Introduction
A number of epidemiological studies, including prospective studies, have found a positive association between blood estrogen levels and breast cancer risk, supporting the notion that estrogen plays a central role in the pathogenesis of this common malignancy (1). The association between endogenous estrogen exposure and breast cancer risk could be explained, in part, by genetic factors that affect estrogen biosynthesis, metabolism, and signal transduction. The CYP19A1 gene plays a central role in estrogen biosynthesis. The gene encodes aromatase, the enzyme that catalyses the conversion of androstenedione to estrone and testosterone to estradiol in both ovarian granulosa cells and peripheral adipose tissue. Several studies have described an overexpression of the CYP19A1 gene in human breast tumors and surrounding tissue, suggesting that aromatase plays a role in the in situ production of estrogen in breast tissues. It has been hypothesized that CYP19A1 gene polymorphisms may affect estrogen biosynthesis, and thus these polymorphisms may modify the risk of breast cancer. Several SNPs in the CYP19A1 gene have been evaluated in relation to breast cancer risk with mixed results (2-11). In this study, we comprehensively evaluated the association between the CYP19A1 gene polymorphisms and breast cancer risk among Chinese women using the data from the Shanghai Breast Cancer Study, a large-scale, population-based case-control study conducted among Chinese women in Shanghai.
Materials and Methods
Cases and controls in this study were participants of the Shanghai Breast Cancer Study. Detailed study methods have been published elsewhere (12, 13). The study included 1,459 women between the ages of 25 and 64 and 1,556 age frequency-matched controls. Blood samples were obtained from 1,193 (82%) cases and 1,310 (84%) controls who completed the in-person interviews. 1,140 cases and 1,244 controls were genotyped successfully in this study.
Haplotype-tagging SNP (htSNP) were selected based on the data provided in a study conducted by Haiman et al. (3). In that study, 25 htSNPs were identified to capture the variation of the CYP19A1 gene. Among the 25 htSNPs, 2 SNPs had a minor allele frequency of <1% in the Japanese population and 4 SNPs were African-American-specific polymorphisms. Thus, 19 htSNPs were identified to capture the variations of the CYP19A1 gene in the Japanese population (3). Because the pattern of genetic variation is similar in Japanese and Chinese populations (14), we used the 19 informative htSNPs reported in Haiman's study for the Japanese population to define haplotypes in our study. We also genotyped rs2304463 and included this SNP in the single SNP analyses. The SNP locus/position, LD block, and locations are shown in Appendix 1. In addition, we included the (TTTA)n repeat polymorphism in intron 4 in the study.
Appendix I .
SNPs included in the haplotype analysis of the CYP19A1 gene and genotyping methods
| SNP | Block | Positiona | Location | Method | Primers or ABI Assay ID | Probes (VIC/FAM) or ABI assay ID |
|---|---|---|---|---|---|---|
| rs2446405 | 1 | 49225931 | promoter | TaqMan | GGAGGGTGAATCATTCCAAGTACAG CTTCCTGACTTGCACCATTTTCATT |
CTTGGCTCATATTATT TTGGCTCAAATTATT |
| rs2445765 | 1 | 49214036 | promoter | TaqMan | GGGACGTCAATATGGTGCAATTTT CGCAGGTCCCATGTTAAGAAC |
CTTTGACACTGCATTTT TTTGACAGTGCATTTT |
| rs2470144 | 1 | 49200863 | intron | TaqMan | GGTATAATGGGAAGGCCAGCAA GGTGGTATTTGAAGGGAGTTCTCT |
AAATTTCCCTCCATCAGTG AATTTCCCTCCGTCAGTG |
| rs1004984 | 1 | 49192667 | intron | Masscode | gacgatgccttcagcacaCAGAGGGAGCAGGGTGAG ATAATTCAGGCCCTTCACAATC |
gggacggtcggtagatATCCCCCATGACTGCCTACTGTTG gctggctcggtcaagaATCCCCCATGACTGCCTACTGTTA |
| rs1902584 | 1 | 49190792 | intron | TaqMan | C 1664181 10 | C 1664181 10 |
| rs28566535 | 2 | 49180279 | intron | TaqMan | C_1664178_10 | C_1664178_10 |
| rs12900137 | 2 | 49178491 | intron | TaqMan | GAGCCAACGAAAGCAAACGT CCACCAATCCAAACAAAACCAAACA |
CTACTAATCATGGATCTTCATG CTAATCATGGATGTTCATG |
| rs730154 | 2 | 49170342 | promoter | TaqMan | GACCAGGCACCCCATCTG GCCGGTTCCAGCAAAACTTC |
ACCCCCATGCTCCAT CCCCACGCTCCAT |
| rs936306 | 2 | 49158736 | 3′UTR | TaqMan | C 1664161 10 | C 1664161 10 |
| rs1902586 | 2 | 49149991 | intron | TaqMan | C_11484670_1 | C_11484670_1 |
| rs749292 | 3 | 49137869 | intron | TaqMan | TCTGCCAGTCCTTCTTCAAACC GGCTTAGGGCCTGATAGAAATTGTG |
TCGGAGTCGAGGATT TCGGAGTCAAGGATT |
| rs6493494 | 3 | 49128972 | intron | TaqMan | CTTGGCTTCCTGGACATTGTG CGCTGTGTGGGATTGATCCT |
TCCAGCGCCTGAGC CTCCAGCACCTGAGC |
| rs1008805 | 3 | 49128737 | intron | TaqMan | TTGGAAGTAATAGCAGGCCTAGGTA CCTTACCGAATCACTACCCTTCAC |
CTTCCTGCGTCCTGC CTTCCTGTGTCCTGC |
| rs12907866 | 4 | 49124592 | intron | TaqMan | GGGCCTTATCAGGTGTCTAGCATAT GCTCTTGCTGGAGACTGAATCATAA |
CAAAACCTCAATAAAC CAAAACCTCGATAAAC |
| rs727479 | 4 | 49113685 | intron | TaqMan | GTGGAATAAAGAGAAGGGATAAATACAAGACA TCTGGAACATCTTCTTCACTGCTTT |
ACTTTGTTTCCGCCATGC CACTTTGTTTCCTCCATGC |
| rs2414096 | 4 | 49108917 | intron | TaqMan | GGAGAATGTCCAATCCAAGAACATCT TTCAAAGACCCATTGCCTGACT |
AAGACTCCGTTTAAGAAA AAAAGACTCCATTTAAGAAA |
| rs2304463 | 4 | 49087258 | intron | Masscode | AGCTAACTCTGGCACCTTAACA gacgatgccttcagcacaAGTTTAGACATCTAGCGAAACAGA |
gggacggtcggtagatTCACTTACTCATAAGCACCAATGT gctggctcggtcaagaTCACTTACTCATAAGCACCAATGG |
| rs700519 | 4 | 49087106 | coding | RFLP | CGCTAGATGTCTAAACTGAG CATATGTGGCATGGGAATTA |
Restrction enzyme; HgaI |
| rs10046 | 4 | 49082124 | exon | TaqMan | C 8234731 1 | C 8234731 1 |
| rs4646 | 4 | 49081982 | exon | TaqMan | C 8234730 1 | C 8234730 1 |
Chromosome position was based on NCBI build 35.
Two SNPs (rs1004984 and rs230463) were genotyped in 2004 by BioServe Biotechnologies, Ltd (Laurel, MD) using Masscode assay. One SNP (rs700519) was genotyped in 2002 using the PCR-RFLP method and the genotypes were confirmed by direct sequencing using BigDye Terminator Chemistry on an ABI PRISM® 3700 automated DNA Analyzer. Genotypes for the other 17 SNPs were conducted from 2003 to 2004 using the TaqMan genotyping assay in ABI PRISM 7900 Sequence Detection Systems (Applied Biosystems, Foster City, CA). Details of the genotyping methods are described in Appendix I. The genotyping for the (TTTA)n repeat polymorphism in intron 4 was performed by detection of fluorescent amplimers on an ABI 3700 automated DNA sequencer as reported earlier (13) using the following primers: F: 5′-GAGGTTACAGTGAGCCAAG-3′ and R: 5′-gtgtcCAGGTACTTAGTTAGCTAC-3′. Sequenced alleles enabled distinction of amplimer size variation as a function of STR allele length and of the adjacent 3 bp insertion/deletion located approximately 50 bp upstream of the (TTTA)n repeat. Quality control (QC) samples were included in the genotyping assays. The consistency rate for QC samples was 98.7%. In addition, we genotyped rs1902584 in 45 DNA samples of the Chinese participants used in the International HapMap project (http://www.hapmap.org) and 24 DNA samples used in the Perlegen (http://genome.perlegen.com) database as an additional quality control. The concordance rates between the data generated in our lab and the data from the HapMap and Perlegen was 100%.
The Chi-squared test was used to evaluate case-control differences in the distributions of CYP19A1 alleles and genotypes. The haplotype blocks were determined according to the method described by Haiman et al. (3). Haplotypes for the CYP19A1 gene within each haplotype block were derived using the software PHASE (version 2.1), and the overall association between haplotypes within each block and breast cancer risk was evaluated with the permutation test (15, 16). The risk of breast cancer associated with each haplotype as compared with the most common haplotype under different genetic modes (additive, dominant, and recessive) was estimated using logistic regression models with the HAPSTAT method recently developed by DY Lin et al.(17-19). The potential confounding effect of major demographic factors and known breast cancer risk factors were adjusted for using logistic models. Adjustments for these factors did not result in any appreciable changes in the risk estimates. Thus, we report results without adjustment for these factors.
Results
The distributions of selected demographic characteristics and major risk factors for breast cancer in the Shanghai Breast Cancer Study have been previously reported (12). The Hardy-Weinberg equilibrium (HWE) of all SNPs was examined in controls. The SNP rs12907866 was not in HWE (p<10−10) and was excluded from subsequent analyses. The other 19 SNPs were in HWE (p-values with Bonferroni correction>0.05). Overall, no apparent association of any SNP with breast cancer risk was observed. Similarly, no statistically significant association with any SNP was found in either pre- or post-menopausal women (data not shown).
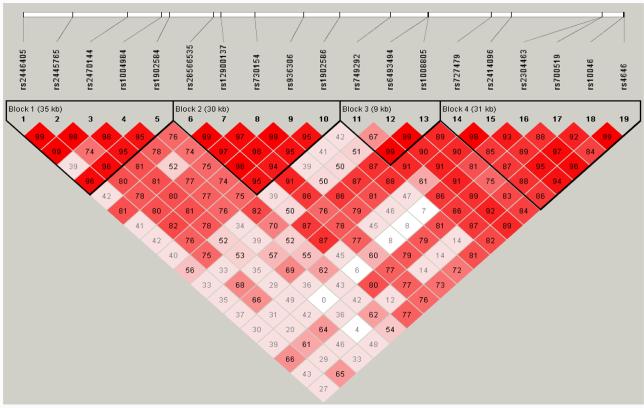
The linkage disequilibrium plot is presented in Figure 1. Four haplotype blocks were identified in the CYP19A1 gene among Chinese women. In each block, several common haplotypes with 5% or higher frequency accounted for between 91.0% to 99.9% of all haplotypes (Table 1). Also presented in Table 1 are the association results of breast cancer risk with common haplotypes in each haplotype block under additive models. No apparent association was found in the analysis including all women nor in analyses stratified by menopausal status. We performed a heterogeneity test by menopausal status, and found no statistically significant heterogeneity (p>0.05). Analyses under dominant or recessive models also showed no statistically significant associations of CYP19A1 haplotypes with breast cancer risk either in the analyses including all women or in analyses conducted in pre- or post-menopausal women (data not shown). We also examined the interaction between BMI and CYP19A1 haplotypes in relation to breast cancer risk under additive, dominant, and recessive models. No significant interactions were found either in the analyses including all women or in analyses stratified by menopausal status (data not shown).
Figure 1.
LD plot for SNPs in the CYP19A1 gene. The value within each diamond is D' between pairs of SNPs, estimated based on control subjects. The red-to-white gradient reflects higher to lower LD values (red=high, white=low).
Table 1.
Association between breast cancer risk and CYP19A1 haplotypes by blocks, the Shanghai Breast Cancer Study, 1996-1998
| Haplotype | All Subjects |
Pre-menopausal Womena |
Post-menopausal Womena |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases N=1140 (%) |
Controls N=1244 (%) |
OR (95% CI) | Cases N=760 (%) |
Controls N=792 (%) |
OR (95% CI) | Cases N=375 (%) |
Controls N=448 (%) |
OR (95% CI) | ||
| Block 1: rs2446405, rs2445765, rs2470144, rs1004984, rs1902584 | ||||||||||
| AGTGA | 38.3 | 38.1 | 1.00 (reference) | 37.1 | 37.0 | 1.00(reference) | 40.0 | 39.8 | 1.00 (reference) | |
| TGCGA | 24.6 | 25.8 | 0.97 (0.84-1.12) | 25.9 | 26.6 | 0.98(0.82-1.18) | 22.0 | 24.0 | 0.93 (0.72-1.21) | |
| TCCAT | 14.1 | 13.5 | 1.06 (0.88-1.27) | 14.0 | 13.0 | 1.10(0.88-1.37) | 14.1 | 14.0 | 1.03 (0.76-1.39) | |
| AGCAA | 9.2 | 9.7 | 0.93 (0.75-1.14) | 9.0 | 10.0 | 0.88(0.68-1.15) | 9.6 | 9.0 | 1.03 (0.72-1.47) | |
| TCCAA | 8.4 | 7.7 | 1.06 (0.85-1.32) | 8.3 | 8.0 | 1.03(0.78-1.35) | 8.9 | 7.3 | 1.13 (0.77-1.64) | |
| TCCGA | 3.8 | 3.8 | 0.96 (0.70-1.31) | 3.9 | 3.7 | 1.04(0.70-1.55) | 3.7 | 4.4 | 0.86 (0.51-1.44) | |
| pb=0.13 | p=0.09 | p=0.77 | ||||||||
| Block 2: rs28566535, rs12900137, rs730154, rs936306, rs1902586 | ||||||||||
| AGTCG | 66.5 | 64.1 | 1.00 (reference) | 67.1 | 64.1 | 1.00(reference) | 64.5 | 63.6 | 1.00 (reference) | |
| CCCTA | 16.0 | 17.1 | 0.92 (0.78-1.07) | 15.6 | 16.1 | 0.95(0.78-1.16) | 16.3 | 18.2 | 0.88 (0.67-1.15) | |
| CGCTA | 15.2 | 15.5 | 0.96 (0.82-1.13) | 14.8 | 15.9 | 0.90(0.73-1.10) | 16.7 | 15.4 | 1.08 (0.83-1.42) | |
| p=0.46 | p=0.89 | p=0.55 | ||||||||
| Block 3: rs749292, rs6493494, rs1008805 | ||||||||||
| AAA | 39.1 | 39.0 | 1.00 (reference) | 37.0 | 38.2 | 1.00(reference) | 41.2 | 38.7 | 1.00 (reference) | |
| GGG | 29.7 | 29.2 | 1.02 (0.89-1.18) | 30.7 | 28.5 | 1.11(0.93-1.33) | 26.7 | 29.6 | 0.85 (0.67-1.09) | |
| GGA | 17.2 | 16.8 | 1.01 (0.85-1.20) | 16.2 | 15.9 | 1.05(0.85-1.31) | 18.3 | 17.5 | 0.95 (0.71-1.26) | |
| AGA | 7.5 | 8.2 | 0.91 (0.72-1.15) | 8.2 | 9.4 | 0.88(0.66-1.17) | 7.9 | 7.3 | 0.96 (0.65-1.42) | |
| GAA | 6.4 | 6.8 | 0.95 (0.74-1.21) | 7.6 | 7.6 | 1.01(0.75-1.37) | 5.9 | 6.9 | 0.81 (0.53-1.24) | |
| p=0.71 | p=0.80 | p=0.55 | ||||||||
| Block 4: rs727479, rs2414096, rs700519, rs10046, rs4646 | ||||||||||
| AACAC | 45.0 | 45.1 | 1.00 (reference) | 44.3 | 44.8 | 1.00(reference) | 45.5 | 44.9 | 1.00(reference) | |
| CGCGA | 25.3 | 24.0 | 1.07 (0.93-1.24) | 25.8 | 24.1 | 1.10(0.93-1.32) | 23.9 | 23.4 | 1.01 (0.79-1.30) | |
| AGTGC | 14.3 | 13.3 | 1.09 (0.91-1.30) | 14.3 | 12.8 | 1.16(0.93-1.44) | 13.7 | 14.0 | 0.97 (0.72-1.31) | |
| AGCAC | 7.6 | 8.6 | 0.92 (0.74-1.14) | 7.3 | 8.7 | 0.86(0.66-1.14) | 8.4 | 8.3 | 0.98 (0.68-1.42) | |
| AGCGA | 2.5 | 2.3 | 1.12 (0.76-1.64) | 2.9 | 2.6 | 1.23(0.78-1.94) | 1.9 | 2.0 | 0.94 (0.45-1.95) | |
| AGCGC | 1.8 | 2.1 | 0.93 (0.61-1.41) | 1.7 | 2.1 | 0.79(0.46-1.38) | 2.7 | 2.1 | 1.18 (0.61-2.27) | |
| CGCAC | 1.1 | 1.7 | 0.69 (0.41-1.14) | 1.2 | 1.6 | 0.78(0.41-1.50) | 1.2 | 2.1 | 0.50 (0.22-1.17) | |
| p=0.16 | P=0.12 | p=0.58 | ||||||||
Nine subjects without information on menopausal status were excluded from the stratified analyses.
p: derived from permutation test for overall association.
No interaction was statistically significant at p<0.05 for genetic variables and menopausal status.
We also evaluated the associations of the (TTTA)n repeat polymorphism with breast cancer risk. A total of 7 (TTTA)n repeat alleles were observed in our study population, ranking from 7 repeats to 13 repeats. Alleles with 7, 11, or 12 repeats were common. A 3-bp deletion polymorphism was reported approximately 50 bp upstream of the (TTTA)n polymorphic site. Virtually all alleles with this 3-bp deletion had 7 (TTTA)n repeats. No significant association with any repeat allele was found either in the analyses including all women or in analyses stratified by menopausal status (data not shown).
Discussion
In this study, we constructed common haplotyes from 19 SNPs in the CYP19A1 gene for 1,140 breast cancer cases and 1,244 controls among Chinese women. Three to five common haplotypes accounted for >90% of the observed haplotypes in this Chinese population, which is consistent with observations in other ethnic groups (3).
Several tissue-specific promoters, including adipose and breast cancer tissue promoters, are located between promoter I.1 and exon 2 (approximately 89kb upstream of exon 2). Haplotype blocks 1 to 3 are located in this regulatory region. Few studies have evaluated the association of genetic polymorphisms in this region with breast cancer risk. In the report of Haiman et al. (3), four common haplotypes (1d, 2b, 2d, and 3c) in blocks 1 to 3 were significantly associated with increased breast cancer risk when analyses combined subjects in all ethnic groups. They also observed significant associations of breast cancer risk among Japanese subjects (347 cases and 420 controls) with four common haplotypes in block 1 (1d, OR=1.44; 95% CI, 1.07-1.93), block 2 (2b, OR=1.42; 95% CI: 1.13-1.80; 2c, OR=1.43; 95%CI: 1.03-1.98), and block 3 (3c, OR=1.40; 95% CI: 1.07-1.83). These positive associations, however, were not replicated in our study. Our results are supported by two very recent large-scale studies involving haplotype analyses (10, 11). In a large-scale study conducted within the NCI Breast and Prostate Cancer Cohort Consortium, Haiman et al. (10) observed no significant associations with any SNPs or common haplotypes of the CYP19A1 gene and breast cancer risk, although genetic variation in CYP19A1 produces measurable differences in estrogen levels among post-menopausal women. Olson et al. (11) also failed to detect any association between the CYP19A1 gene haplotype-tagging SNPs and breast cancer risk. Additionally, two recent studies reported that CYP19A1 polymorphisms were not associated with breast density (20, 21).
Using the single polymorphism approach, several SNPs of CYP19A1 have been studied to evaluate their association with breast cancer risk with conflicting results. The Arg/Cys or Cys/Cys genotypes of the Arg264Cys (rs700519) polymorphism in exon 7 were associated with increased risk of breast cancer when compared to the Arg/Arg genotype among Hawaiian and Japanese (3) and Korean women (4). Our study, along with several other studies (5-7), however, found a null association. Miyoshi et al. (6) found that carrying the Arg allele in the Trp39Arg polymorphism of exon 2 conferred significant protection against the development of breast cancer in Japanese women. This association, however, was not confirmed by another study (3) or by our study. A C-to-T polymorphism in the 3′UTR (rs10046) of exon 10 has also been associated with breast cancer risk (8). This finding, however, was not confirmed by another study (9). A 12-repeat allele in the tetranucleotide polymorphism [(TTTA)12] located in intron 4 was associated with increased breast cancer risk in a case-control study conducted among Norwegian women (22). In the Nurses' Health Study conducted in the United States, the (TTTA)10 but not the (TTTA)12 allele was associated with breast cancer risk (23). These findings, however, were not confirmed by other studies (24-26). Our data also showed a null association between the (TTTA)n repeat polymorphism and breast cancer risk. Many of the above studies had small sample sizes or used a hospital-based study design.
To our knowledge, this is the first large-scale study to comprehensively evaluate the association of CYP19A1 polymorphisms with breast cancer risk in Chinese women. In addition, most previous studies have been conducted in post-menopausal women, while our study provides evidence that CYP19A1 gene polymorphisms are not associated with breast cancer risk among pre-menopausal women. The participation rate of our study was high, minimizing the potential selection bias that is common to many case-control studies. Chinese women living in Shanghai are relatively homogenous in ethnic background, because more than 98% of them are classified in a single ethnic group (Han Chinese). The sample size of this study is large, which allowed for a careful analysis of CYP19A1 gene polymorphisms and breast cancer risk. Our study includes a large number of loci (19 SNPs and the (TTTA)n repeat) and our estimates of haplotype frequencies should be accurate. Our study has 80% statistical power to detect an odds ratio of 1.41 for any genotype or haplotype with 10% frequency and an odds ratio of 1.29 for any genotype or haplotype with 20% frequency at a significance level of 0.05 under an additive genetic model.
In summary, our large-scale, comprehensive study failed to identify an overall association of breast cancer risk with common CYP19A1 gene variants among Chinese women. However, we cannot rule out the possibility that the CYP19A1 gene may interact with environmental exposure in the development of breast cancer. Further studies are needed to explore the CYP19A1 gene-environment interaction in relation to breast cancer risk.
Acknowledgments
We thank Ms. Qing Wang and Ms. Regina Courtney for their excellent technical laboratory assistance and Ms. Bethanie Hull for technical assistance in manuscript preparation. We thank Dr. Christopher A. Haiman at the University of Southern California for sharing information about primers and probes of several SNPs. This study would not have been possible without the support of all of the study participants and research staff of the Shanghai Breast Cancer Study. This research was supported by research grants (R01CA64277 and R01CA90899) from the National Cancer Institute.
Sources of Support: This research was supported by research grants (R01CA64277 and R01CA90899) from the National Cancer Institute.
The abbreviations used are
- SNP
single nucleotide polymorphism
- LD
linkage disequilibrium
- htSNP
haplotype-tagging SNP
- UTR
untranslated region
- OR
odds ratio
- CI
confidence interval
- BMI
body mass index
- WHR
waist-to-hip ratio
References
- 1.Colditz GA, Baer H, Tamimi RM. Breast Cancer. In: Schottenfeld D D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. Oxford University Press; New York: 2006. pp. 995–1012. [Google Scholar]
- 2.Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:843–54. [PubMed] [Google Scholar]
- 3.Haiman CA, Stram DO, Pike MC, Kolonel LN, Burtt NP, Altshuler D, et al. A comprehensive haplotype analysis of CYP19 and breast cancer risk: the Multiethnic Cohort. Hum Mol Genet. 2003;12:2679–92. doi: 10.1093/hmg/ddg294. [DOI] [PubMed] [Google Scholar]
- 4.Lee KM, Abel J, Ko Y, Harth V, Park WY, Seo JS, et al. Genetic polymorphisms of cytochrome P450 19 and 1B1, alcohol use, and breast cancer risk in Korean women. Br J Cancer. 2003;88:675–8. doi: 10.1038/sj.bjc.6600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Probst-Hensch NM, Ingles SA, Diep AT, Haile RW, Stanczyk FZ, Kolonel LN, et al. Aromatase and breast cancer susceptibility. Endocr Relat Cancer. 1999;6:165–73. doi: 10.1677/erc.0.0060165. [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi Y, Iwao K, Ikeda N, Egawa C, Noguchi S. Breast cancer risk associated with polymorphism in CYP19 in Japanese women. Int J Cancer. 2000;89:325–8. doi: 10.1002/1097-0215(20000720)89:4<325::aid-ijc2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe J, Harada N, Suemasu K, Higashi Y, Gotoh O, Kawajiri K. Arginine-cysteine polymorphism at codon 264 of the human CYP19 gene does not affect aromatase activity. Pharmacogenetics. 1997;7:419–24. doi: 10.1097/00008571-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen VN, Harada N, Yoshimura N, Haraldsen E, Lonning PE, Erikstein B, et al. Genetic variants of CYP19 (aromatase) and breast cancer risk. Oncogene. 2000;19:1329–33. doi: 10.1038/sj.onc.1203425. [DOI] [PubMed] [Google Scholar]
- 9.Haiman CA, Hankinson SE, Spiegelman D, Brown M, Hunter DJ. No association between a single nucleotide polymorphism in CYP19 and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:215–6. [PubMed] [Google Scholar]
- 10.Haiman CA, Dossus L, Setiawan VW, Stram DO, Dunning AM, Thomas G, et al. Genetic variation at the CYP19A1 locus predicts circulating estrogen levels but not breast cancer risk in postmenopausal women. Cancer Res. 2007;67:1893–7. doi: 10.1158/0008-5472.CAN-06-4123. [DOI] [PubMed] [Google Scholar]
- 11.Olson JE, Ingle JN, Ma CX, Pelleymounter LL, Schaid DJ, Pankratz VS, et al. A comprehensive examination of CYP19 variation and risk of breast cancer using two haplotype-tagging approaches. Breast Cancer Res Treat. 2007;102:237–47. doi: 10.1007/s10549-006-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87:295–300. doi: 10.1002/1097-0215(20000715)87:2<295::aid-ijc23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Cai Q, Gao YT, Wen W, Shu XO, Jin F, Smith JR, et al. Association of breast cancer risk with a GT dinucleotide repeat polymorphism upstream of the estrogen receptor-alpha gene. Cancer Res. 2003;63:5727–30. [PubMed] [Google Scholar]
- 14.Altshuler D, Brooks L, Chakravarti A, Collins F, Daly M, Donnelly P. A haplotype map of the human genome. Nature. 2005;437:1299–320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DY, Zeng D, Millikan R. Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol. 2005;29:299–312. doi: 10.1002/gepi.20098. [DOI] [PubMed] [Google Scholar]
- 18.Zeng D, Lin DY, Avery CL, North KE, Bray MS. Efficient semiparametric estimation of haplotype-disease associations in case-cohort and nested case-control studies. Biostatistics. 2006;7:486–502. doi: 10.1093/biostatistics/kxj021. [DOI] [PubMed] [Google Scholar]
- 19.Lin D, Zeng D. Likelihood-Based Inference on Haplotype Effects in Genetic Association Studies. J Am Stat Assoc. 2006;101:89–118. [Google Scholar]
- 20.Haiman CA, Hankinson SE, De Vivo I, Guillemette C, Ishibe N, Hunter DJ, et al. Polymorphisms in steroid hormone pathway genes and mammographic density. Breast Cancer Res Treat. 2003;77:27–36. doi: 10.1023/a:1021112121782. [DOI] [PubMed] [Google Scholar]
- 21.Olson JE, Ma CX, Pelleymounter LL, Schaid DJ, Pankratz VS, Vierkant RA, et al. A comprehensive examination of CYP19 variation and breast density. Cancer Epidemiol Biomarkers Prev. 2007;16:623–5. doi: 10.1158/1055-9965.EPI-06-0781. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen VN, Andersen TI, Lindblom A, Erikstein B, Magnus P, Borresen-Dale AL. A rare CYP19 (aromatase) variant may increase the risk of breast cancer. Pharmacogenetics. 1998;8:43–8. doi: 10.1097/00008571-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Haiman CA, Hankinson SE, Spiegelman D, De Vivo I, Colditz GA, Willett WC, et al. A tetranucleotide repeat polymorphism in CYP19 and breast cancer risk. Int J Cancer. 2000;87:204–10. [PubMed] [Google Scholar]
- 24.Siegelmann-Danieli N, Buetow KH. Constitutional genetic variation at the human aromatase gene (Cyp19) and breast cancer risk. Br J Cancer. 1999;79:456–63. doi: 10.1038/sj.bjc.6690071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Healey CS, Dunning AM, Durocher F, Teare D, Pharoah PD, Luben RN, et al. Polymorphisms in the human aromatase cytochrome P450 gene (CYP19) and breast cancer risk. Carcinogenesis. 2000;21:189–93. doi: 10.1093/carcin/21.2.189. [DOI] [PubMed] [Google Scholar]
- 26.Baxter SW, Choong DY, Eccles DM, Campbell IG. Polymorphic variation in CYP19 and the risk of breast cancer. Carcinogenesis. 2001;22:347–9. doi: 10.1093/carcin/22.2.347. [DOI] [PubMed] [Google Scholar]