. Leukemia. 2003;17:1783–1793. doi: 10.1038/sj.leu.2403032. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970;227:1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D'Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007a;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Franklin RA, Abrams SL, Chappell WH, Wong EW, Lehmann BD, Terrian DM, Basecke J, Stivala F, Libra M, Evangelisti C, Martelli AM. Targeting the RAF/MEK/ERK, PI3K/AKT and P53 pathways in hematopoietic drug resistance. Adv Enzyme Regul. 2007b;47:64–103. doi: 10.1016/j.advenzreg.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier RW, Bielke W, Chen T, Niklaus G, Friis RR, Tobler A. Lyn, a src-like tyrosine-specific protein kinase, is expressed in HL60 cells induced to monocyte-like or granulocyte-like cells. Biochem Biophys Res Commun. 1992;185:91–95. doi: 10.1016/s0006-291x(05)80959-3. [DOI] [PubMed] [Google Scholar]
- Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermel CH, McLemore ML, Liu F, Pereira S, Woloszynek J, Lowell CA, Link DC. Src family kinases are important negative regulators of G-CSF-dependent granulopoiesis. Blood. 2006;108:2562–2568. doi: 10.1182/blood-2006-05-024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella M, Estrov Z, Kornblau SM, Carter BZ, Konopleva M, Tari A, Schober WD, Harris D, Leysath CE, Lopez-Berestein G, Huang Z, Andreeff M. Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood. 2002;99:3461–3464. doi: 10.1182/blood.v99.9.3461. [DOI] [PubMed] [Google Scholar]
- Milella M, Konopleva M, Precupanu CM, Tabe Y, Ricciardi MR, Gregorj C, Collins SJ, Carter BZ, D'Angelo C, Petrucci MT, Foa R, Cognetti F, Tafuri A, Andreeff M. MEK blockade converts AML differentiating response to retinoids into extensive apoptosis. Blood. 2007;109:2121–2129. doi: 10.1182/blood-2006-05-024679. [DOI] [PubMed] [Google Scholar]
- Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E, Andreeff M. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MB, Dyer KF, Grandis JR, Johnson DE. Differential activation of apoptosis regulatory pathways during monocytic vs granulocytic differentiation: a requirement for Bcl-X(L)and XIAP in the prolonged survival of monocytic cells. Leukemia. 2003;17:390–400. doi: 10.1038/sj.leu.2402779. [DOI] [PubMed] [Google Scholar]
- Miranda MB, Johnson DE. Signal transduction pathways that contribute to myeloid differentiation. Leukemia. 2007;21:1363–1377. doi: 10.1038/sj.leu.2404690. [DOI] [PubMed] [Google Scholar]
- Miranda MB, McGuire TF, Johnson DE. Importance of MEK-1/-2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002;16:683–692. doi: 10.1038/sj.leu.2402400. [DOI] [PubMed] [Google Scholar]
- Miranda MB, Xu H, Torchia JA, Johnson DE. Cytokine-induced myeloid differentiation is dependent on activation of the MEK/ERK pathway. Leuk Res. 2005;29:1293–1306. doi: 10.1016/j.leukres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Katamine S, Kohno T, Moriuchi R, Miyamoto T, Tomonaga M. fgr proto-oncogene is expressed during terminal granulocytic differentiation of human promyelocytic HL60 cells. Exp Hematol. 1993;21:366–371. [PubMed] [Google Scholar]
- Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J Immunol. 1999;162:1120–1126. [PubMed] [Google Scholar]
- Mueller BU, Pabst T, Osato M, Asou N, Johansen LM, Minden MD, Behre G, Hiddemann W, Ito Y, Tenen DG. Heterozygous PU.1 mutations are associated with acute myeloid leukemia. Blood. 2002;100:998–1007. doi: 10.1182/blood.v100.3.998. [DOI] [PubMed] [Google Scholar]
- Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62:4236–4243. [PubMed] [Google Scholar]
- Nagar B, Hantschelc O, Young MA, Scheffzek K, Veach D, Bornmann W, Clarkson B, Superti-Furga G, Kuriyan J. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–871. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- Nakada S, Kawano T, Saito-akita S, Iwase S, Horiguchi-Yamada J, Ohno T, Yamada H. MEK and p38MAPK inhibitors potentiate TNF-alpha induced apoptosis in U937 cells. Anticancer Res. 2001;21:167–171. [PubMed] [Google Scholar]
- Nawata R, Yujiri T, Nakamurac Y, Ariyoshi K, Takahashi T, Sato Y, Oka Y, Tanizawa Y. MEK kinase 1 mediates the antiapoptotic effect of the Bcr-Abl oncogene through NF-kappaB activation. Oncogene. 2003;22:7774–7780. doi: 10.1038/sj.onc.1206901. [DOI] [PubMed] [Google Scholar]
- Notario V, Gutkind JS, Imaizumi M, Katamine S, Robbins KC. Expression of the fgr protooncogene product as a function of myelomonocytic cell maturation. J Cell Biol. 1989;109:3129–3136. doi: 10.1083/jcb.109.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC, Deininger MW, Druker BJ. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Hayakawa F, Miyata Y, Watamoto K, Emi N, Abe A, Kiyoi H, Towatari M, Naoe T. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia. 2007;21:403–410. doi: 10.1038/sj.leu.2404547. [DOI] [PubMed] [Google Scholar]
- Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi PP, Grignani F, Alcalay M, Mencarelli A, Biondi A, LoCoco F, Grignani F, Pelicci PG. Structure and origin of the acute promyelocytic leukemia myl/RAR alpha cDNA and characterization of its retinoid-binding and transactivation properties. Oncogene. 1991;6:1285–1292. [PubMed] [Google Scholar]
- Papin C, Eychene A, Brunet A, Pages G, Pouyssegur J, Calothy G, Barnier JV. B-Raf protein isoforms interact with and phosphorylate Mek-1 on serine residues 218 and 222. Oncogene. 1995;10:1647–1651. [PubMed] [Google Scholar]
- Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) Embo J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- Radomska HS, Basseres DS, Zheng R, Zhang P, Dayaram T, Yamamoto Y, Sternberg DW, Lokker N, Giese NA, Bohlander SK, Schnittger S, Delmotte M-H, Davis RJ, Small D, Hiddemann W, Gilliland DG, Tenen DG. Block of C/EBP alpha function by phosphorylation in acute myeloid leukemia with FLT3 activating mutations. J Exp Med. 2006;203:371–381. doi: 10.1084/jem.20052242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redner RL. Variations on a theme: the alternate translocations in APL. Leukemia. 2002;16:1927–1932. doi: 10.1038/sj.leu.2402720. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E, Sebolt-Leopold J, Konopleva M, Andreeff M. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia. 2005;19:1543–1549. doi: 10.1038/sj.leu.2403859. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Xue J, Corey SJ. Src family tyrosine kinases are activated by Flt3 and are involved in the proliferative effects of leukemia-associated Flt3 mutations. Exp Hematol. 2005;33:469–479. doi: 10.1016/j.exphem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Roginskaya V, Zuo S, Caudell E, Nambudiri G, Kraker AJ, Corey SJ. Therapeutic targeting of Src-kinase Lyn in myeloid leukemic cell growth. Leukemia. 1999;13:855–861. doi: 10.1038/sj.leu.2401429. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ross SE, Radomska HS, Wu B, Zhang P, Winnay JN, Bajnok L, Wright WS, Schaufele F, Tenen DG, MacDougald OA. Phosphorylation of C/EBPalpha inhibits granulopoiesis. Mol Cell Biol. 2004;24:675–686. doi: 10.1128/MCB.24.2.675-686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980;77:6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhinia E, Faranghpour M, Liu Yin JA, Brady G, Hoyland JA, Byers RJ. Routine expression profiling of microarray gene signatures in acute leukaemia by real-time PCR of human bone marrow. Br J Haematol. 2005;130:233–248. doi: 10.1111/j.1365-2141.2005.05594.x. [DOI] [PubMed] [Google Scholar]
- Schindler T, Sicheri F, Pico A, Gazit A, Levitzki A, Kuriyan J. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol Cell. 1999;3:639–648. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- Seth A, Gonzalez FA, Gupta S, Raden DL, Davis RJ. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992;267:24796–24804. [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Shelly C, Petruzzelli L, Herrera R. PMA-induced phenotypic changes in K562 cells: MAPK-dependent and -independent events. Leukemia. 1998;12:1951–1961. doi: 10.1038/sj.leu.2401221. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Rettenmier CW. The fms gene and the CSF-1 receptor. Cancer Surv. 1986;5:221–232. [PubMed] [Google Scholar]
- Sherr CJ, Roussel MF, Rettenmier CW. Colony-stimulating factor-1 receptor (c-fms) J Cell Biochem. 1988;38:179–187. doi: 10.1002/jcb.240380305. [DOI] [PubMed] [Google Scholar]
- Shonai T, Adachi M, Sakata K, Takekawa M, Endo T, Imai K, Hareyama M. MEK/ERK pathway protects ionizing radiation-induced loss of mitochondrial membrane potential and cell death in lymphocytic leukemia cells. Cell Death Differ. 2002;9:963–971. doi: 10.1038/sj.cdd.4401050. [DOI] [PubMed] [Google Scholar]
- Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- Small D. FLT3 mutations: biology and treatment. Hematology Am Soc Hematol Educ Program. 2006:178–184. doi: 10.1182/asheducation-2006.1.178. [DOI] [PubMed] [Google Scholar]
- Smart JE, Oppermann H, Czernilofsky AP, Purchio AF, Erikson RL, Bishop JM. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src) Proc Natl Acad Sci U S A. 1981;78:6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaddon J, Smith ML, Neat M, Cambal-Parrales M, Dixon-McIver A, Arch R, Amess JA, Rohatiner AZ, Lister TA, Fitzgibbon J. Mutations of CEBPA in acute myeloid leukemia FAB types M1 and M2. Genes Chromosomes Cancer. 2003;37:72–78. doi: 10.1002/gcc.10185. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, Neel BG, Birge RB, Fajardo JE, Chou MM, Hanafusa H, Schaffhausen B, Cantley LC. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Staber PB, Linkesch W, Zauner D, Beham-Schmid C, Guelly C, Schauer S, Sill H, Hoefler G. Common alterations in gene expression and increased proliferation in recurrent acute myeloid leukemia. Oncogene. 2004;23:894–904. doi: 10.1038/sj.onc.1207192. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- Takeya T, Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983;32:881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kurokawa M, Ueki K, Tanaka K, Imai Y, Mitani K, Okazaki K, Sagata N, Yazaki Y, Shibata Y, Kadowaki T, Hirai H. The extracellular signal-regulated kinase pathway phosphorylates AML1, an acute myeloid leukemia gene product, and potentially regulates its transactivation ability. Mol Cell Biol. 1996;16:3967–3979. doi: 10.1128/mcb.16.7.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tian SS, Tapley P, Sincich C, Stein RB, Rosen J, Lamb P. Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5,and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood. 1996;88:4435–4444. [PubMed] [Google Scholar]
- Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11:479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- Treinies I, Paterson HF, Hooper S, Wilson R, Marshall CJ. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal To stimulate DNA synthesis. Mol Cell Biol. 1999;19:321–329. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–482. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ward AC, Monkhouse JL, Csar XF, Touw IP, Bello PA. The Src-like tyrosine kinase Hck is activated by granulocyte colony-stimulating factor (G-CSF) and docks to the activated G-CSF receptor. Biochem Biophys Res Commun. 1998;251:117–123. doi: 10.1006/bbrc.1998.9441. [DOI] [PubMed] [Google Scholar]
- Williams JC, Weijland A, Gonfloni S, Thompson A, Courtneidge SA, Superti-Furga G, Wierenga RK. The 2.35 A crystal structure of the inactivated form of chicken Src: a dynamic molecule with multiple regulatory interactions. J Mol Biol. 1997;274:757–775. doi: 10.1006/jmbi.1997.1426. [DOI] [PubMed] [Google Scholar]
- Willman CL, Stewart CC, Griffith JK, Stewart SJ, Tomasi TB. Differential expression and regulation of the c-src and c-fgr protooncogenes in myelomonocytic cells. Proc Natl Acad Sci U S A. 1987;84:4480–4484. doi: 10.1073/pnas.84.13.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willman CL, Stewart CC, Longacre TL, Head DR, Habbersett R, Ziegler SF, Perlmutter RM. Expression of the c-fgr and hck protein-tyrosine kinases in acute myeloid leukemic blasts is associated with early commitment and differentiation events in the monocytic and granulocytic lineages. Blood. 1991;77:726–734. [PubMed] [Google Scholar]
- Wilson MB, Schreiner SJ, Choi HJ, Kamens J, Smithgall TE. Selective pyrrolo-pyrimidine inhibitors reveal a necessary role for Src family kinases in Bcr-Abl signal transduction and oncogenesis. Oncogene. 2002;21:8075–8088. doi: 10.1038/sj.onc.1206008. [DOI] [PubMed] [Google Scholar]
- Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64:6461–6468. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–3172. [PubMed] [Google Scholar]
- Yu C, Dai Y, Dent P, Grant S. Coadministration of UCN-01 with MEK1/2 inhibitors potently induces apoptosis in BCR/ABL+ leukemia cells sensitive and resistant to ST1571. Cancer Biol Ther. 2002a;1:674–682. doi: 10.4161/cbt.319. [DOI] [PubMed] [Google Scholar]
- Yu C, Dasmahapatra G, Dent P, Grant S. Synergistic interactions between MEK1/2 and histone deacetylase inhibitors in BCR/ABL+ human leukemia cells. Leukemia. 2005;19:1579–1589. doi: 10.1038/sj.leu.2403868. [DOI] [PubMed] [Google Scholar]
- Yu C, Krystal G, Varticovksi L, McKinstry R, Rahmani M, Dent P, Grant S. Pharmacologic mitogen-activated protein/extracellular signal-regulated kinase kinase/mitogen-activated protein kinase inhibitors interact synergistically with STI571 to induce apoptosis in Bcr/Abl-expressing human leukemia cells. Cancer Res. 2002b;62:188–199. [PubMed] [Google Scholar]
- Yu C, Wang S, Dent P, Grant S. Sequence-dependent potentiation of paclitaxel-mediated apoptosis in human leukemia cells by inhibitors of the mitogen-activated protein kinase kinase/mitogen-activated protein kinase pathway. Mol Pharmacol. 2001;60:143–154. doi: 10.1124/mol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Yu G, Glazer RI. Purification and characterization of p93fes- and p60src-related tyrosine protein kinase activities in differentiated HL-60 leukemia cells. J Biol Chem. 1987;262:17543–17548. [PubMed] [Google Scholar]
- Yuan Y, Zhou L, Miyamoto T, Iwasaki H, Harakawa N, Hetherington CJ, Burel SA, Lagasse E, Weissman IL, Akashi K, Zhang D-E. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci U S A. 2001;98:10398–10403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood. 2006;107:1847–1856. doi: 10.1182/blood-2005-04-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]