Abstract
Background
Methamphetamine (MA) abuse causes damage to structures within the human cerebrum, with particular susceptibility to white matter (WM). Abnormalities have been reported in anterior regions with less evidence of changes in posterior regions. MA abusers have also shown deficits on attention tests that measure response conflict and cognitive control.
Methods
We examined cognitive control using a computerized measure of the Stroop selective attention task and indices of WM microstructure obtained from diffusion tensor imaging (DTI) in the callosal genu and splenium of 37 currently abstinent MA abusers and 17 non-substance abusing controls. Measurements of Fractional Anisotropy (FA), apparent diffusion coefficient (ADC) of callosal fibers and diffusion tensor eigenvalues were obtained in all subjects.
Results
The MA abusers exhibited greater Stroop reaction time interference (i.e., reduced cognitive control) [p=.04] compared to controls. After correcting for multiple comparisons, FA within the genu correlated significantly with measures of cognitive control in the MA abusers [p=.04, bonferroni corrected] but not in controls [p=.26]. Group differences in genu, but not splenium, FA were trend significant [p=.09].
Conclusions
MA abuse appears to alter anterior callosal WM microstructure with less evidence of change within posterior callosal WM microstructure. DTI indices within the genu, but not splenium, correlated with measures of cognitive control in chronic MA abusers.
Keywords: methamphetamine, diffusion tensor imaging, corpus callosum, cognitive control, Stroop, substance abuse
Introduction
In the past decade the use of the stimulant methamphetamine (MA) has increased in the general population, with worldwide abuse of amphetamines surpassing that of cocaine and opiates combined(1). It is now estimated that approximately 5% of the adult population in the United States have used MA on at least one occasion with worldwide use estimated to be 33 million users (2, 3). Human imaging studies of chronic MA abusers have shown metabolic and neurochemical changes consistent with damage in both gray (GM) and white matter (WM) tissue within the cerebral cerebrum (4–11). Studies of MA exposure using animal models have suggested that chronic exposure may destroy axonal arbors of dopamine neurons with sparing of the cell bodies themselves (12, 13). Compared to the rich body of published animal studies on WM damage following MA exposure, only a small number of studies in humans have been published (4, 5, 11, 14). Although WM tracts within the corpus callosum have been shown to be significantly influenced by cortical damage (15), substance abuse (16–18) and aging (18–20), to the best of our knowledge there has only been one previously published diffusion tensor imaging (DTI) study in human MA abusers that examined changes to WM microstructure (5).
DTI has emerged as a promising imaging technique to examine WM abnormalities in both clinical (17, 21–23) and non-clinical populations (18, 19, 20, 22, 24). DTI measures the pattern of WM microstructure in neural regions by examining the restricted flow directions of water molecules in axonal pathways and WM substrates (25, 26). Restricted diffusion within WM has a preferential orientation and can be measured within a voxel as fractional anisotropy (FA). Diffusivity can also be measured independent of orientation within a voxel and expressed as apparent diffusion coefficient (ADC). Information derived from the DTI measures can then be correlated with cognitive measures in order to predict the relationship between changes in WM microstructure and behavior (19, 27–32).
Study Rationale and Hypotheses
The goal of the present study was to examine in currently abstinent MA abusers the relationship between behavioral regulation (i.e., cognitive control) and WM microstructure within callosal regions as measured by DTI. Cognitive control can be defined as the ability to flexibly adapt behavior to current demands, by promoting task-relevant information and behaviors over temporally-extended periods and in the face of interference or competition. (33). Based on our previous findings (34), we hypothesized that MA abusers would display performance deficits on the Stroop attention test, a sensitive task that requires subjects to inhibit a prepotent but task-irrelevant response (i.e., word reading) in order to successfully perform the task. (35, 36). Based on our published Magnetic Resonance Spectroscopy (MRS) findings of abnormal metabolite levels in anterior GM brain regions (8, 9, 37), as well as findings of below-normal glucose metabolic rate in the infragenual region of the anterior cingulate cortex (ACC) measured via Positron Emission Tomography (PET) (7) we anticipated that performance on the Stroop task would correlate with DTI indices in anterior, but not posterior regions of the corpus callosum. The hypotheses related to the genu were further influenced by reports of WM abnormalities in anterior brain regions of MA abusers (4–6, 14) and findings of widespread WM hypertrophy following MA abuse (11).
Materials and Methods
Participants
Two groups were studied: 37 MA abusers and 17 age-matched non-substance abusing controls. The MA abusers were recruited from substance abuse treatment centers and residential housing programs in the Sacramento area and met DSM-IV criteria for lifetime MA dependence determined from the Structured Clinical Interview (SCID) (38). Random urine screens were performed at the referring sites to verify drug abstinence and none of the screens yielded positive results. For the MA abusers, inclusion criteria were: 1) lifetime diagnosis of MA dependence; and 2) age range between 18 and 55 years.
The controls were recruited from the local community and met the same criteria as the patients, except for the history of MA dependence. Exclusion criteria for both groups were: 1) substance dependence other than MA (except nicotine) within the past year; 2) alcohol abuse within the past 5 years; 3) treatment for non-drug related DSM-IV Axis I psychiatric disorders; 4) medical or neurological illness or trauma which would affect the CNS (e.g. stroke or seizure disorder); 5) reported history of a seropositive test for HIV; 6) severe hepatic, endocrine, renal disease, or history of loss of consciousness of over 30 min; 7) clear neurological sequelae of head trauma; and 8) metal implantation that would preclude MRI procedure. On average the controls and MA abusers did not differ in age [F (1,52)=2.84, p=.10] or years of parental education [F (1,52)=.41, p=.52], but differed in years of education [F (1,52) =8.29, p =.005 ] and estimates of premorbid intelligence as assessed by the National Adult Reading Test (NART) (39) [F (1,52) =4.61, p=.04]. All MA abusers had been drug abstinent a minimum of 3 weeks. Substance use characteristics of the MA abusers are outlined in Table 1. All subjects signed informed consent approved by the University of California, Davis Institutional Review Board and were paid a modest stipend for their participation in the study.
Table 1.
Demographic and drug use characteristics of methamphetamine (MA) abusers and control subjects.
| Methamphetamine Abusers
(n = 37) |
Control Subjects
(n = 17 ) |
|
|---|---|---|
| Demographic Variables | ||
| Age, y, mean (SD) | 36.29 (8.7) | 32.18 (7.5) |
| Range | 19 to 51 years | 19 to 46 years |
| Females | 24 (65%) | 8 (47%) |
| Subject’s education, y, mean (SD) | 13.19 (1.4) ††† | 14.48 (1.7) |
| Parental education, y, mean (SD) | 13.54 (3.2) | 14.12 (2.7) |
| NART | 107.76 (5.2) †† | 111.19 (5.6) |
| Race | ||
| White (non-Hispanic) | 30 (81%) | 8 (47%) |
| White (Hispanic) | 5 (14%) | 5 (29%) |
| African American | 1 (3%) | 1 (3%) |
| Other | 1 (3%) | 3 (18%) |
| Right-handed | 34 (92%) | 13 (76%) |
| Methamphetamine Use Variables | ||
| Duration, y, mean (SD) | 11.61 (7.1) | -- |
| Range | 1.5 to 31 years | |
| Months Abstinent, mean (SD) | 20.98 (31.9) | -- |
| Range | 3 weeks to 10 years | |
| Age of first use, y, mean (SD) | 19.92 (6.9) | -- |
| Range | 16 to 41 years of age | |
| Tobacco smokers | 30 (81%) | 4 (24%) |
| History of Cannabis Abuse | 20 (54%) | -- |
Significantly different from control group, p < .05
Significantly different from control group, p < .01
Stroop Attention Test
Apparatus
Stimuli were presented on a 14″ VGA color monitor. An IBM compatible computer controlled stimuli presentation and data collection. Voice responses were recorded via a voice-operated relay interfaced to the computer. Response timing was set to 1-millisecond (msec) resolution.
Stimuli
Four colors were employed in this experiment: red, green, blue, and yellow. The incongruent stimuli were created by printing each of the 4 color names in the three other ink-colors. The congruent stimuli were created by printing each of the four color names in its own ink-color. The neutral stimuli consisted of strings of XXXXs of variable length printed in one of the 4 ink-colors. Each letter within the stimulus words was upper case and subtended 1 degree of visual angle vertically. There were two blocks of trials, each one composed of 162 stimuli. 1
Procedure
Subjects were instructed to name aloud the color of ink that the words were printed in while ignoring the word itself. They were given instructions to respond as quickly as possible without making too many errors. Each trial began with a fixation point of 500 msec followed by the stimulus at the center of the screen. The onset of the subject’s voice triggered the voice-operated relay switch (recorded to the nearest msec) and terminated the stimulus display on the screen. The fixed time interval between the subjects’ response and the next trial was 494 msec. Any response stimulus interval that exceeded 494 msec was excluded from the analysis. These excluded trials accounted for less than 1% of the trials and did not differ between groups.
Image Acquisition
Structural whole brain MRI scans were acquired with a neuro-optimized 1.5T GE Signa NV/i MRI system (GE Medical Systems, Waukesha, WI) having a gradient specification of 40 mT/m peak strength and 150 mT/m/ms peak slew rate, and running version LX 84M4 operating system software.
Sagittal Scout Sequence
The midsagittal slice from a sagittal Fast Spin Echo (FSE) sequence (TR=2500 ms, TE=85 msec, slice thickness=3 mm, skip 1.5 mm, NEX=1, time < 2 min) was used to compute axial slice positions based on the identification of the AC-PC line.
Axial Fast Spin Echo (FSE) Dual-echo Sequence
19 oblique slices (FOV=24 cm, 256 x 256 matrix, TR=3500ms, TE=17ms/115ms, echo train length=20, slice thickness=5 mm, skip=0 mm, NEX=2) were acquired parallel to the AC-PC line for PD/T2 image contrasts.
Diffusion Weighted spin echo EPI sequence (DW-EPI)
DWI sequence with b=0 and 900 s/mm2, TE=90.3ms, TR=8000ms, (2 images of b=0, and 4 images for each of the 6 gradient direction) FOV=24cm, Freq. Encode=128, Phase Encode=128, Slice Thickness=5mm, Spacing=0, Scan time=3min29sec. Diffusion was measured in six non-collinear directions (x,y,z) = (1,1,0), (1,−1,0), (1,0,1), (1, 0, −1), (0,1,1) (0,1,−1) as well as a non-diffusion weighted acquisition (b=0) (40). Due to the presence of geometric image distortion, DTI quantification was always preceded by a distortion correction scheme based on the Haselgrove technique (41). In the technique we used, a reference image was produced by taking the simple average over all of the diffusion weighted images at each slice location (42). The average image had low intensity cerebral spinal fluid (CSF) that was similar in intensity to the CSF of the individual diffusion weighted images. In the average image, the edge of the brain was less sharply defined compared to that in the b=0 image or the Flair EPI image (43). However, this deficiency, when the image was used as a reference, was not significant in that the estimation of shear, magnification, and translation parameters produced registration of all diffusion weighted images within one pixel width. To produce this accurate registration, the process of forming the average image, and then estimating shear, magnification and translation factors for each of the individual diffusion weighted image to match the average image was performed twice (i.e., two iterations). This comparable performance using the average image as reference has been validated by the developers (42).
Regions of Interest
Regions of interest (ROIs) for both the genu and splenium of the corpus callosum were defined on the oblique AC/PC axial slice of the PD and T2 image sets. (See Figure 1). To avoid a bias in placement the DTI images were not used to identify the ROIs. Similar to the method of Foong et al (44), the borders of the genu and splenium were traced manually by two experienced investigators on the 5mm slice in which the structures showed maximal thickness. The raters (TEN, RS) were blind to the diagnosis of the study subjects and interclass correlation coefficients for FA inter-rater reliability were .986 and .967 for the genu and splenium respectively.
Figure 1.
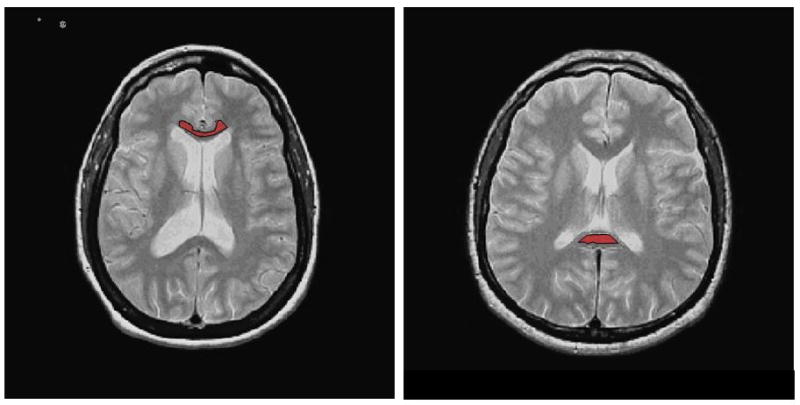
Anterior (Genu) and Posterior (Splenium) eroded Regions of Interest (ROIs) selected on Proton Density (PD) weighted images of one control subject.
Data Analysis
Diffusion anisotropy
After geometric distortion correction, ADC maps derived from images from each of the 6 gradient directions were calculated offline using a C-language program called Tensorcalc (45). At each voxel, the values from the ADC maps were then used to derive a symmetric 3x3 diffusion tensor matrix for the voxel. An eigensystem decomposition of the diffusion tensor matrix provided three eigenvalues (λ1, λ2 and λ3) and their corresponding eigenvectors. The eigenvalue λ1 represents the diffusion along the direction of the axonal fiber while, the other two eigenvalues (λ2 and λ3) represent diffusion perpendicular to the tract axis. λT was calculated using the following formula λT = (λ2 + λ3)/2. The derived eigenvalues and eigenvectors at each voxel were then used to calculate FA. The FA mainly reflects whether the diffusion is anisotropic, by being closer to the maximum value of 1, or isotropic, by being closer to minimum value of 0.
For each subject, the ROI was defined, and analysis performed, using a customized script written in MATLAB (Mathworks, Natick, MA). Mean and Standard Deviation (SD) of FA, ADC (mean diffusivity, which is an average of 3 principal ADC directions), λ1, and λT measures were obtained in ROIs defined within the genu and splenium of the callosal WM as described above. The defined ROIs were eroded (reduced in size) by one voxel around the perimeter of the ROI to help insure that the ROI contained only WM regions, and no regions of WM-GM transition. Prior to the mean and SD calculations, the center of gravity and the boundaries of each segmented FSE brain image and corresponding DTI map were matched to maximize their registration. FSE image and DTI map features were aligned to within 1.875mm (or two voxels in FSE map), and it was judged that no additional processing to further align the images and maps was required.
Results
Imaging
The MA abusers exhibited lower mean FA in the genu (641.8) compared to controls (673.9). However, after employing Bonferroni corrections for multiple comparisons, FA differences between the MA abusers and Controls in the genu were only trend significant [p=.09]. Group differences in FA within the splenium did not reach statistical significance [p=. 88]. No group differences were observed in ADC [genu; p=.37: splenium; p= .84], λ1 [genu; p=. 94: splenium; p=.93] or λT [genu; p= .25: splenium; p=.91]. Correlational analyses were carried out to examine the relationship between mean DTI indices across callosal regions (genu vs splenium) separately in the MA abusers and controls. No significant correlations were observed between callosal regions in FA [p = 0.77], ADC [p= .87], λ1 [p=.68] or λT [p=.75] in the MA abusers and none reached significance in the controls.
Behavioral Analysis
Stroop Attention Test
Mean reaction times (RTs) for correct responses for every condition were computed for each subject. Median analyses were also conducted for comparison. Analysis of variance (ANOVA) for repeated measures was used to analyze the data in a 2 x 3 mixed ANOVA with group as a between-subjects factor (patients vs controls) and wordtype (incongruent vs congruent vs neutral) as a within-subjects variable. Further analyses were conducted to examine the effect of error responses.
RT Analyses
Mean RT analyses revealed main effects of Stroop wordtype [F (2,104) =369.41, p<. 0001] as well as an interaction between group and wordtype (incongruent vs neutral) between the MA abusers and controls [F (1, 52) = 4.51, p = .04] with the MA abusers exhibiting greater Stroop interference compared to controls. Median analyses revealed similar results. A calculation of the effect size of the group difference revealed a medium effect size of .66 (Cohen’s d). No other main effects or interactions reached significance. Group differences in interference endured with education [p =.04] as a covariate. When NART scores were employed as a covariate the difference became trend significant [p=.06].
Error Analyses
Analyses revealed a main effect of wordtype [F (2,104) =51.69, p <. 0001]. All groups made significantly more errors in the incongruent condition (14%) than in either the neutral condition (2 %) or the congruent condition (1 %). No other effects were significant. Analyses revealed no evidence of a speed versus accuracy trade-off in the MA abusers or the controls. In fact there was a positive correlation between RTs and errors (MA: r=.14, p=.41; Controls: r= .31, p=.27).
Correlations between DTI Indices and Behavioral Measures
Pearson correlations were used to examine the relationship between DTI indices (FA and ADC). Analyses revealed that longer RTs in the Stroop task (i.e., greater interference) correlated with lower FA within the genu of the corpus callosum [r= .369; p=.04, corrected, bonferroni method) but not in the splenium [r= .20; p = .17]. Although the correlation was significant only in the genu, a statistical test of the difference between the slopes did not reach significance (p=.155). A multiple regression analysis with the Stroop Interference as a dependent variable and genu and splenium FA as predictors revealed that the genu FA, t = −.2.384; p = .0229, contributed to Stroop interference over and above the contribution of splenium FA (t= −1.312; p = .19). Correlations between Stroop RT interference and FA measures endured when years of MA use [p=.03], months of MA abstinence [p=.02] and age of first MA use [p=.03] were employed as covariates. In contrast to the FA indices, the ADC values failed to correlate with Stroop interference in either callosal region [genu: p=.45; splenium: p= .18]. The eigenvalues also failed to correlate with Stroop interference in either region. None of the DTI measures correlated significantly with either years of MA use or months drug abstinent. None of the correlations reached statistical significance in the controls.
Gender Differences
Because gender differences have been reported in studies of WM changes in MA abusers (4) as well as DTI studies of other clinical populations (16, 46, 47), we conducted post-hoc analyses of the DTI measures obtained in male and female MA abusers who participated in this study. No gender differences were observed in anterior [F (1, 35) = 1.15; p=.29] or posterior FA [F (1, 35) = 1.28; p=.26]. Although no gender differences emerged in ADC within the genu [p=.15}, there was a significant gender effect within the splenium [p=.003], with the male MA abusers exhibiting greater ADC compared to the female MA abusers. Furthermore, the male MA abusers exhibited greater λ1 (p=.01) eigenvalues compared to the female MA abusers within the splenium. No gender differences were observed among the controls on any of the DTI indices.
Discussion
The key finding in the current study is that our cognitive measure (i.e., Stroop interference) correlated with FA in the genu and not the splenium of the MA abusers. Data from the current study are consistent with a recent DTI study conducted in abstinent MA abusers (5), in which lower FA within frontal regions correlated with worse performance on the Wisconsin Card Sorting Test, a task of frontal executive function. A pair of recent DTI studies of cocaine abusers reported similar findings in that they observed significant correlations between FA in anterior white matter and measures of impulsivity (48, 49) and suggest that the findings are applicable to a broader population of stimulant abusers (48, 50). Furthermore, PET studies of MA abusers have also reported correlations between measures of attention and the ACC (51). Although FA group differences within the genu were only trend significant, correlational findings between genu FA and our behavioral measure in the MA abusers suggest that disruption of neural function in the Rostral Anterior Cingulate Cortex and adjacent WM produces a disruption in the pathways that are involved in behavioral regulation.
One possible reason for this correlation with anterior structures is the connectivity pattern of the genu itself. The fibers that cross through the genu connect right and left regions of the dorsolateral prefrontal cortex, an area with strong interconnections to the ACC both regions which are thought to mediate top-down cognitive control (52). Another possibility for the correlation with the genu may be that axons within the genu are usually thinner in diameter and less myelinated than those in the splenium and thus may be more susceptible to damage following long-term drug abuse (53, 54). In contrast, the fibers that cross through the splenium connect regions within visual association areas (55), regions that are certainly involved in the execution of any visual selection task, but may not be recruited heavily by demanding tasks of behavioral control, such as the Stroop task.
Findings of reduced anisotropy may occur as a result of demyelination, as well as from axonal damage (56). In the current study group differences in genu FA only reached a statistical trend and no group differences in FA were observed within the splenium. Morphological studies of animals who have been exposed to MA strongly indicate that abnormally low presynaptic DA & 5-HT axonal markers are related to overt destruction of DA and 5-HT axon terminals as well as the axon proper, typically sparing the nerve cell bodies. (12, 13). While the exact mechanism of MA damage to axonal fibers is unknown, there is abundant evidence from animal studies that MA exposure causes both acute disintegration and more chronic fragmentation in the WM fibers of frontal, parietal and temporal cortices as well as in the hippocampus and cingulum bundle (57).
Although no gender differences emerged on the DTI indices within the genu of the callosal region, differences did emerge within the splenium. Male MA abusers exhibited greater ADC and λ1 eigenvalues. These group differences do not appear to be a result of drug use patterns as the male and female MA abusers in this study did not differ in years of MA abuse, length of drug abstinence or age at first use. They also did not differ in years of education, estimates of premorbid IQ (e.g. NART score) or Stroop interference scores. These findings are challenging to interpret from a functional perspective as the behavioral measure did not correlate with DTI indices within the splenium. Further studies are needed to examine gender differences in WM microstructure.
The lack of aging effects in the current study differs from previous DTI studies in which aging effects on WM structure are reported (20, 58, 59). One reason for this discrepancy might be the limited age range of the subjects in the current study (19–51 years of age) compared to other aging studies of WM change which often span 60-years (20, 58).
Limitations
One possible limitation of the current study is the possibility of pre-existing abnormalities in the MA abusers. To minimize the possibility that group differences were due to this, we excluded those abusers who had pre-existing non-drug-related Axis I disorders. As IQ measures have also been shown to be related to DTI measures (60), it is also possible that group differences in NART scores might have been a contributing factor to the FA differences between the groups. However, a post-hoc regression analysis revealed that when Stroop Interference was employed as the dependent variable and FA genu and NART as simultaneous predictors that genu FA, t = −.2.603; p = .0136, contributed to Stroop interference over and above the contribution of NART score (t = 1.47; p = .15).
Comorbid substance use is another factor that could influence the results. To minimize the effects of comorbid substance abuse, we studied subjects whose primary drug of choice was MA and whose dependence of other substances was greater than 5 years prior to the time of this study. However, given the high percentage of MA abusers who also abuse cannabis combined with reports of altered brain function associated with cannabis abuse, (61), we conducted post-hoc analyses on those MA dependent subjects who met DSM-IV lifetime criteria for past cannabis abuse (N=20) compared to those who did not (N=17). No group differences were observed in DTI indices or cognitive performance. Another limitation of the current study is that a high % of our MA subjects were smokers (81%). As nicotine consumption has been shown to impact both neurochemical concentrations, we conducted post-hoc analyses on those MA abusers who were smokers versus MA abusers who were non-smokers (62–64). The results showed no group differences in FA [p=.94], however the % of non-smokers was small (19%). Finally, although recent DTI studies have employed more than 6 directions, a large number of published studies have applied diffusion gradients in 6 non-collinear directions as we did. (18, 19, 21, 32, 44, 46, 47, 65–70). Thus the approach utilized in the current study shares a consistent and established methodology within the DTI literature.
Conclusion
Addiction is hypothesized to be caused by long-lasting changes in the brain that appear to compromise decision making processes by limiting the cognitive control necessary to inhibit the maladaptive choices (i.e., drug seeking behavior) (37, 71–74). The specific findings from this study that link abnormalities in WM microstructure (i.e., genu of the corpus callosum) with deficits on tasks that measure cognitive control and conflict resolution are consistent with this hypothesis. In making any decision, an individual is usually confronted with many alternatives; choices which can generate response conflict. It may be that disruptions in WM integrity contribute to maladaptive decision making. Although most individuals resolve conflict through decisions that result in positive outcomes, a disturbing feature of substance abusers is that they make decisions that are often associated with negative outcomes (75).
Figure 2.
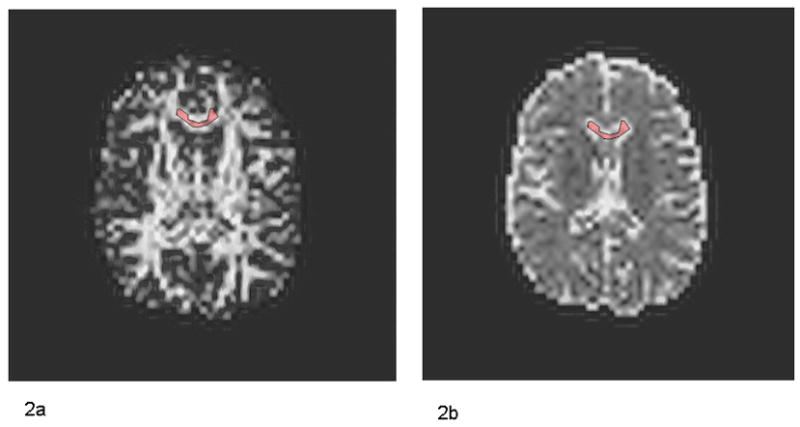
Fractional Anisotropy (FA) Image (2a) and Apparent Diffusion Coefficient (ADC) image (2b) of one control subject.
Figure 3.
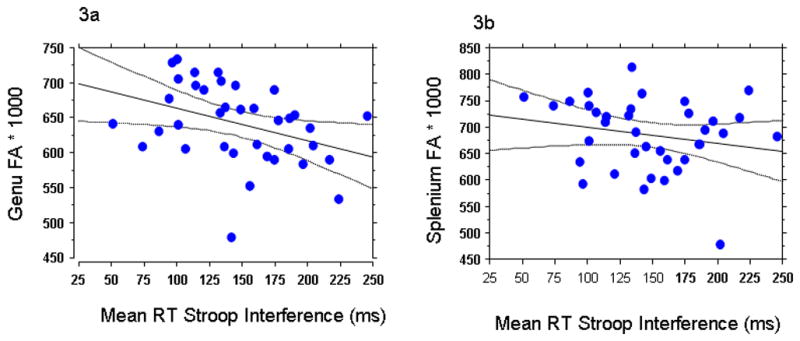
Correlational scatterplots between millisecond (ms) reaction time (RT) Stroop interference and Fractional Anisotropy (FA) *1000 values in the (3a) genu (r= .37, p=.04, corrected, bonferroni method)) and (3b) splenium (r=.23, p=.17) of 37 methamphetamine (MA) abusers.
Table 2.
Mean Corpus Callosum Fractional Anisotropy (FA) Values (x 1000), Apparent Diffusion Coefficient (ADC), λ1 and λT between 37 methamphetamine (MA) abusers and 17 control subjects.
| DTI Index
mean, (SEM) |
Methamphetamine Abusers
(n = 37) |
Control Subjects
(n = 17 ) |
|---|---|---|
| FA Genu | 641.8 (9.24) ††† | 673.9 (11.74) |
| FA Splenium | 686.2 (11.04) | 683.0 (20.04) |
| ADC Genu | .86 x10−3 mm2/s. (.02) | .83 x10−3 mm2/s. (.01) |
| ADC Splenium | 1.06 x10−3 mm2/s. (.04) | 1.04 x10−3 mm2/s. (.07) |
| λ1 Genu | 1.61 (.04) | 1.61 (.02) |
| λ1 Splenium | 1.95 (.04) | 1.94 (.08) |
| λT. Genu | .48 (.02) | .44 (.01) |
| λT. Splenium | .59 (.04) | .60 (.07) |
Trend Significant, p = .09. Effect size = .66.
Acknowledgments
We would like to thank John Ryan for his expert technical assistance with MR data collection. We are also very appreciative of the support of Cameron Carter, MD.
Disclosure/Conflict of Interest The authors declare that this work was funded by NIDA grants, DA16293-01 to RS, DA14359 to TEN and DA10641 to GPG. The authors reported no biomedical financial interests or potential conflicts of interest.
A subset of the participants also participated in a published MRS study (Salo et al, 2007).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nations U; Crime UNOoDa. 2004 World Drug Report. Vol. 1. Vienna: United Nations; 2004. The world drug problem: A status report; pp. 25–26. [Google Scholar]
- 2.WHO; Organization WH Other psychoactive substances. 2004. [Google Scholar]
- 3.Roehr B. Half a million Americans use methamphetamine every week. BMJ (Clinical research ed. 2005:476. doi: 10.1136/bmj.331.7515.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, et al. Increased white matter hyperintensities in male methamphetamine abusers. Drug and alcohol dependence. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006:1–11. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 6.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 7.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Archives of general psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Archives of general psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 9.Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, et al. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry research. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 10.Taylor MJ, Alhassoon OM, Schweinsburg BC, Videen JS, Grant I. MR spectroscopy in HIV and stimulant dependence HNRC Group. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 2000;6:83–85. doi: 10.1017/s1355617700611104. [DOI] [PubMed] [Google Scholar]
- 11.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain research. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 13.Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain research. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- 14.Oh JS, Lyoo IK, Sung YH, Hwang J, Kim J, Chung A, et al. Shape changes of the corpus callosum in abstinent methamphetamine users. Neuroscience letters. 2005;384:76–81. doi: 10.1016/j.neulet.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 15.de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. Journal of neuropathology and experimental neurology. 1985;44:578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. NeuroImage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. European journal of radiology. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- 21.Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biological psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 22.Lim KO, Helpern JA. Neuropsychiatric applications of DTI - a review. NMR in biomedicine. 2002;15:587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- 23.Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- 24.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. Ajnr. 2006;27:533–547. [PMC free article] [PubMed] [Google Scholar]
- 25.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and biobehavioral reviews. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 29.Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- 30.Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, et al. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harvard review of psychiatry. 2002;10:324–336. doi: 10.1080/10673220216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubicki M, Westin CF, McCarley RW, Shenton ME. The application of DTI to investigate white matter abnormalities in schizophrenia. Annals of the New York Academy of Sciences. 2005;1064:134–148. doi: 10.1196/annals.1340.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism, clinical and experimental research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- 33.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 34.Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry research. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 35.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 36.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 37.Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biological psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of general psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 39.Nelson HE. The National Adult Reading Test (NART) Windsor, Canada: Nelson Publishing Company; 1982. [Google Scholar]
- 40.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 41.Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med. 1996;36:960–964. doi: 10.1002/mrm.1910360620. [DOI] [PubMed] [Google Scholar]
- 42.Hedehus M, Skare S. DTI post-processing using “tensorcalc” software 2000 [Google Scholar]
- 43.de Crespigny AJ, Moseley ME. Eddy Current Induced Image Warping in Diffusion Weighted EPI. ISMRM 6th Meeting; Sydney. 1998. [Google Scholar]
- 44.Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. Journal of neurology, neurosurgery, and psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J Magn Reson. 2000;147:340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- 46.Kumra S, Ashtari M, McMeniman M, Vogel J, Augustin R, Becker DE, et al. Reduced frontal white matter integrity in early-onset schizophrenia: a preliminary study. Biological psychiatry. 2004;55:1138–1145. doi: 10.1016/j.biopsych.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 47.Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, et al. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport. 2003;14:2469–2473. doi: 10.1097/00001756-200312190-00035. [DOI] [PubMed] [Google Scholar]
- 48.Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug and alcohol dependence. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 50.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry research. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 51.London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 52.Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two Hemispheres-One Brain: Functions of the Corpus Callosum. New York: Alan R. Liss, Inc.; 1986. pp. 47–74. [Google Scholar]
- 53.Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. Neuroreport. 1996;7:1761–1764. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- 54.LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rockland KS, Pandya DN. Topography of occipital lobe commissural connections in the rhesus monkey. Brain research. 1986;365:174–178. doi: 10.1016/0006-8993(86)90736-5. [DOI] [PubMed] [Google Scholar]
- 56.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 57.Zhou FC, Bledsoe S. Methamphetamine causes rapid varicosis, perforation and definitive degeneration of serotonin fibers: An immunocytochemical study of serotnin trasnporter. Neuroscience-Net. 1996:1–17. [Google Scholar]
- 58.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- 59.Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 60.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voytek B, Berman SM, Hassid BD, Simon SL, Mandelkern MA, Brody AL, et al. Differences in regional brain metabolism associated with marijuana abuse in methamphetamine abusers. Synapse (New York, NY. 2005;57:113–115. doi: 10.1002/syn.20155. [DOI] [PubMed] [Google Scholar]
- 62.Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. The American journal of psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- 63.Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcoholism, clinical and experimental research. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 64.Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol (Fayetteville, NY. 2006;39:1–11. doi: 10.1016/j.alcohol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, et al. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet. 2003;118:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- 66.Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. The American journal of psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. J Magn Reson Imaging. 2003;18:427–433. doi: 10.1002/jmri.10377. [DOI] [PubMed] [Google Scholar]
- 69.Schulte T, Muller-Oehring EM, Salo R, Pfefferbaum A, Sullivan EV. Callosal involvement in a lateralized stroop task in alcoholic and healthy subjects. Neuropsychology. 2006;20:727–736. doi: 10.1037/0894-4105.20.6.727. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- 71.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 73.Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- 74.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of general psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 75.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]


