Abstract
Gender-based differences in the incidence of hypertensive and coronary artery disease, the development of atherosclerosis, and myocardial remodeling after infarction are attributable to the indirect effect of estrogen on risk factor profiles such as cholesterol levels, glucose metabolism, and insulin levels. More recent evidence, however, suggests that activated estrogen receptor (ER) mediates signaling cascades that culminate in direct protective effects such as vasodilation, inhibition of response to vessel injury, limiting myocardial injury after infarction, and attenuating cardiac hypertrophy. Although the ER is usually thought of as a ligand-dependent transcription factor, it can also rapidly mobilize signals at the plasma membrane and in the cytoplasm. Thus, a greater understanding of ER function and regulation may lead to the development of highly specific therapeutics that mediate the prevention and treatment of cardiovascular diseases.
INTRODUCTION
Gender-based differences in the incidence of hypertensive and coronary artery disease, the development of atherosclerosis, and myocardial remodeling after infarction are attributable to the indirect effect of estrogen on risk factor profiles, such as cholesterol levels, glucose metabolism, and insulin levels (1–3), as well as its direct effects on the myocardium, vascular smooth muscle and endothelium. Although estrogen receptor (ER) is typically thought of as a ligand-dependent transcription factor, it also modulates the activity of intracellular second messengers and membrane-associated signaling complexes. In the heart and vasculature, these non-nuclear signaling pathways mediate rapid vasodilation (4), inhibition of response to vessel injury (5–10), reduction in myocardial injury after infarction (11, 12), and attenuation of cardiac hypertrophy (13, 14).
ESTROGEN RECEPTOR STRUCTURE AND FUNCTION
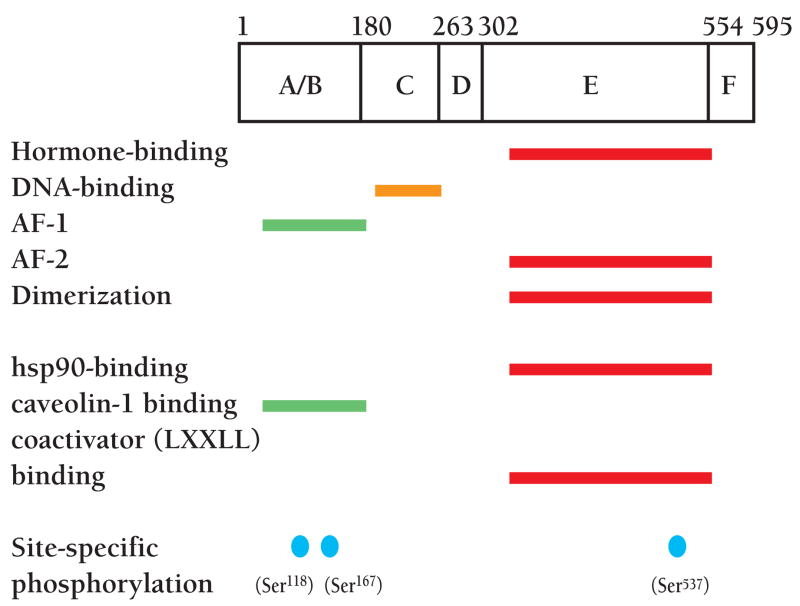
Both subtypes of ER, ERα and ERβ, are members of the nuclear receptor superfamily (15, 16). They are synthesized from separate genes and are structurally and functionally distinct. Classically, ER regulates gene expression in target tissues in a ligand-dependent manner: the binding of estradiol (E2) releases ER from an inhibitory complex and allows for receptor homodimerization and translocation into the nucleus (1, 2, 17). The receptor then binds a palindromic estrogen response element (ERE) located in the promoter region of target genes. The concerted actions of the ligand-independent activation function domain (AF-1) in the N terminus (Figure 1) and the ligand-dependent AF-2 region in the hormone-binding domain lead to the recruitment of tissue-, cell-, and promoter-specific co-regulator complexes to the ERE, resulting in transactivation or transrepression (18, 19).
Figure 1.
Functional regions of the human estrogen receptor α (ERα). These domains include a ligand-independent transactivation function domain (AF-1), DNA-binding domain, hormone-binding domain and ligand-dependent transactivation function domain (AF-2). Putative regions of interaction with other proteins and sites of phosphorylation by various kinases are also shown.
Gene deletion or mutation studies have underlined the importance of ER in cardiovascular physiology (20). Early studies of ovariectomized mice demonstrated that E2 inhibits the proliferation of intimal and medial vascular smooth muscle (5), suggesting a direct protective effect of estrogen on endothelium and vascular smooth muscle cells (VSMCs). In ERα and ERβ double-knockout mice, however, E2 inhibits VSMC proliferation but not medial thickening, suggesting that a leakily expressed splice-variant of ERα could mediate partial protection (21, 22). The more recent production of complete ERα-null mice (23), which exhibit increased medial area, VSMC proliferation, and deposition of proteoglycans in response to vascular injury, has confirmed the role of ERα in vascular protection (24). The effects also extend to the myocardium. For example, ERα-deficient hearts subjected to whole-organ ischemia and reperfusion (25) exhibit greater ischemia and higher incidence of arrhythmias than that observed in wild-type hearts. The process may involve nitric oxide (NO), which ameliorates coronary dysfunction and reduces tissue edema by decreasing microvascular permeability, because ERα-deficient hearts also demonstrate decreased NO release.
In 1975, Pietras and Szego first described membrane binding sites for estrogen and described a non-genomic mechanism for calcium influx in endometrial cells (26). More recent studies have added to our current understanding of the highly tissue-specific, non-nuclear ERα signaling network. Though there is also evidence that ERβ has an important function in the vasculature (27, 28), we focus on ERα because of the greater number of observations that have been made. Defining the cascades through which ERα elicits its pleiotropic cellular effects and understanding the dysregulation of the network in disease states promises to uncover novel targets for pharmacological intervention.
NON-NUCLEAR ACTIVITY OF ESTROGEN
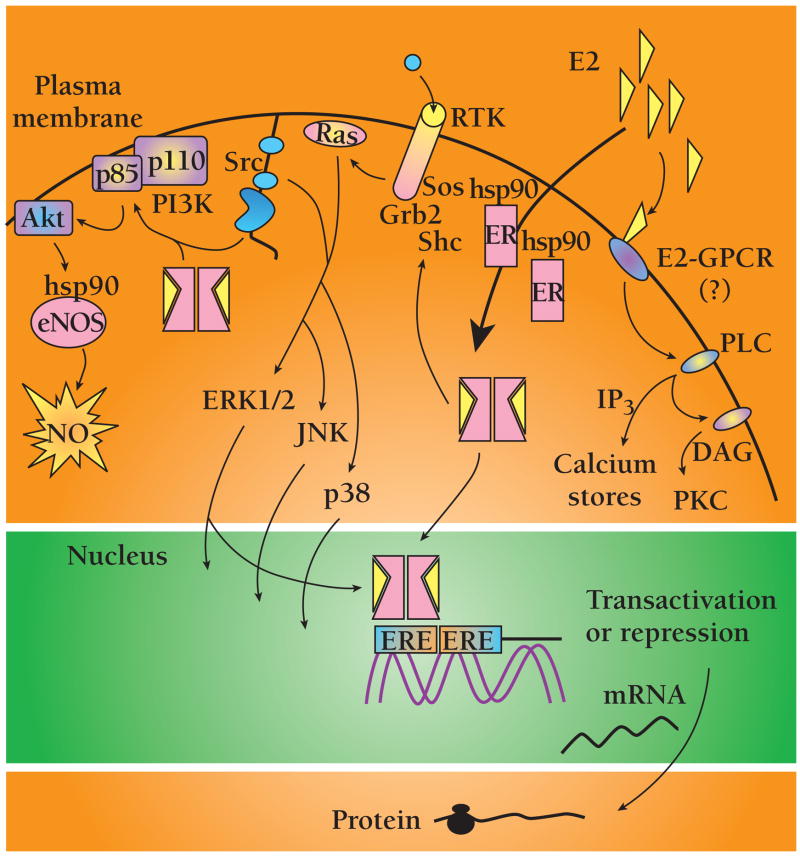
Estrogenic transcription-dependent effects, such as those that contribute prominently in organogenesis and function of the reproductive system, become evident hours after stimulation. Non-nuclear (alternatively referred to as “non-transcriptional” or “non-genomic”) estrogenic action peaks minutes after stimulation in multiple cell types. Other characteristics include immunity to inhibitors of DNA transcription or protein synthesis (actinomycin D or cycloheximide) and recruitment of membrane or cytosol-localized signaling components. These include the second messengers calcium and nitric oxide (NO), receptor tyrosine kinases including the epidermal growth factor receptor (EGFR) and insulin-like growth factor-1 (IGF-1) receptor (IGF1R), G protein coupled receptors (GPCRs), and protein kinases including phosphatidylinositol-3′ kinase (PI3K), the serine-threonine kinase Akt, mitogen-activated protein kinase (MAPK) family members, the non-receptor tyrosine kinase Src, and protein kinases A and C (PKA and PKC, respectively) (Figure 2) (for reviews see 17, 29, 30).
Figure 2.
Selected nuclear and non-nuclear activities of ERα. Details are described in the text. Abbreviations: endothelial nitric oxide synthase (eNOS), nitric oxide (NO), heat shock protein 90 (HSP90), phosphatidylinositol-3′ kinase (PI3K), son of sevenless (Sos), growth factor receptor binding protein 2 (Grb2), G protein coupled receptor (GPCR), protein kinase A (PKA), protein kinase C (PKC), extracellular-regulated kinases 1 and 2 (ERK1/2), Jun N-terminal kinase (JNK), 38-kDa isoform of MAPK (p38), estrogen response element (ERE).
SIGNALING CASCADES ACTIVATED BY ESTROGEN
The PI3K-Akt signaling cascade is one downstream target of non-nuclear estrogenic signaling (31–33). In the vasculature, short-term exposure to E2 leads to NO-dependent vasodilation (34). The secretion of NO by healthy vessels relaxes smooth muscle cells and inhibits platelet activation in a cyclic guanosine 3′, 5′-mono-phosphate (cGMP)-dependent mechanism. In cultured endothelial cells, estrogen enhances NO release within minutes without altering expression of endothelial nitric oxide synthase (eNOS) (33, 35). E2 activates eNOS activity in a biphasic manner through MAPK and PI3K-Akt pathways, leading to enhanced NO release (32). Myocardial protection by high-dose corticosteroids during ischemia-reperfusion injury also appears to be mediated by PI3K-Akt (36). In both cases, ERα and glucocorticoid receptors activate PI3K by associating with the p85α regulatory subunit in a ligand-dependent manner (32). Furthermore, the 90-kDa heat shock protein (HSP90) interacts with both eNOS and Akt and modulates eNOS activity by acting as a scaffold to regulate Akt-dependent phosphorylation of eNOS (37).
MAPK family members are common targets of non-nuclear estrogenic signaling. Induction of eNOS and inducible NOS (iNOS) expression in cardiac myocytes is blocked by the MAPK inhibitor PD98059 (38). This may be clinically relevant since NO inhibits the activation of caspases and prevents the development of congestive heart failure (39). Estrogen also activates extracellular-regulated kinases 1 and 2 (ERK1/2) in cardiomyocytes (38), colon cancer (40), breast cancer (41), and bone (42, 43), and inhibits ERK1/2 in VSMCs (44) and lung myofibroblasts (45). In the heart, ERα also selectively activates the 38-kDa isoform of MAPK (p38) to modulate the development of pressure-overload hypertrophy (13, 14, 46, 47), which is consistent with recruitment of p38 in other models of cardiac hypertrophy (48, 49). In endothelial cells, estrogen prevents disruption of the actin cytoskeleton during ischemia, prevents cell death, and enhances injury-dependent angiogenesis by rapidly and selectively activating the anti-apoptotic β isoform of p38 (p38β) and inhibiting pro-apoptotic p38α, leading to the increased expression of MAPK-activated protein kinase-2 (MAPKAP-2) kinase and phosphorylation of HSP27 (50). Downstream effects include preservation of stress fiber formation and membrane integrity, prevention of hypoxia-induced apoptosis, and induction of both endothelial cell (EC) migration and the formation of primitive capillary tubes (50).
It is possible that ERα might direct the activation of more receptor-proximal signaling complexes located at the plasma membrane. When overexpressed in cells, ligand-bound ERα induces the rapid phosphorylation of IGF1R and the activation of ERK1/2. Because these receptors co-immunoprecipitate in a ligand-dependent manner, a direct physical interaction between ERα and IGF1R could conceivably mediate the activation of ERK1/2 (51). In breast cancer cell lines, ligand-bound ERα promotes the rapid phosphorylation of the proteins Src and Shc, resulting in the formation of a Shc-Grb2- (growth factor receptor binding protein 2)-Sos (son of sevenless) complex (52), leading to downstream activation of Ras, Raf, and MAPK. Similarly, in both breast cancer and prostate cancer cells, E2 treatment induces the association of ERα phospho-Tyr537 with the Src SH2 (Src homology 2) domain, leading to activation of the Src-Ras-ERK pathway and cell cycle progression (53, 54). Additionally, in breast cancer cells, Src modulates PI3K-Akt signaling through a reversible cross-talk mechanism whereby the ligand-bound ER forms a ternary complex composed of ERα, PI3K, and Src (55). Cross-talk between PI3K and Src has also been observed in osteoclasts and bone marrow cells (56, 57).
Non-nuclear signaling can also amplify the nuclear, transcriptional activity of ERα. For example, in lactotroph cells, E2 rapidly activates ERK1/2, leading to increased transcription of the prolactin (PRL) gene, thus creating an additive effect on PRL expression by complementing the direct ERE-dependent transcriptional activation of PRL by ERα (58). Non-nuclear ERα activity can also elicit ERE-independent transcriptional activation. In cardiac myocytes, E2 rapidly increases ERK1/2-dependent expression of the early growth response-1 gene (egr-1) by inducing the recruitment of serum response factor (SRF) to serum response elements (SREs) in the egr-1 promoter (59).
Growth factors such as EGF and IGF-1 can stimulate the nuclear activity of ERα through a non-nuclear, E2-independent mechanism. Through the cross-talk of molecular networks, mitogenic extracellular signals are translated into cell cycle progression or, in cancer cells, into hormone-independent proliferation (60). EGF- and IGF-1–mediated stimulation of MAP kinases result in the direct phosphorylation of ERα on Ser118 (42, 61, 62), which enhances the binding of p68 RNA helicase (63), and promotes AF-1-dependent transcriptional activity in uterine (64, 65) and ovarian adenocarcinoma cells (66). Nuclear coregulator proteins can also be phosphorylated by ERK1/2 leading to increased transcriptional activity (67). Lastly, Src may enhance AF-1 function of ERα through either a Src, Raf-1, mitogen-activated ERK kinase (MEK) and ERK pathway that leads to phosphorylation of Ser118, or a pathway that includes Src, MEK kinase (MEKK), Jun N-terminal kinase (JNK) kinase (JNKK), and JNK, and that regulates AF-1-associated coactivators (68).
MECHANISMS FOR ERα ACTIVITY AT THE PLASMA MEMBRANE
Membrane binding sites for E2 were first implicated in 1977 (26), and additional indirect evidence for a membrane-associated ERα comes from immunohistochemistry (69, 70), overexpressed nuclear receptors (71), or studies with membrane-impermeable ligands (72–74). The trafficking of ERα to different cellular compartments may be regulated by the nature of the stimulation; for example, in VSMCs transfected with ERα, MAPK activation mediates the nuclear translocation of ERα from the membrane fraction by both E2-dependent and -independent mechanisms (75). However, because ERα has no intrinsic kinase or phosphatase activity, does not have hydrophobic stretches that could represent transmembrane domains, and lacks myristoylation and palmitoylation sequences that could anchor it to the membrane, membrane localization of the receptor seems unlikely. Alternatively, the receptor may associate with membrane caveolae: in fractionated plasma membranes from endothelial cells (ECs), ERα is localized to caveolae, and E2 stimulates eNOS in isolated caveolae in an ERα- and calcium-dependent manner (76–78). There is evidence that within the caveolae of ECs, HSP90, eNOS, and cav-1 (caveolin-1, the coat protein of caveolae) exist in a heterotrimeric complex that modulates eNOS activity depending on intracellular calcium levels (79, 80).
Non-nuclear ERα signaling also involves membrane-associated heterotrimeric G proteins. In Chinese hamster ovary (CHO) cells transfected with ERα cDNA, treatment of membrane fractions with estrogen activated Gαq and Gαs and rapidly stimulated inositol phosphate production and adenylyl cyclase activity, respectively (71). G protein activation also occurs in ECs, where E2 activation of eNOS can be inhibited with the ER antagonist ICI 182,780, RGS-4 (a regulator of G protein signaling specific for Gαi and Gαq), or pertussis toxin (specific for Gαi) (81).
POSSIBLE NEW ESTROGEN RECEPTORS
Non-nuclear signaling may involve a receptor altogether distinct from the classical ERα. In macrophage cells, E2 and E2-BSA induce a rise in intracellular calcium that is inhibitable with pertussis toxin (82, 83). The existence of an E2-GPCR in the hippocampus has also been hypothesized, where E2 stimulation potentiates kainate-induced currents through modulation of PKA activity (84).
The most notable evidence that estrogen’s non-nuclear effects are mediated by a receptor distinct from ERα or ERβ has come from studies in the cerebral cortex, where estrogen rapidly stimulates tyrosine phosphorylation of Src, leading to subsequent Shc–Grb2 complex formation upstream of ERK and B-Raf activation (85, 86). The pathway is not inhibitable by ICI 182,780 in cortical explants from ERα-deficient mice, suggesting that a new receptor, responsive to E2 but insensitive to ICI 182,780, mediates non-nuclear neuronal differentiation.
The nature of the ERα that mediates the non-nuclear effects of estrogen clearly requires further definition: the distinction between classical and atypical ERα might be made using cells cultured from complete ERα knockout mice, and ERα-truncated mutants might provide insight into the specific domains that mediate non-nuclear effects.
NON-NUCLEAR PHARMACOLOGICAL TARGETS
Nonetheless, an increasingly detailed understanding of the ER signaling network and its pleiotropic cellular effects have made the receptor an attractive pharmacological target. Selective estrogen receptor modulators (SERMs) are ER ligands which can have varying degrees of agonist or antagonist activities depending on the cell, promoter and coregulator context (87, 88) (Table 1).
TABLE 1.
TISSUE-SPECIFIC EFFECTS OF SELECTED SERMS
| Breast | Uterus | Vasculature | ||
|---|---|---|---|---|
| Indirect effect | Direct effect | |||
| Tamoxifen | − | + | Lowers total cholesterol, no effect on HDL | ? |
| ? | ||||
| GW5638 | − | − | Lowers total cholesterol | ? |
| EM-800 | − | − | Lowers total cholesterol and triglycerides | NO-mediated vasodilation |
| Raloxifene | − | − | Lowers total cholesterol and triglycerides, no effect on HDL | NO-mediated vasodilation |
| ROS reduction | ||||
| LY117018 | ? | − | Lowers total cholesterol | NO-mediated vasodilation |
Tamoxifen, the prototypical SERM, renders indirect cardiovascular protective effects by reducing the amounts of serum total cholesterol and low-density lipoprotein (LDL) (89). Unfortunately, its strong agonist activity in the endometrium leads to endometrial hyperplasia and low-grade cancers. Raloxifene, a non-steroidal compound, is similar to tamoxifen but it is less agonistic in the endometrium (90). Though administered primarily for bone preservation, raloxifene also reduces serum triglycerides and serum fibrinogen levels (91). Like estrogen, raloxifene and its analog LY117018 (92) stimulate eNOS activity in endothelial cells through PI3K- and ERK-dependent pathways, respectively (93), both of which may be involved in coronary artery relaxation (94). Raloxifene also improves endothelium-dependent vasorelaxation in hypertensive rats by enhancing the expression and activity of NOS (95).
EM-800, a non-steroidal compound, has higher affinity for ERα than any other SERM (96). In addition to demonstrating potent antitumor activity in the uterus and breast, EM-800 may also prevent bone loss and lower serum cholesterol and triglyceride levels (97). Furthermore, in vitro studies in endothelial cells suggest that EM-800, like E2, enhances NO release by sequential activation of MAPKs and PI3K-Akt, implicating a direct vascular protective effect (98).
The tissue specificity of SERMs suggests context-specific regulatory mechanisms that depend on the ligand, the promoter of the target gene, and the combination and exchange of co-regulators (99, 100). Breast cancer and pituitary lactotroph tumors, for example, demonstrate enhanced apoptosis and tumor shrinkage when transfected with adenovirus constructs containing dominant-negative ERα mutants (101). Because dominant-negative ERα and antiestrogens both recruit transcriptionally repressive proteins to the ERα DNA-binding complex (102, 103), the co-regulatory proteins that govern ERα activity, in addition to the receptor itself, represent promising therapeutic targets.
SUMMARY
We are just beginning to appreciate the complexity of ERα signaling. Future research efforts will undoubtedly reveal the intricacies of expression and translocation of endogenous ERα and possibly the identity of a new receptor that binds E2 and activates non-nuclear signaling. Furthermore, the activity of coregulators and their role in distinguishing the nuclear and non-nuclear activities of ERα remain to be defined. A full understanding of these highly cell- and promoter-specific mechanisms will allow the development of specific agonists and antagonists that selectively elicit only the beneficial effects of estrogen.
Acknowledgments
J.K. Liao is an Established Investigator of the American Heart Association. K.J. Ho is a Howard Hughes Medical Institute Medical Student Fellow. We thank Dr. A. Senes and S. Gainsbourg for assistance in preparing the manuscript. We apologize to all authors whose work could not be cited due to space limitations.
Biography

James K. Liao, MD, (left) is the Director of the Vascular Medicine Research Unit at the Brigham and Women’s Hospital, and is a Associate Professor of Medicine at Harvard Medical School. Address comments to JKL. E-mail jliao@rics.bwh.harvard.edu. Fax 617-768-8425 Karen J. Ho, MD, (right) is a member of the Vascular Medicine Research Unit, Brigham and Women’s Hospital and Harvard Medical School.
References
- 1.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 2.Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: Regulatory network and function. Cardiovasc Res. 2002;53:709–719. doi: 10.1016/s0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson JC. Cardiovascular effects of oestrogens. J Steroid Biochem Mol Biol. 2000;74:387–393. doi: 10.1016/s0960-0760(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 4.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan TR, Jr, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O’Donnell TF, Jr, Mendelsohn ME. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 8.Akishita M, Ouchi Y, Miyoshi H, Kozaki K, Inoue S, Ishikawa M, Eto M, Toba K, Orimo H. Estrogen inhibits cuff-induced intimal thickening of rat femoral artery: Effects on migration and proliferation of vascular smooth muscle cells. Atherosclerosis. 1997;130:1–10. doi: 10.1016/s0021-9150(96)06023-6. [DOI] [PubMed] [Google Scholar]
- 9.Oparil S, Levine RL, Chen SJ, Durand J, Chen YF. Sexually dimorphic response of the balloon-injured rat carotid artery to hormone treatment. Circulation. 1997;95:1301–1307. doi: 10.1161/01.cir.95.5.1301. [DOI] [PubMed] [Google Scholar]
- 10.White CR, Shelton J, Chen SJ, Darley-Usmar V, Allen L, Nabors C, Sanders PW, Chen YF, Oparil S. Estrogen restores endothelial cell function in an experimental model of vascular injury. Circulation. 1997;96:1624–1630. doi: 10.1161/01.cir.96.5.1624. [DOI] [PubMed] [Google Scholar]
- 11.McHugh NA, Cook SM, Schairer JL, Bidgoli MM, Merrill GF. Ischemia- and reperfusion-induced ventricular arrhythmias in dogs: Effects of estrogen. Am J Physiol. 1995;268:H2569–H2573. doi: 10.1152/ajpheart.1995.268.6.H2569. [DOI] [PubMed] [Google Scholar]
- 12.Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17β-estradiol: Role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96:1953–1963. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]
- 13.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol. 1993;110:719–723. doi: 10.1111/j.1476-5381.1993.tb13871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: Gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: Many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 17.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;276:36865–36868. doi: 10.1074/jbc.R100041200. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 20.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 21.Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, Mendelsohn ME. Effects of estrogen on the vascular injury response in estrogen receptor α, β double knockout mice. Circ Res. 2001;89:534–539. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 22.Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF. The AF-1 activation-function of ERα may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci USA. 2002;99:2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 24.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 25.Zhai P, Eurell TE, Cooke PS, Lubahn DB, Gross DR. Myocardial ischemia-reperfusion injury in estrogen receptor-α knockout and wild-type mice. Am J Physiol Heart Circ Physiol. 2000;278:H1640–H647. doi: 10.1152/ajpheart.2000.278.5.H1640. [DOI] [PubMed] [Google Scholar]
- 26.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson K, Gustafsson JA. Role of estrogen receptor β in estrogen action. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Bian Z, Lu P, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 29.Collins P, Webb C. Estrogen hits the surface. Nat Med. 1999;5:1130–1131. doi: 10.1038/13453. [DOI] [PubMed] [Google Scholar]
- 30.Moggs JG, Orphanides G. Estrogen receptors: Orchestrators of pleiotropic cellular responses. EMBO Rep. 2001;2:775–781. doi: 10.1093/embo-reports/kve185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3′-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Simoncini T, Hafezi–Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3′-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- 34.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafezi-Moghadam A, Simoncini T, Yang E, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of HSP90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 38.Nuedling S, Kahlert S, Loebbert K, Meyer R, Vetter H, Grohe C. Differential effects of 17β-estradiol on mitogen-activated protein kinase pathways in rat cardiomyocytes. FEBS Lett. 1999;454:271–276. doi: 10.1016/s0014-5793(99)00816-9. [DOI] [PubMed] [Google Scholar]
- 39.Mital S, Barbone A, Addonizio LJ, Quaegebeur JM, Mosca RJ, Oz MC, Hintze TH. Endogenous endothelium-derived nitric oxide inhibits myocardial caspase activity: Implications for treatment of end-stage heart failure. J Heart Lung Transplant. 2002;21:576–585. doi: 10.1016/s1053-2498(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 40.Di Domenico M, Castoria G, Bilancio A, Migliaccio A, Auricchio F. Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res. 1996;56:4516–4521. [PubMed] [Google Scholar]
- 41.Castoria G, Barone MV, Di Domenico M, Bilancio A, Ametrano D, Migliaccio A, Auricchio F. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 1999;18:2500–2510. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shimizu T, Kato S, Kawashima H. Rapid activation of MAP kinase by estrogen in the bone cell line. Biochem Biophys Res Commun. 1997;235:99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- 43.Jessop HL, Sjoberg M, Cheng MZ, Zaman G, Wheeler-Jones CP, Lanyon LE. Mechanical strain and estrogen activate estrogen receptor α in bone cells. J Bone Miner Res. 2001;16:1045–1055. doi: 10.1359/jbmr.2001.16.6.1045. [DOI] [PubMed] [Google Scholar]
- 44.Hwang KC, Lee KH, Jang Y. Inhibition of MEK1,2/ERK mitogenic pathway by estrogen with antiproliferative properties in rat aortic smooth muscle cells. J Steroid Biochem Mol Biol. 2002;80:85–90. doi: 10.1016/s0960-0760(01)00169-8. [DOI] [PubMed] [Google Scholar]
- 45.Flores-Delgado G, Bringas P, Buckley S, Anderson KD, Warburton D. Nongenomic estrogen action in human lung myofibroblasts. Biochem Biophys Res Commun. 2001;283:661–667. doi: 10.1006/bbrc.2001.4827. [DOI] [PubMed] [Google Scholar]
- 46.Scheuer J, Malhotra A, Schaible TF, Capasso J. Effects of gonadectomy and hormonal replacement on rat hearts. Circ Res. 1987;61:12–19. doi: 10.1161/01.res.61.1.12. [DOI] [PubMed] [Google Scholar]
- 47.van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17β-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- 48.Sugden PH, Clerk A. Stress-responsive” mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. 1998;83:345–352. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 49.Clerk A, Michael A, Sugden PH. Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: A role in cardiac myocyte hypertrophy? J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razandi M, Pedram A, Levin ER. Estrogen signals to the preservation of endothelial cell form and function. J Biol Chem. 2000;275:38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- 51.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 52.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of rapid estrogen action to MAPK activation by ERα–Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 53.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 54.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3′-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 57.Kubota Y, Tanaka T, Kitanaka A, Ohnishi H, Okutani Y, Waki M, Ishida T, Kamano H. Src transduces erythropoietin-induced differentiation signals through phosphatidylinositol 3′-kinase. EMBO J. 2001;20:5666–5677. doi: 10.1093/emboj/20.20.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol Endocrinol. 2000;14:1872–1881. doi: 10.1210/mend.14.11.0551. [DOI] [PubMed] [Google Scholar]
- 59.de Jager T, Pelzer T, Muller-Botz S, Imam A, Muck J, Neyses L. Mechanisms of estrogen receptor action in the myocardium. Rapid gene activation via the ERK1/2 pathway and serum response elements. J Biol Chem. 2001;276:27873–27880. doi: 10.1074/jbc.M010984200. [DOI] [PubMed] [Google Scholar]
- 60.Kato S, Masuhiro Y, Watanabe M, Kobayashi Y, Takeyama KI, Endoh H, Yanagisawa J. Molecular mechanism of a cross-talk between oestrogen and growth factor signalling pathways. Genes Cells. 2000;5:593–601. doi: 10.1046/j.1365-2443.2000.00354.x. [DOI] [PubMed] [Google Scholar]
- 61.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 62.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 63.Endoh H, Maruyama K, Masuhiro Y, et al. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor α. Mol Cell Biol. 1999;19:5363–5372. doi: 10.1128/mcb.19.8.5363. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Aronica SM, Katzenellenbogen BS. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993;7:743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- 65.Ignar-Trowbridge DM, Teng CT, Ross KA, Parker MG, Korach KS, McLachlan JA. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol Endocrinol. 1993;7:992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- 66.Ignar-Trowbridge DM, Pimentel M, Parker MG, McLachlan JA, Korach KS. Peptide growth factor cross-talk with the estrogen receptor requires the A/B domain and occurs independently of protein kinase C or estradiol. Endocrinology. 1996;137:1735–1744. doi: 10.1210/endo.137.5.8612509. [DOI] [PubMed] [Google Scholar]
- 67.Rowan BG, Weigel NL, O’Malley BW. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 68.Feng W, Webb P, Nguyen P, Liu X, Li J, Karin M, Kushner PJ. Potentiation of estrogen receptor activation function 1 (AF-1) by Src/JNK through a serine118-independent pathway. Mol Endocrinol. 2001;15:32–45. doi: 10.1210/mend.15.1.0590. [DOI] [PubMed] [Google Scholar]
- 69.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 70.Ropero AB, Soria B, Nadal A. A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol Endocrinol. 2002;16:497–505. doi: 10.1210/mend.16.3.0794. [DOI] [PubMed] [Google Scholar]
- 71.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 72.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 73.Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- 74.Morey AK, Razandi M, Pedram A, Hu RM, Prins BA, Levin ER. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. Biochem J. 1998;330:1097–1105. doi: 10.1042/bj3301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Q, Ebling H, Mittler J, Baur WE, Karas RH. MAP kinase mediates growth factor-induced nuclear translocation of estrogen receptor α. FEBS Lett. 2002;516:1–8. doi: 10.1016/s0014-5793(02)02432-8. [DOI] [PubMed] [Google Scholar]
- 76.Chambliss KL, Shaul PW. Rapid activation of endothelial NO synthase by estrogen: Evidence for a steroid receptor fast-action complex (SRFC) in caveolae. Steroids. 2002;67:413–419. doi: 10.1016/s0039-128x(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 77.Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor α localized in caveolae. Biochem Biophys Res Commun. 1999;263:257–262. doi: 10.1006/bbrc.1999.1348. [DOI] [PubMed] [Google Scholar]
- 78.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 79.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide-synthase–caveolin regulatory cycle. J Biol Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- 80.Gratton JP, Fontana J, O’Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric–oxide synthase (eNOS), HSP90, and caveolin-1 complex in vitro. Evidence that HSP90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 81.Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Gαi. J Biol Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 82.Benten WP, Stephan C, Lieberherr M, Wunderlich F. Estradiol signaling via sequestrable surface receptors. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- 83.Guo Z, Krucken J, Benten WP, Wunderlich F. Estradiol–induced nongenomic calcium signaling regulates genotropic signaling in macrophages. J Biol Chem. 2002;277:7044–7050. doi: 10.1074/jbc.M109808200. [DOI] [PubMed] [Google Scholar]
- 84.Moss RL, Gu Q. Estrogen: Mechanisms for a rapid action in CA1 hippocampal neurons. Steroids. 1999;64:14–21. doi: 10.1016/s0039-128x(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 85.Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD. Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: The initial event in E2 activation of the MAPK cascade? Endocrinology. 2001;142:5145–5148. doi: 10.1210/endo.142.12.8546. [DOI] [PubMed] [Google Scholar]
- 86.Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-α knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Osborne CK, Zhao H, Fuqua SA. Selective estrogen receptor modulators: Structure, function, and clinical use. J Clin Oncol. 2000;18:3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 88.Burger HG. Selective oestrogen receptor modulators. Horm Res. 2000;53(Suppl 3):25–29. doi: 10.1159/000023528. [DOI] [PubMed] [Google Scholar]
- 89.Williams JK, Wagner JD, Li Z, Golden DL, Adams MR. Tamoxifen inhibits arterial accumulation of LDL degradation products and progression of coronary artery atherosclerosis in monkeys. Arterioscler Thromb Vasc Biol. 1997;17:403–408. doi: 10.1161/01.atv.17.2.403. [DOI] [PubMed] [Google Scholar]
- 90.Dardes RC, Schafer JM, Pearce ST, Osipo C, Chen B, Jordan VC. Regulation of estrogen target genes and growth by selective estrogen-receptor modulators in endometrial cancer cells. Gynecol Oncol. 2002;85:498–506. doi: 10.1006/gyno.2002.6659. [DOI] [PubMed] [Google Scholar]
- 91.Walsh BW. The effects of estrogen and selective estrogen receptor modulators on cardiovascular risk factors. Ann NY Acad Sci. 2001;949:163–167. doi: 10.1111/j.1749-6632.2001.tb04015.x. [DOI] [PubMed] [Google Scholar]
- 92.Hisamoto K, Ohmichi M, Kanda Y, et al. Induction of endothelial nitric-oxide synthase phosphorylation by the raloxifene analog LY117018 is differentially mediated by Akt and extracellular signal-regulated protein kinase in vascular endothelial cells. J Biol Chem. 2001;276:47642–47649. doi: 10.1074/jbc.M103853200. [DOI] [PubMed] [Google Scholar]
- 93.Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105:1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- 94.Figtree GA, Lu Y, Webb CM, Collins P. Raloxifene acutely relaxes rabbit coronary arteries in vitro by an estrogen receptor-dependent and nitric-oxide-dependent mechanism. Circulation. 1999;100:1095–1101. doi: 10.1161/01.cir.100.10.1095. [DOI] [PubMed] [Google Scholar]
- 95.Wassmann S, Laufs U, Stamenkovic D, Linz W, Stasch JP, Ahlbory K, Rosen R, Bohm M, Nickenig G. Raloxifene improves endothelial dysfunction in hypertension by reduced oxidative stress and enhanced nitric oxide production. Circulation. 2002;105:2083–2091. doi: 10.1161/01.cir.0000014618.91633.67. [DOI] [PubMed] [Google Scholar]
- 96.Martel C, Provencher L, Li X, St Pierre A, Leblanc G, Gauthier S, Merand Y, Labrie F. Binding characteristics of novel nonsteroidal antiestrogens to the rat uterine estrogen receptors. J Steroid Biochem Mol Biol. 1998;64:199–205. doi: 10.1016/s0960-0760(97)00192-1. [DOI] [PubMed] [Google Scholar]
- 97.Labrie F, Labrie C, Belanger A, Simard J, Giguere V, Tremblay A, Tremblay G. EM–652 ( SCH57068), a pure SERM having complete antiestrogenic activity in the mammary gland and endometrium. J Steroid Biochem Mol Biol. 2001;79:213–225. doi: 10.1016/s0960-0760(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 98.Simoncini T, Varone G, Fornari L, Mannella P, Luisi M, Labrie F, Genazzani AR. Genomic and nongenomic mechanisms of nitric oxide synthesis induction in human endothelial cells by a fourth-generation selective estrogen receptor modulator. Endocrinology. 2002;143:2052–2061. doi: 10.1210/endo.143.6.8749. [DOI] [PubMed] [Google Scholar]
- 99.Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: Selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279–285. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 100.McDonnell DP, Chang CY, Norris JD. Capitalizing on the complexities of estrogen receptor pharmacology in the quest for the perfect SERM. Ann NY Acad Sci. 2001;949:16–35. doi: 10.1111/j.1749-6632.2001.tb03999.x. [DOI] [PubMed] [Google Scholar]
- 101.Lee EJ, Jakacka M, Duan WR, Chien PY, Martinson F, Gehm BD, Jameson JL. Adenovirus-directed expression of dominant negative estrogen receptor induces apoptosis in breast cancer cells and regression of tumors in nude mice. Mol Med. 2001;7:773–782. [PMC free article] [PubMed] [Google Scholar]
- 102.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 103.Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA. 1999;96:6947–6952. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]