Abstract
Platelets are the chief effector cells in hemostasis and have additional major functions in inflammation, vascular integrity, and tissue repair. Platelets and the lungs have interrelated activities. Previous studies provide evidence that platelets contribute to pulmonary vascular barrier function and are required for defense against pulmonary hemorrhage, and that the lungs can influence platelet number and distribution. There is also evidence that platelets contribute to pathologic syndromes of pulmonary inflammation and thrombosis. Thus, platelets have an “amicus or adversary” relationship with the lung. Recent observations and discoveries have established new paradigms relevant to influences of platelets on lung cell and molecular biology. These new findings are in a variety of areas including thrombopoieis, nontraditional activities of platelets, new synthetic capabilities and mechanisms of post-translational gene expression, interactions of platelets with endothelial cells and contributions to alveolar capillary barrier permeability, interactions of platelets with myeloid leukocytes, and platelet involvement in stem cell signaling and vascular repair. These issues are considered in a translational approach, with an emphasis on acute lung injury and the acute respiratory distress syndrome.
Keywords: platelets, inflammation, thrombosis, lung injury
CLINICAL RELEVANCE
The studies reviewed here will alter paradigms regarding the contributions and activites of platelets in acute lung injury and other syndromes of pulmonary inflammation and thrombosis.
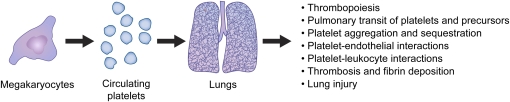
Platelets are anucleate cells of myeloid origin that circulate in a resting state in the blood (1). In response to activating signals, the quiescent platelet phenotype changes and these versatile effectors of host defense perform major hemostatic, inflammatory, and reparative functions (2, 3). Platelets and the lung—a complex multicellular organ that accomplishes gas exchange, immune surveillance, and other physiologic processes—have interrelated activities (Figure 1). Platelets and platelet precursors transit the normal and injured mammalian lung. Inflammatory diseases and other pulmonary disorders cause accumulation of platelets in the lung, and remote tissue injury and systemic conditions such as sepsis can activate platelets in the circulation and cause them to sequester in pulmonary vascular beds. Activated platelets release paracrine mediators that can alter pulmonary function in a variety of ways. In addition, adhesion molecules and other factors with signaling properties are locally displayed on surfaces of activated platelets, where they can bind to ligands on target cells and trigger functional responses in a spatially localized, juxtacrine fashion (2, 4). These adhesion and signaling molecules allow platelets to interact not only with pulmonary endothelium and, potentially, other resident lung cells, but also with one another and with leukocytes in pulmonary vessels.
Figure 1.
Platelets and the lungs interact in physiologic and pathologic conditions. Platelets, platelet precursors, and the lungs have activities that lead to multiple levels of interaction under physiologic condition and in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Each of the processes and interactions listed is considered in the text.
A variety of platelet–lung interactions were identified in early studies (5) and summarized and cataloged over a decade ago (reviewed in Ref. 6). More recently, new discoveries and insights into previously unrecognized features of platelet function have emerged. These observations alter concepts and paradigms regarding contributions of platelets to respiratory cell and molecular biology and to pulmonary diseases. This translational review will consider some of these recent findings, concentrating on those that are particularly relevant to the clinical spectrum of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (7). Because of space limitations we refer to published work in a focused and eclectic, rather than comprehensive, fashion. Roles of platelets in ALI/ARDS have been reviewed previously (6, 8, 9) and additional relevant reviews are cited when possible in our discussion of specific topics.
PLATELET ONTOGENY AND THROMBOPOIESIS: MARROW, BLOOD, AND LUNG
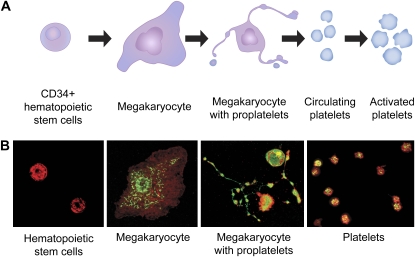
Hematopoietic stem cells differentiate to immature megakaryocytes, which are lineage-committed parent cells that then mature and spawn platelets in the process of thrombopoiesis (1, 10). Platelet production can increase by as much as 20-fold in conditions of peripheral demand and inflammation (11). Discovery of the cytokine thrombopoietin, and identification of transcription factors that influence megakaryocyte differentiation and the expression of platelet-specific proteins, were critical in understanding the regulation of thrombopoiesis (10, 12). Two theories of terminal platelet formation have been proposed based on studies conducted in model systems and in vivo over many years (11). In one, cytoplasmic fragmentation of megakaryocytes in the bone marrow and pulmonary capillary bed is thought to generate mature, circulating platelets (13, 14). A potential problem with this idea is that the intracellular composition of mature platelets might then be predicted to be random unless megakaryocyte cytoplasmic composition is uniform and fragmentation is a stereotyped process. A second model proposes that differentiated marrow megakaryocytes extend multiple elongated processes termed proplatelets; platelets form at the ends of proplatelets after regulated translocation of intracellular constituents to proplatelet tips via microtubular tracks (reviewed in Refs. 1 and 10) (Figure 2). Studies of differentiation of mouse and human hematopoietic stem cells to megakaryocytes in vitro support this second model (1, 10, 15–18). The two models of thrombopoieis are not necessarily mutually exclusive (13).
Figure 2.
Thrombopoiesis involves regulated proplatelet formation and release of mature platelets. (A) CD34+ hematopoietic stem cells differentiate to megakaryocytes in the marrow. Differentiated megakaryocytes extend proplatelets and translocate constituents of mature platelets to proplatelet tips in a highly regulated fashion. Platelets are “spawned” from proplatelet tips. Mature platelets then circulate in the blood for approximately 7 days, unless they are activated and deposited at sites of vascular injury, inflammation, and/or microvascular sequestration. There is evidence that thrombopoiesis occurs in the normal and injured lung. See text for details. (B) Key steps in thrombopoiesis are illustrated by photomicrographs from a model of proplatelet formation based on differentiation of CD34+ human hematopoietic stem cell precursors isolated from umbilical cord blood. See Refs. 17 and 18 for details. Mature platelets freshly isolated from blood are shown in the right-hand panel. The photomicrographs are reprinted from Refs. 17 and 18 with permission.
A recent study using intravital microcopy to examine exposed bone marrow of thrombopoietin-treated transgenic mice reported proplatelet formation in vivo (19), confirming previous evidence for this mechanism (1, 10, 13). Sessile megakaryocytes labeled with an expressed florescent protein were observed to extend proplatelets into marrow sinusoidal blood vessels, where shear forces appeared to free them from the parent cells. Circulating cells with proplatelet morphology were present in the systemic blood of these animals, suggesting the possibility that generation of single, mature platelets continues in peripheral vessels. Additional findings supported observations dating to the first half of the 20th century indicating that platelet counts are higher in pulmonary venous samples compared with pulmonary arterial blood, suggesting that circulating proplatelets undergo processing to two or more mature platelets in the lungs (5, 11, 19, and references cited therein). This study did not directly address the molecular basis for the fact that normal wild-type mice have circulating platelet counts 4- to 5-fold greater than those in humans (20).
How thrombopoiesis is altered in inflammatory lung diseases, and what the consequences are, remain largely unknown. Circulating platelet numbers may influence the natural history of a variety of pulmonary syndromes, including cystic fibrosis (21), asthma (22), pulmonary hypertension (23), and ALI/ARDS. Increased platelet turnover rates, altered platelet life span, thrombocytopenia, and thrombocytosis have each been reported in patients with ALI and/or ARDS (14, 24–26). Whether or not proplatelets circulate and give rise to individual platelets in an altered fashion in the blood in ALI/ARDS is unknown. Furthermore, there are additional mechanisms of platelet generation in the peripheral circulation (H. Schwertz and coworkers, unpublished data) that may be influenced by inflammatory lung injury.
There is evidence that the lung is a reservoir for megakaryocytes (10, 13) in addition to being a site of proplatelet processing. Megakaryocytes are reported to circulate and accumulate in the lungs of humans and rodents (27). A pathologist with a busy lung biopsy practice might note pulmonary intravascular megakaryocytes in several specimens a day across the spectrum of normal and diseased lungs, and would also identify them in autopsy examinations (T. Colby, M.D., personal communication). A recent retrospective analysis of thorascopic biopsy specimens from patients in the fibroproliferative phase of ARDS indicated that megakaryocytes are present in microvessels of the injured lung, and suggested that intrapulmonary megakaryocytes may influence circulating platelet numbers (14). Patients with thrombocytopenia had greater mortality than those with thrombocytosis in this series. Generalization of these observations is complicated by the fact that few of these patients had common causes of ALI/ARDS such as sepsis, trauma, or aspiration (7), and drug-induced diffuse alveolar damage was the etiology in approximately a quarter of the small group of subjects (14). Furthermore, the histochemical marker used, GP IIIa (CD61; β3), is not specific for megakaryocytes and platelets, although it is present on these cells complexed to GPIIb (αIIb). Nevertheless, these observations suggest that megakaryocytes are present in the lungs of patients in the subacute phase of ALI/ARDS. There is evidence that human megakaryocytes and a megakaryocytic cell line adhere to activated endothelial cells (28), which are present in macro- and microvessels in ALI/ARDS (29), providing a mechanism for targeting and localization of megakaryocytes to the injured pulmonary vascular bed. The molecular signals that might lead to migration and/or in situ differentiation of megakaryocytes in the injured lung are unknown, and it is also unknown if the injured lung provides organ-specific signals that alter thrombopoieis in the marrow. The number and activity of megakaryocytes (marrow, lung, or both), proplatelets, and platelets, and their contributions to pulmonary dysfunction and repair, are likely to be dynamic variables in the acute edematous phase versus the fibroproliferative phase of ALI/ARDS and in patients with resolving ALI (7, 30). These issues are also largely unexplored.
TRADITIONAL AND NONTRADITIONAL ROLES OF PLATELETS IN HEMOSTASIS AND INFLAMMATION RELEVANT TO ALI/ARDS
Platelets are the chief cellular effectors of hemostasis. A primary function of platelets, well known to clinicians and investigators alike, is to limit hemorrhage after trauma and vascular injury (reviewed in Refs. 3 and 31). The lung is a site of hemorrhage in thrombocytopenic patients. In addition, thrombocytopenia is a relative contraindication to lung biopsy and other invasive procedures. Bleeding and therapeutic hemostasis are daily issues in the ICU. Thus, practicing chest and critical care physicians consider hemostatic functions of platelets on a regular basis.
Platelets initially adhere to exposed subendothelial matrix at sites of endothelial disruption via the glycoprotein Ib/V/IX complex, which recognizes von Willebrand factor and other ligands (31). Several surface integrins and collagen receptors also tether platelets to matrix. Activation of platelets via G protein–coupled receptors that recognize thrombin, adenosine diphosphate, endogenously generated thromboxane A2, and other agonists then rapidly amplifies the initial adhesion, and mediates aggregation and recruitment of additional platelets to the site of injury. Cellular activation converts integrin αIIBβ3 (glycoprotein IIb/IIIA), a major cell-specific integrin on megakaryocytes and platelets, to a state that binds fibrinogen, fibrin, and other ligands, mediating homotypic aggregation and additional adhesive events. Each of these responses occurs within seconds to minutes after activation. Genetic or pharmacologic interruption of one or more of these rapid steps causes bleeding, providing clear evidence for critical roles of platelets in physiologic hemostasis. Each step is a major target for current therapeutic strategies in thrombotic diseases (3, 31). In addition to rapid formation of a hemostastic barrier and provision of cellular components to the clot scaffold, platelets also mediate clot retraction. This is a more prolonged process that has largely been defined in vitro, and that is thought to stabilize clots and initiate their remodeling (3, 18).
Endothelial cells can regulate rapid hemostatic responses of platelets. At least three molecular systems contribute to the antithrombotic armamentarium of endothelium: cyclooxygenase/prostacyclin, L-arginine/nitric oxide, and ecto-adenosine diphosphatase (31). Because of the potency of these endothelial mechanisms as regulators of platelet activation and responsiveness, platelets were previously thought to deposit exclusively on exposed subendothelial structures and matrix at sites of primary hemostasis and clot formation, and to be repelled from endothelial surfaces. Exposed subendothelial matrix is clearly a favored site of platelet deposition in the flowing blood (32). Nevertheless, more recent observations, largely in rodent models, indicate that platelets can adhere to injured or inflamed endothelial cells (reviewed in Refs. 33 and 34). This may occur in the pulmonary circulation (35). Activated platelets that accumulate at sites of vascular injury or disruption can deliver signals that induce inflammatory responses of endothelial cells, a potential amplification mechanism in hemostasis, thrombosis, and inflammation.
Platelets have activities relevant to hemostasis beyond the traditional and well-known responses of adhesion, aggregation, hemostatic plug formation, and clot retraction at sites of vascular injury. Activated platelets provide a surface for catalysis of the extrinsic clotting cascade by the tissue factor/factor VIIa complex (36). This is critical in clot propagation and stabilization. Microparticles bearing tissue factor (TF) released from activated platelets or other cells may also be involved in clot propagation (36, 37). The sources of TF displayed by activated platelets and platelet microparticles have been controversial. TF-rich microparticles derived from stimulated monocytes can bind to activated platelets via PSGL-1 on the microparticle and P-selectin on the platelet (reviewed in Ref. 36). Recent observations, however, indicate that activated human platelets synthesize TF and may also be a primary source of this procoagulant protein in hemostasis and thrombosis (38, 39). TF synthesis by activated platelets is regulated by novel signal-dependent pre-mRNA splicing and translation pathways (38) (also see below). These observations have the potential to alter paradigms in the field because they suggest that TF may contribute to both initiation and amplification of the clotting cascade if it is rapidly synthesized and displayed by activated platelets at sites of primary hemostasis (36, 38). In parallel, there is evidence that activated platelets provide signals that induce TF synthesis by monocytes (40, 41), which also accumulate in clots and at sites of vascular injury (42 and references cited therein). Thus, activated platelets have recently identified activities that can influence clot formation via mechanisms that complement their rapid deposition in a hemostatic plug. These activities, or others such as inflammatory functions (see below), could provide new molecular targets in the treatment of venous thrombosis and thromboembolism (42) or acute lung injury (43).
While platelets are largely known for their immediate hemostatic activities, this is too restrictive a view of their functions in health and disease. They have nontraditional activities that, while secondary to clotting, nevertheless are tightly linked to hemostasis and to maintenance of tissue integrity (44). As a clear example, platelets are remarkably diverse inflammatory and immune effector cells, and they respond to, and deliver, signals that link inflammation and hemostasis (2, 21, 22, 33, 34, 45). This may be an evolutionarily conserved capacity that originated at an ancient time when host defense was accomplished by one, or a small number, of multifunctional cells with antimicrobial and wound repair activities; precursors of modern platelets then became progressively more specialized for hemostasis, but retained and evolved molecular systems for interacting with leukocytes and performing inflammatory and immune functions (2, 45). An important and relatively recent discovery that supports this possibility is the observation that mouse and human platelets have functional Toll-like receptors (TLRs) that recognize bacterial lipopolysaccharides and other microbial products (46–50). TLR expression presents a new facet in the repertoire by which platelets interact with, and respond to, microorganisms and their molecular signals, contributing to antimicrobial host defense (51). In addition, TLR signaling mediates inflammatory and thrombotic responses in sepsis and other pathologic conditions (52). Other signal transduction systems in platelets, such as those activated by thrombin or PAF (3), also link hemostasis and inflammation under physiologic and pathologic conditions (52).
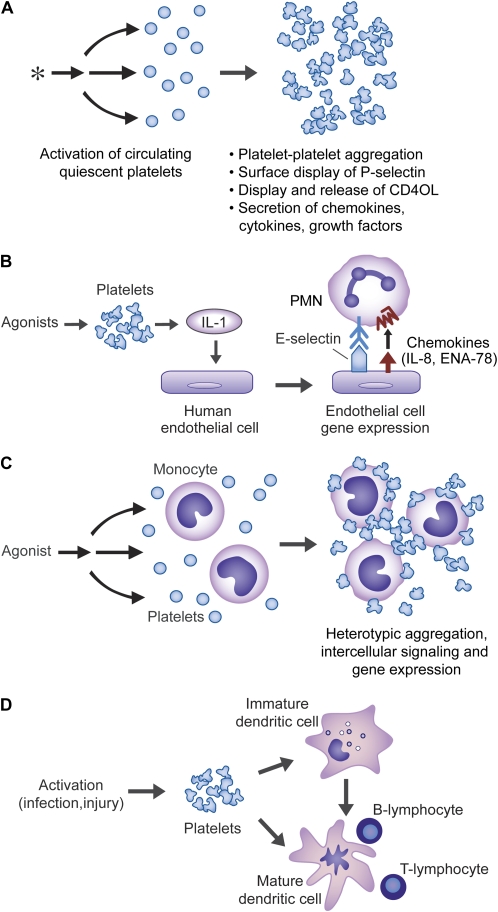
In addition to TLR signaling, platelets have other inflammatory capacities. They release a variety of preformed chemokines and cytokines, providing a mechanism for rapid contributions to acute inflammatory responses (45) (Figure 3). In addition, they rapidly synthesize the eicosanoid thromboxane A2, which has immunomodulatory as well as hemostatic activities (53). Unexpectedly, platelets also synthesize interleukin-1β (IL-1β) (Figure 3) (17, 54), a pleiotropic inflammatory regulator (55) with multiple actions in lung injury and repair (56–59). IL-1β synthesized by activated platelets can induce inflammatory responses of human endothelial cells (Figure 3). Synthesis of IL-Iβ by activated human platelets occurs via signal-dependent pre-mRNA splicing and translation (see below).
Figure 3.
Platelets have activities that span the continuum from acute inflammation to adaptive immune responses. (A) Activated platelets can mediate inflammatory and immune responses using a variety of molecular mechanisms, including release of stored chemokines (β-thromboglobulin, platelet factor 4, ENA-78, Gro α, RANTES, SDF1α, and others), cytokines (HMBG1), and growth factors (PDGF, others). Activated platelets also synthesize lipid mediators including thromboxane A2 and PAF, which have inflammatory and immune activities. The spectrum of inflammatory factors released from activated platelets is reviewed in Refs. 34 and 45. (B) In addition to lipid mediators, platelets rapidly synthesize IL-1β. This occurs via a regulated post-transcriptional pathway. IL-1β can then remain cell associated or be released in solution or in association with microvessicles. When synthesized by activated platelets in vitro, IL-1β is deposited in fibrin clots; if this occurs in vivo, clots and thrombi may act as local reservoirs for the cytokine. Platelet-derived IL-1β can signal human endothelial cells, leading to synthesis of adhesion molecules and chemokines that mediate PMN accumulation and activation (54). Platelets can also induce inflammatory responses by display of CD40 ligand (CD40L), which is recognized by CD40 on the surfaces of endothelial cells (34). (C) Activated platelets display P-selectin, which can mediate their adhesion to monocytes by binding to PSGL-1 on the leukocyte surfaces. P-selectin acts in concert with other adhesion molecules on the platelet plasma membrane and with chemokines and cytokines released by the platelets to mediate intercellular signaling and inflammatory gene expression by the target leukocytes. Activated platelets can also bind to PMNs (see Figure 4) and lymphocytes and alter their responses (reviewed in Refs. 2 and 45). (D) Activated platelets can signal functional changes in dendritic cells using multiple molecular pathways, and can potentially also influence differentiation of monocyte precursors to dendritic cells or macrophages (reviewed in Ref. 45). This figure was modified from Ref. 45 with permission.
Activated platelets interact directly with polymorphonuclear leukocytes (PMNs, neutrophils) and monocytes using P-selectin and other cell surface adhesion molecules and paracrine factors (2, 45). Physiologically relevant changes in function of the interacting cells result from these adhesive and signaling interactions. These include altered gene expression, with the potential to change patterns of expressed inflammatory and hemostastic factors in the local milieu (see below). Platelets also have the potential to directly or indirectly alter the ontogeny and function of macrophages and dendritic cells (45, 60–64). Dendritic cells are major effectors of “command and control” in interactions between innate and acquired immunity (65). They can also transition to macrophages (66). Thus platelets have molecular systems that influence the full spectrum of the inflammatory continuum, from innate to acquired immune responses (45) (Figure 3). Because of this, they have the capacity to alter events in a broad group of inflammatory alveolar, airway, and pulmonary vascular diseases.
PLATELETS IN CLINICAL ALI AND ARDS
Dysregulated hemostasis and inflammation are pivotal pathophysiologic mechanisms in ALI/ARDS, and have emerged as therapeutic targets in these syndromes (7, 30, 43). There is clinical evidence that platelets, as potent hemostatic and inflammatory cells, directly contribute to these central mechanisms.
Autopsy and biopsy studies of the lungs of patients with ALI/ARDS demonstrate intravascular microthrombi and fibrin deposition. In a study of 30 patients with ARDS, 23% developed disseminated intravascular coagulation (DIC), all of whom died (24). Autopsies were conducted in five of the seven fatal cases, and each revealed microthrombi. The lung was the most commonly involved organ, followed by the kidney and skin. Autopsies of patients with ARDS who did not develop overt DIC also revealed microthrombi in the lungs. The authors concluded that deposition of platelets on damaged pulmonary microvascular endothelium is common in ARDS regardless of whether or not it is accompanied by overt manifestations of the clinical and laboratory syndrome of DIC (24).
In a classic series of autopsy studies, Bachofen and Weibel reported platelets, intravascular fibrin deposits, and organized microthrombi in alveolar capillaries in the acute, exudative stage of ARDS (67), which is characterized by altered alveolar capillary membrane barrier function and increased pulmonary capillary permeability (7, 30). Morphometic analysis demonstrated increased numbers of platelets and leukocytes in lung capillaries. In some histologic samples platelets were apparently exiting pulmonary microvessels and entering the interstitial space (67). Subsequently, platelet–fibrin thrombi in pulmonary arteries, arterioles, and capillaries were detected in autopsy studies that used both microscopy and postmortem balloon angiography (68–70). Macrothrombi (in pulmonary arteries > 1 mm in diameter) were frequent in patients dying in the acute phase of ARDS and corresponded to vascular filling defects detected by bedside balloon occlusion pulmonary angiography in 48% of patients with ARDS (70). Although macrothrombi were particularly frequent in the acute phase, both macro- and microthrombi were present in all stages of the syndrome (68, 70). Open lung biopsy samples from patients with ALI also contained macro- and microthrombi (71). None of these autopsy or biopsy studies commented specifically on the presence of megakaryocytes (see above).
Consistent with morphologic observations, autologous radiolabeled platelets sequestered in the lungs, liver, and spleen when infused into patients with ALI (6, 25). Thus, there is anatomic and functional evidence demonstrating that platelets deposit in the acutely injured lung (8). The exact contributions of platelets to microthrombi formation, fibrin deposition, and thrombus remodeling in ALI/ARDS remain to be determined. Of interest, the influence of variables such as fraction of inspired oxygen (72) and protective versus damaging patterns of ventilation (30) on platelet accumulation in the injured human lung have not been defined.
As noted previously, thrombocytopenia occurs frequently in ALI/ARDS, with a variable incidence depending on the underlying clinical “trigger” (6). In addition to more common causes of diffuse alveolar damage such as trauma and sepsis (7), thrombocytopenia and ALI have also recently been associated with Severe Acute Respiratory Syndrome (SARS) and avian influenza A(H5N1) (73, 74). At the other end of the spectrum, thrombocytosis occurs in some patients with ALI/ARDS (see above). Interestingly, thrombocytosis, which also occurs in a variety of other inflammatory pulmonary syndromes, is a reported cause of pseudohypoxemia. The apparent impact of the circulating platelet number on oxygen exchange in subjects with septic shock or severe community-acquired pneumonia, which are important underlying conditions that contribute to ALA/ARDS, appears to be variable (F. Bozza and colleagues, unpublished observations).
In addition to alterations in circulating platelet number, there is evidence for altered phenotype and activation state of these cells in ALI/ARDS (6, 9). Circulating platelets from subjects with ARDS were reported to display P-selectin (formerly called GMP-140), a marker of activation, on their surfaces (75). P-selectin on activated platelets mediates adhesive interactions with neutrophils and monocytes (see below). PMN adhesiveness in pulmonary artery blood samples from patients with ARDS was influenced by platelet number (26), suggesting that platelet activation and altered platelet–leukocyte interactions may determine sequestration of these cells and other adhesive events in pulmonary vascular beds. Specific receptors on platelets from subjects with sepsis, one of the most common causes of ALI/ARDS (7, 30), were occupied by platelet-activating factor (PAF) (76), an agonist implicated in sepsis-induced ALI (52). Systemic and/or local intrapulmonary generation of platelet agonists such as thrombin, PAF, and other factors would provide mechanisms for platelet activation, increased adhesiveness, and additional functional responses such as degranulation in conditions that underlie ALI/ARDS (Figures 1, 3, and 4). Consistent with the latter possibility, bronchoalveolar lavage (BAL) samples from subjects with ARDS contain markers of platelet activation and release, but the levels do not clearly distinguish them from samples collected from subjects with interstitial lung disease or congestive heart failure (77, 78). Beta thromboglobulin, a platelet chemokine, was detected in the blood of patients with ALI and subjects with predisposing conditions without respiratory failure (79).
Figure 4.
Platelet–PMN interactions influence acute lung injury in patients and experimental models. (A) When platelets are activated by prothrombotic and inflammatory signals, platelet–platelet, platelet–endothelial, and platelet–PMN interactions occur in pulmonary arterioles, capillaries, and venules. Several molecular interactions mediate adhesion of activated human and/or murine platelets to PMNs. These include P-selectin/PSGL-1; integrin αmβ2 (MAC-1) on the PMN interacting with integrin αIIbβ3 or ICAM-2 on the platelet with fibrinogen as an intermediate; integrin αmβ2 on the PMN binding to GPIbα or junctional adhesion molecular 3 on the platelet; ICAM-2 on the platelet binding to integrin αLβ2 (LFA-1) on the PMN (reviewed in Ref. 34). Platelets also interact with monocytes under these conditions (see Figure 3). These events likely contribute to physiologic responses in lung infection and trauma, but can also mediate acute lung injury. (B) Platelets and PMNs accumulate in macro- and microvessels in the lungs of patients with ALI/ARDS, and lungs of animals in models of ALI. Sequestration of platelets and PMNs is frequently associated with fibrin deposition and endothelial injury. (C) In mouse models of ALI, platelets facilitate accumulation of PMNs in pulmonary capillaries and alveolar spaces, and contribute to alveolar-capillary membrane injury. Platelet–PMN interactions mediated by P-selectin/PSGL-1 are involved, as are other molecular and signaling pathways (see text for details).
Recent studies of transfusion-related acute lung injury (TRALI) provide topical observations relevant to platelet participation in ALI. TRALI is a syndrome of noncardiogenic pulmonary edema that is temporally related to transfusion of blood products, usually occurring within 30 minutes to 6 hours of their administration (80). All plasma-containing blood products have been associated with TRALI, including platelet transfusions (80). Clinical and experimental studies indicate that TRALI is caused by infused antineutrophil antibodies and/or biologically active lipids in the plasma of the transfusate, acting together with “primed,” adherent PMNs (80–82). Stored platelet concentrates are a source of lipids that can induce ALI in rats pretreated with LPS (82), consistent with this concept. It is not clear if transfused platelets themselves and/or endogenous activated platelets in the circulation of patients with TRALI play direct roles in the lung injury, although recent animal models suggest potential mechanisms (see below). If PMNs are activated by antineutrophil antibodies (81) and/or platelet-derived lipid mediators (82), they can then potentially activate platelets (reviewed in Refs. 6 and 83) contributing to alveolar capillary membrane injury. In an experimental model, infusion of a neutrophil agonist induced neutropenia, thrombocytopenia, and platelet sequestration in the lung (84), consistent with this possibility.
SYNTHETIC FUNCTIONS OF ACTIVATED PLATELETS: KNOWN PATHWAYS AND NEW BIOLOGY
Platelets rapidly synthesize thromboxane A2 from arachidonic acid via the cyclooxygenase pathway, and also use 12 lipoxygenase to metabolize arachidonate to 12- hydroxy–eicosanoids (6, 21). Thromboxane A2 has prothrombotic and proinflammatory activities, as previously outlined, and platelet 12 lipoxygenase can mediate transcellular metabolism and production of lipoxins in platelet–neutrophil interactions (21). Platelets also synthesize PAF (3). In addition to lipids, activated platelets produce superoxide and other reactive oxygen species (ROS) via NADPH oxidase activity (31). The generation of superoxide by activated platelets enhances their accumulation in growing thrombi and may impair the inhibitory activities of NO locally released by endothelial cells (31). The capacity to synthesize thromboxane, ROS, and lipoxins provides mechanisms by which activated platelets can modify vasoactive, inflammatory, and hemostatic events in ALI/ARDS (6, 85).
While synthesis of biologically active lipids and ROS are established functions of activated platelets, it has only recently been discovered that mature, circulating platelets synthesize proteins in response to thrombin and other physiologically relevant activating signals. This de novo protein synthesis occurs via a repertoire of post-transcriptional pathways, consistent with the fact that platelets are anucleate and do not transcribe messenger mRNAs (with the exception of mitochondrial transcription). The general term assigned to synthesis of specific proteins by activated, but not resting, platelets is signal-dependent translation (reviewed in Ref. 44). The first example was synthesis of B cell lymphoma-3 (BCL-3) by activated human platelets, a process that is under specialized translational control by the intracellular kinase mammalian target of Rapamycin (mTOR) (86) (reviewed in Ref. 44). Synthesis of Bcl-3 by activated human and murine platelets regulates clot retraction in ex vivo assays (18), demonstrating that signal-dependent translation of previously transcribed but basally “silenced” mRNAs by activated platelets has physiologic relevance (44). An important point is that signal-dependent synthesis of Bcl-3 and other key proteins is rapidly initiated (86). Expedited synthesis of the protein products is a biologic advantage of translational control of previously transcribed mRNAs, and is one that is clearly advantageous to platelets since they are rapid response cells in injury and hemostasis. In addition, however, signal-dependent translation of new proteins by activated human platelets continues for several hours, indicating that platelet responses are not limited to adhesion, aggregation, and degranulation in the first few minutes of hemostasis (44, 45).
As noted above, activated human platelets also synthesize IL-1β and TF (17, 38, 39, 54). Remarkably, in both of these cases intron-containing pre-mRNA transcripts for each gene product are present in resting, unstimulated platelets and are spliced to mature, translatable mRNAs in a signal-dependent fashion in response to cellular activation (17, 38). Extranuclear splicing had not previously been observed in human cells before these observations. Based on studies of model megakaryocytes, the pre-mRNAs are translocated to proplatelets during thrombopoiesis (17, 38). In response to signals that induce splicing, activated platelets then translate the processed, mature mRNAs to biologically active TF, which mediates a procoagulant platelet surface, and to pro–IL-1β, a portion of which can be converted to active IL-1β (17, 38, 54). TF and IL-1β have many established and postulated activities in experimental and clinical ALI; therefore, their synthesis by activated platelets has the potential to influence key events in this setting. In addition, it is possible that signal-dependent translation and synthesis of Bcl-3 by activated platelets (18, 87) may modify fibrin deposits and intravascular thrombi in the injured lung (43) (Figure 4). Other products in the proteome of activated human platelets have also been reported to be synthesized in response to cellular stimulation, and many newly synthesized proteins are yet to be identified (45, 87). Some of these may have pathophysiologically relevant activities in acute lung injury syndromes.
In addition to acting as templates for new protein synthesis, the transcriptome of platelets from subjects with ALI/ARDS may provide unique diagnostic information. In a recent report, analysis of platelets from patients with myocardial infarction revealed differences compared with the mRNA profile in platelets from subjects with stable coronary artery disease (88). Similar analysis may be informative and useful in samples from subjects with ALI/ARDS and key predisposing conditions such as sepsis, in which platelet activation is frequent and may be ubiquitous (89).
PLATELETS, ENDOTHELIAL CELLS, AND ALVEOLAR CAPILLARY MEMBRANE PERMEABILITY
Platelets can interact with and signal endothelial cells in a variety of ways (6, 34, 90). In this section we focus on interactions that influence alveolar capillary barrier function because of its central importance in ALI/ARDS, particularly in the early edematous phase (7, 30).
Clinical and experimental observations indicate that platelets are important, and potentially essential, for systemic and pulmonary vascular integrity. As an example, thrombocytopenia is associated with pleural effusion—which was shown to be a measure of vascular leakage—in Dengue syndromes (91). Extensive evidence for a contribution by platelets to basal vascular integrity has been reviewed (6). It includes the observation that infusion of small numbers of platelets insufficient to alter the bleeding time improved vascular fragility in patients with leukemia. In studies of systemic tissues, vascular permeability increased when experimental animals were made thrombocytopenic, and perfusion of isolated organs with platelets corrected ultrastructural alterations in endothelium associated with altered barrier function. Studies of the lungs in both large and small animal models indicated that platelets contribute to pulmonary vascular integrity and to alveolar–capillary barrier function. Platelets were proposed to take up and eliminate noxious barrier-disrupting agents such as ROS, to plug paracellular gaps, and/or to provide factors that decrease endothelial permeability and stimulate endothelial survival and barrier function. Experimental evidence suggested that serotonin, epinephrine, and adenosine released from activated platelets induce endothelial stress fiber organization and/or replication of endothelial cells, depending on the specific factor studied. These and other observations indicated that platelets are critical for vascular barrier function; nevertheless, the conclusion was that the mechanisms by which platelets preserve vascular integrity remained elusive (6).
More recent studies have identified several phospholipids with signaling properties for endothelial cells that are released from platelets (92, and references cited therein). One of these, sphingosine-1-phosphate (S1P), was reported to promote endothelial barrier integrity in vitro and in vivo, including improvement of barrier function in models of increased pulmonary capillary permeability. S1P is recognized by a family of five surface G protein–coupled SIP receptors, which are also called Edg receptors (92). S1P released from activated platelets was shown to interact with endothelial cells (93), and to induce adherens junction assembly and cytoskeletal rearrangement in an in vitro model of angiogenesis using cultured human umbilical vein endothelial cells (94). Adherens junctions and tight junctions are critical in regulating endothelial permeability and polarity, and can be modified by inflammatory mediators and hemostatic signals in pulmonary vascular injury (29). S1P induced rapid and sustained increases in transmonolayer electrical resistance in cultured human and bovine pulmonary artery and microvascular endothelial cells by engaging S1P receptors (92), paralleling improved barrier function in “leaky” human umbilical vein endothelial cells in the angiogenesis model (94). Reduction in S1P receptor Type 1 (S1PR1) by selective “knockdown” using RNA interference altered human endothelial cell function and modified parallel TNF-α signaling (95). In addition, endothelial barrier function was reported to be improved by activated protein C, which is used therapeutically in the treatment of sepsis, by a mechanism that involves transactivation of S1PR1 by protease-activated receptor 1 (96).
Exogenous administration of S1P was later shown to incrementally reduce pulmonary microvascular permeability and alter indices of alveolar inflammation in mice and dogs subjected to LPS challenge and in a model of ventilator-induced lung injury (97, 98). Administration of S1P also reduced extravasation of labeled albumin and accumulation of myeloperoxidase, a PMN marker, in the kidneys of LPS-infused mice in these experiments. In aggregate together with in vitro studies, these observations suggested that S1P may be useful as a therapeutic agent in syndromes of vascular barrier dysfunction (99), and also provided a previously unrecognized mechanism by which platelets may contribute to vascular integrity. Importantly, steady-state plasma levels of S1P are significantly higher than the Kd for SIP receptors, suggesting that endothelial cell S1P receptors are tonically ligated by endogenous S1P under physiologic conditions (99). Nevertheless, this may not be the case in thrombocytopenic patients. In addition to clinical issues of this sort, a number of mechanistic and physiologic issues regarding the activities of S1P in altered lung barrier function remain to be resolved (99).
It is not clear if platelets also influence alveolar epithelial function. This question is important because epithelial cells, together with endothelial cells, are critical in regulation of alveolar capillary membrane permeability and alveolar fluid clearance (30, 100). Because platelet products are found in the alveolar fluid in ALI (see above), activated platelets may deliver paracrine signals such as IL-1β or other soluble inflammatory factors (34, 45) to the alveolar epithelium.
In addition to releasing molecular factors that provide direct signals to alveolar capillary endothelial or epithelial cells, platelets may alter alveolar barrier function by interacting with leukocytes. Examples in the next section illustrate this point.
PLATELETS, LEUKOCYTES, AND ALI
As emphasized previously, platelets have molecular systems and functional activities that link hemostasis and inflammation. Platelet–leukocyte interactions are central in this repertoire (Figures 3 and 4). Activated platelets adhere to myeloid leukocytes in the circulating blood and in microvascular beds, forming heterotypic aggregates (2, 45). In some clinical conditions detection of circulating platelet–monocyte aggregates by flow cytometry may be the most sensitive measure of platelet activation (101). Activated platelets adhere to monocytes and neutrophils (Figures 3 and 4) by binding of P-selectin, which is translocated from α granules to the platelet plasma membrane, to P-selectin glycoprotein ligand 1 (PSGL-1) on the leukocyte surfaces (reviewed in Refs. 34 and 45). Integrin α11bβ3 and glycoprotein Ib on activated platelets, together with integrin αmβ2 (MAC-1, CD11b/CD18) on the myeloid leukocytes, also contribute to these adhesive interactions (34, 102, 103). Platelets facilitate recruitment of PMNs to the surfaces of inflamed endothelial cells in experimental models (104). In addition, adherent, activated platelets deposited on exposed vascular matrix provide a surface for rolling and attachment of myeloid leukocytes in experimental studies (2, 34). Whether this occurs in the human lung vessels is unknown. In an experimental model, PMNs sequestered in lung microvessels facilitated deposition of platelets, suggesting that neutrophil–platelet interactions influence the local deposition of platelets in alveolar inflammation (84).
Adhesion of activated platelets to PMNs and monocytes is more than a physical association. Platelets also provide juxtacrine and paracrine signals that can induce functional alterations in the leukocytes (6, 83, 105). As an example, activated platelets release the chemokine RANTES, which can deliver signals to monocytes via CC chemokine receptors on their surfaces (106). In addition, PSGL-1 on monocytes and PMNs can transmit juxtacrine signals when it is engaged by P-selectin on activated platelets (2, 105). One consequence of signaling of myeloid leukocytes by activated platelets is the expression of genes that encode inflammatory proteins and enzymes, including IL-8, monocyte chemoattractant protein-1, cyclooxygenase-2, and others (2, 105, 107). There is also evidence for reciprocal signaling between activated platelets and leukocytes and, as mentioned previously, transcellular metabolism (6, 83). These processes are influenced by cell–cell adhesion and signaling mediated by P-selectin, other adhesion molecules, and soluble factors.
Platelet interactions with myeloid leukocytes were previously implicated in ALI/ARDS based on a variety of observations (6, 8). In addition, platelets are found together with PMNs and monocytes in lung vessels in histologic samples from subjects with ALI/ARDS (see above). Early laboratory studies suggested a role for platelets in acute lung injury; for example, platelets were reported to be required for complement-mediated ALI in mice (108). Recent experimental studies add to the body of evidence indicating that platelet–leukocyte interactions are important in ALI syndromes.
A murine model of lung injury induced by hydrochloric acid (HCL), used as a surrogate for human ALI/ARDS triggered by aspiration, indicated that platelet–PMN interactions are determinants of leukocyte accumulation, increased alveolar–capillary permeability, and impaired gas exchange (109). Platelet–neutrophil aggregates were detected in the blood and in lung microvessels within 30 minutes of acid injury. Platelet depletion reduced PMN accumulation in microvascular, interstitial, and alveolar compartments, an experimental finding with potential clinical correlates, since adhesiveness of PMNs was related to platelet numbers in pulmonary blood samples from patients with ARDS (26). Of note, engagement of PSGL-1 on human PMNs triggers release of IL-8 (110). IL-8 mediates PMN accumulation and alveolar capillary membrane injury in experimental acid aspiration (111).
Platelet depletion reduced the total protein recovered in BAL samples and improved the PaO2/FiO2 ratio in HLC-treated mice compared with those with normal platelet counts and, in limited experiments, improved these variables in a “two hit” model of sepsis (109). In addition, an anti–P-selectin mAb administered 15 minutes after acid injury also reduced PMN accumulation and protein content in alveolar fluid, and improved the PaO2/FiO2 ratio and ultimate survival. These results were, in general, consistent with previous studies indicating that blockade or knockout of P-selectin often protects animals from, or improves, inflammatory damage in models of ALI (104). Studies of chimeric mice indicated that P-selectin expressed by platelets—rather than by endothelial cells—was largely responsible for accumulation of PMNs, altered alveolar barrier function, and impaired gas exchange resulting from acid injury, although endothelial P-selectin contributed to intravascular PMN sequestration (109). Additional experiments using acid-challenged animals, cultured microvascular endothelial cells, and human platelets and PMNs identified thromboxane synthesis mediated by platelet–PMN interactions and signaling via thromboxane receptors as central components in this model.
Platelets were also reported to contribute to inflammatory lung injury in murine models of other syndromes, including hemorrhagic shock. Platelet depletion reduced infiltration of PMNs in the lungs of mice subjected to hemorrhage, and improved lung edema assessed by histologic measurements (112). In the same study, similar improvements in liver inflammation were seen in animals given an anti-platelet antibody before hemorrhage shock. Importantly, some but not all anti-platelet antibodies induce systemic changes and lung injury in mice (113), indicating that the method and timing of platelet depletion are important factors in these experimental models. In earlier large animal models (dogs, sheep), platelet depletion with anti-platelet sera had variable effects on pulmonary responses to LPS infusion (114, 115).
Observations suggesting that platelets are central effectors of experimental alveolar injury (108, 109, 112) pose a potential quandary when considered in the context of studies indicating that platelets are essential for normal alveolar capillary membrane integrity and that platelet products improve alveolar barrier function (discussed above). It is possible that negative and injurious effects mediated by platelet–PMN interactions under conditions of injury (109) outweigh positive signals delivered to the pulmonary alveolar barrier by circulating platelets alone. PMNs activated by platelets and/or by other signaling pathways after being locally recruited by platelets have potent mechanisms that can increase vascular permeability and injure endothelial and epithelial cells (6, 116–118). Furthermore, it is possible that local release of S1P or other factors that stabilize barrier function by platelets is impaired under some injury conditions. With respect to contributions to basal barrier integrity, reduction of circulating platelets by approximately 40% caused a small but nonsignificant decrease in PaO2/FiO2 ratio in control mice (109), suggesting that this degree of platelet depletion did not alter alveolar barrier function; direct measurements of lung water and barrier properties were not reported, however. Beneficial effects of platelet reduction in the face of acid injury were durable when profound thrombocytopenia was induced in this model (∼ 85% reduction), but the impact of this degree of platelet depletion on lung water and barrier function in control mice was again not investigated.
There are additional complex platelet–PMN interactions in models relevant to ALI/ARDS. Studies employing isolated human cells and a mouse model of endotoxemia indicated that, under conditions chosen to be relevant to sepsis, platelets induce formation of neutrophil extracellular traps (NETs) (119). NETs are extracellular chromatin lattices studded with elastase and other antimicrobial factors that capture and kill bacteria and fungi (120). This is a previously unrecognized mechanism of extracellular antimicrobial defense (121). In flow chamber experiments using human cells, LPS-treated platelets adhered to PMNs immobilized on coverslips and induced NET formation (119). This could also be triggered by plasma by from patients with sepsis, but not by thrombin. This, and features of the adhesive interaction between platelets and PMNs under these conditions, suggest an unconventional mechanism of platelet activation mediated by TLR4. In mice infused with LPS, platelet–PMN interaction occurred in liver sinusoids and pulmonary capillaries and NET-like structures were detected in pulmonary vessels. In the liver, NET formation was associated with evidence of microvascular injury that was prevented by prior depletion of platelets or neutrophils. This, together with experiments using cultured endothelial cells, suggested that platelet-triggered NET formation causes endothelial damage if it occurs in the microcirculation, and that platelet-induced NET generation may be a cause of microvascular dysfunction in sepsis (119, 121). The exact mechanism of endothelial injury was not examined, leaving this question open. NETs have not been observed in lungs or systemic vessels of patients with sepsis, but they have been reported in appendicitis, in the alveolar space in bacterial pneumonia, and in interstitial pneumonia in tissues from human subjects (119, 120, 122).
In considering the mechanisms involved in murine ALI models in the context of clinical ALI/ARDS, it should be remembered that the number of circulating platelets is higher and the number of PMNs lower in mice compared with humans (20, 123). Therefore, the platelet/PMN ratio in systemic and pulmonary blood is substantially different in the two species. In the recent HCL injury model, the ratio in systemic blood of control mice was approximately 250/1 (109), whereas in human systemic blood it is approximately 80–100/1 under basal conditions.
PLATELETS AND REPAIR: INTERACTIONS WITH PROGENITOR AND STEM CELLS
Platelets have the potential to influence repair of alveolar and airway structures, and also to participate in maladaptive repair responses such as fibrosis, by cellular interactions and by releasing paracrine factors. These include platelet-derived growth factor, epidermal growth factor, vascular endothelial growth factor, and members of the transforming growth factor family (reviewed in Refs. 6 and 90). IL-1β and eicosanoids, which are synthesized by activated platelets and by monocytes in response to platelet signals (17, 54, 105, 107), also regulate responses of fibroblasts that are relevant to the fibroproliferative phase of AL1/ARDS (124). Thus, signaling interactions may allow platelets to influence homeostatic repair or, conversely, drive untoward cellular proliferation and fibrosis in the acutely injured lung.
Platelets may also influence repair by interacting with stem and progenitor cells. As an example, platelets were recently reported to bind to bone marrow–derived endothelial progenitor cells and to recruit them to thrombi and sites of vascular injury in murine models (125). Activated platelets adhered to progenitor cells using P-selectin and integrins, and also released stromal cell–derived factor 1α (SDF 1α), which further supported homing and adhesion of progenitor cells. Adherent platelets and fibrin were also reported to induce differentiation of progenitor cells to a phenotype that expressed endothelial markers in an in vitro model (126). In a different murine model, hematopoietic cytokines were reported to induce local “deployment” of SDF 1α from platelets and, via this mechanism, mediate mobilization and recruitment of precursor hemangiocytes to ischemic hindlimb vessels (127). Thus, platelets may have key regulatory activities that are critical in endothelial precursor homing and differentiation and in vascular repair (reviewed in Ref. 128). Whether platelet-mediated fibrin modulation (18) and/or deposition of platelet factors such as IL-1β in the fibrin reservoir (54) at sites of injury condition the local niche and favor endothelial precursor cell homing and differentiation are unknown.
Platelet-directed progenitor cell recruitment may drive vascular injury in addition to mediating vascular repair and development. Platelet signaling is reported to direct progenitor cells along differentiation pathways that lead to foam cell formation under some conditions (128). Foam cell formation is considered to be a maladaptive response in atherosclerosis.
While platelet interactions with endothelial progenitor cells have largely been considered in the context of atherosclerosis and ischemic peripheral vascular injury to date, it is possible that these observations are also relevant to repair versus obstruction and destruction of injured pulmonary vessels. Dysregulated vascular remodeling can be severe in the acutely injured lung, and is a cause of vascular obliteration and pulmonary hypertension in ALI/ARDS (30, 68, 70). The contributions of platelets and their interactions with progenitor cells in pulmonary vascular repair and remodeling in ALI/ARDS are unexplored. It is also unknown if platelets influence homing and/or differentiation of bone marrow–derived mesenchymal stem cells to the injured lung (129), or targeting and differentiation of specific progenitor cell types to the kidney or other damaged tissues in multiple organ injury in the setting of ALI/ARDS. In addition, platelets themselves—engineered from hematopoietic stem cells—could be unique vehicles for delivery of reparative factors in cell-based therapeutic strategies (130). These possibilities are intriguing and would provide new facets to the activities of platelets in host injury, defense, and repair.
Acknowledgments
The authors thank the many postdoctoral fellows, students, and collaborators who contributed to work cited here. They are also indebted to their technical staff for providing invaluable support. The authors appreciate the skill and hard work of Sharren Brewer and Linn Steele in preparation of the manuscript and Diana Lim's creativity and art in constructing the figures. They thank Mark Looney, M.D. and Michael Matthay, M.D. for critical reading of the manuscript and helpful comments and suggestions.
This work was supported by grants from the National Institutes of Health (RO1 HL091754; RO1 AG031065; RO1 HL 066277; R37 HL44525) to A.S.W. and G.A.Z., an Established Investigator Award from the American Heart Association to A.S.W., and an award from the Conselho Nacional de Desenvolvimento Cientifico e Technologico of the Ministry for Science and Technology of Brazil to F.A.B.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0241TR on August 21, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hartwig J, Italiano J Jr. The birth of the platelet. J Thromb Haemost 2003;1:1580–1586. [DOI] [PubMed] [Google Scholar]
- 2.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost 2003;1:1897–1905. [DOI] [PubMed] [Google Scholar]
- 3.Parise LV, Smyth SS, Shet AS, Coller BS. Platelet morphology, biochemistry and function. In: M.A. Lichtman TJK, Kaushansky K, Beutler E, Seligshon U, Prachal J, editors. Williams hematology, 7th ed. New York, NY: McGraw Hill; 2006. pp. 1597–1645.
- 4.Zimmerman GA, McIntyre TM, Prescott SM. Cell-to-cell communication. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations. Philadelphia, PA: Lippincott-Raven; 1997. pp. 289–304.
- 5.Bierman HR. The hematologic role of the lung in man. Am J Surg 1955;89:130–140. [DOI] [PubMed] [Google Scholar]
- 6.Heffner J, Repine JE. Platelets. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations, 2nd ed. Philadelphia: Lippincott-Raven; 1997.
- 7.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 8.Heffner JE, Sahn SA, Repine JE. The role of platelets in the adult respiratory distress syndrome. Culprits or bystanders? Am Rev Respir Dis 1987;135:482–492. [DOI] [PubMed] [Google Scholar]
- 9.Heffner JE, Repine JE. Platelets. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations. New York: Raven Press, Ltd.; 1991. pp. 617–630.
- 10.Patel SR, Hartwig JH, Italiano JE Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest 2005;115:3348–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geddis AE, Kaushansky K. Immunology. the root of platelet production. Science 2007;317:1689–1691. [DOI] [PubMed] [Google Scholar]
- 12.Ravid K. Ets and megakaryocytes: maturation matters. Blood 2006;108:2139. [Google Scholar]
- 13.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol 2000;157:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandal RV, Mark EJ, Kradin RL. Megakaryocytes and platelet homeostasis in diffuse alveolar damage. Exp Mol Pathol 2007;83:327–331. [DOI] [PubMed] [Google Scholar]
- 15.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood 1995;85:402–413. [PubMed] [Google Scholar]
- 16.Cramer EM, Norol F, Guichard J, Breton-Gorius J, Vainchenker W, Masse JM, Debili N. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood 1997;89:2336–2346. [PubMed] [Google Scholar]
- 17.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell 2005;122:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, Kraiss LW, Albertine KH, McIntyre TM, Zimmerman GA. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood 2007;109:1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE Jr, Shivdasani RA, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007;317:1767–1770. [DOI] [PubMed] [Google Scholar]
- 20.Tsakiris DA, Scudder L, Hodivala-Dilke K, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost 1999;81:177–188. [PubMed] [Google Scholar]
- 21.O'Sullivan BP, Michelson AD. The inflammatory role of platelets in cystic fibrosis. Am J Respir Crit Care Med 2006;173:483–490. [DOI] [PubMed] [Google Scholar]
- 22.Herd CM, Page CP. Do platelets have a role as inflammatory cells? In: Joseph M, editor. Immunopharmacology of platelets. San Diego: Academic Press, Inc.; 1995. pp. 1–20.
- 23.Peacock AJ, Egermayer P, Town GI. The role of platelets in pulmonary hypertension. In: Weir EK, Reeve HL, Reeves JT, editors. Interactions of blood and the pulmonary circulation. Armonk, NY: Futura Publishing Company, Inc.; 2002. pp. 187–201.
- 24.Bone RC, Francis PB, Pierce AK. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med 1976;61:585–589. [DOI] [PubMed] [Google Scholar]
- 25.Schneider RC, Zapol WM, Carvalho AC. Platelet consumption and sequestration in severe acute respiratory failure. Am Rev Respir Dis 1980;122:445–451. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman GA, Renzetti AD, Hill HR. Granulocyte adherence in pulmonary and systemic arterial blood samples from patients with adult respiratory distress syndrome. Am Rev Respir Dis 1984;129:798–804. [DOI] [PubMed] [Google Scholar]
- 27.Xiao da W, Yang M, Yang J, Hon KL, Fok FT. Lung damage may induce thrombocytopenia. Platelets 2006;17:347–349. [DOI] [PubMed] [Google Scholar]
- 28.Avraham H, Cowley S, Chi SY, Jiang S, Groopman JE. Characterization of adhesive interactions between human endothelial cells and megakaryocytes. J Clin Invest 1993;91:2378–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez ML, Zimmerman GA. The endothelium in acute respiratory distress syndrome. In: Aird WC, editor. Endothelial biomedicine. Cambridge: Cambridge University Press; 2007. pp. 1178–1192.
- 30.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005;33:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]
- 32.Savage B, Ruggeri ZM. Platelet thrombus formation in flowing blood. In: Michaelson AD, editor. Platelets, 2nd ed. Boston: Academic Press; 2007. pp. 359–376.
- 33.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol 2003;23:2131–2137. [DOI] [PubMed] [Google Scholar]
- 34.Bergmeier W, Wagner DD. Inflammation. In: Michaelson AD, editor. Platelets. Boston: Academic Press; 2007. pp. 713–726.
- 35.Eichhorn ME, Ney L, Massberg S, Goetz AE. Platelet kinetics in the pulmonary microcirculation in vivo assessed by intravital microscopy. J Vasc Res 2002;39:330–339. [DOI] [PubMed] [Google Scholar]
- 36.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007;27:1687–1693. [DOI] [PubMed] [Google Scholar]
- 37.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol 2006;26:2594–2604. [DOI] [PubMed] [Google Scholar]
- 38.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med 2006;203:2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood 2007;109:5242–5250. [DOI] [PubMed] [Google Scholar]
- 40.Lindmark E, Tenno T, Siegbahn A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arterioscler Thromb Vasc Biol 2000;20:2322–2328. [DOI] [PubMed] [Google Scholar]
- 41.Steiner S, Seidinger D, Huber K, Kaun C, Minar E, Kopp CW. Effect of glycoprotein IIb/IIIa antagonist abciximab on monocyte-platelet aggregates and tissue factor expression. Arterioscler Thromb Vasc Biol 2003;23:1697–1702. [DOI] [PubMed] [Google Scholar]
- 42.Prescott SM, Weyrich AS, Zimmerman GA. Classification of venous thromboembolism (VTE). The clot is hot: inflammation, myeloid leukocytes, and venous thromboembolism. J Thromb Haemost 2005;3:2571–2573. [DOI] [PubMed] [Google Scholar]
- 43.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L307–L311. [DOI] [PubMed] [Google Scholar]
- 44.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol 2008;28:s17–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol 2004;25:489–495. [DOI] [PubMed] [Google Scholar]
- 46.Mirlashari MR, Hagberg IA, Lyberg T. Platelet-platelet and platelet-leukocyte interactions induced by outer membrane vesicles from N. meningitidis. Platelets 2002;13:91–99. [DOI] [PubMed] [Google Scholar]
- 47.Zhao L, Ohtaki Y, Yamaguchi K, Matsushita M, Fujita T, Yokochi T, Takada H, Endo Y. LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system. Blood 2002;100:3233–3239. [DOI] [PubMed] [Google Scholar]
- 48.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood 2005;106:2417–2423. [DOI] [PubMed] [Google Scholar]
- 49.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol 2005;83:196–198. [DOI] [PubMed] [Google Scholar]
- 50.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 2006;107:637–641. [DOI] [PubMed] [Google Scholar]
- 51.Yeaman MR, Bayer AS. Antimicrobial host defense. In: Michaelson AD, editor. Platelets, 2nd ed. Boston: Academic Press; 2007. pp. 727–755.
- 52.Zimmerman GA, Albertine KH, McIntyre TM. Pathogenesis of sepsis and septic-induced lung injury. In: Matthay MA, editor. Acute respiratory distress syndrome, Vol. 179. New York, Basel: Marcel Dekker, Inc.; 2003. pp. 245–287.
- 53.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 2001;108:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol 2001;154:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov 2004;3:330–339. [DOI] [PubMed] [Google Scholar]
- 56.Martin TR. Lung cytokines and ARDS: Roger S. Mitchell Lecture. Chest 1999;116:2S–8S. [DOI] [PubMed] [Google Scholar]
- 57.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. Am J Respir Crit Care Med 2001;163:1384–1388. [DOI] [PubMed] [Google Scholar]
- 58.Olman MA, White KE, Ware LB, Simmons WL, Benveniste EN, Zhu S, Pugin J, Matthay MA. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J Immunol 2004;172:2668–2677. [DOI] [PubMed] [Google Scholar]
- 59.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 2005;280:18579–18589. [DOI] [PubMed] [Google Scholar]
- 60.Hilf N, Singh-Jasuja H, Schwarzmaier P, Gouttefangeas C, Rammensee HG, Schild H. Human platelets express heat shock protein receptors and regulate dendritic cell maturation. Blood 2002;99:3676–3682. [DOI] [PubMed] [Google Scholar]
- 61.Li G, Kim YJ, Mantel C, Broxmeyer HE. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J Immunol 2003;171:669–677. [DOI] [PubMed] [Google Scholar]
- 62.Hagihara M, Higuchi A, Tamura N, Ueda Y, Hirabayashi K, Ikeda Y, Kato S, Sakamoto S, Hotta T, Handa S, et al. Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro. J Immunol 2004;172:5297–5303. [DOI] [PubMed] [Google Scholar]
- 63.Kissel K, Berber S, Nockher A, Santoso S, Bein G, Hackstein H. Human platelets target dendritic cell differentiation and production of proinflammatory cytokines. Transfusion. 2006;46:818–827. [DOI] [PubMed] [Google Scholar]
- 64.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol 2007;27:1463–1470. [DOI] [PubMed] [Google Scholar]
- 65.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007;449:419–426. [DOI] [PubMed] [Google Scholar]
- 66.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol 1998;160:4587–4595. [PubMed] [Google Scholar]
- 67.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56. [PubMed] [Google Scholar]
- 68.Tomashefski JF Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 69.Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med 2000;21:435–466. [DOI] [PubMed] [Google Scholar]
- 70.Tomashefski JF Jr. Pulmonary pathology of the acute respiratory (diffuse alveolar damage). In: Matthay MA, editor. Acute respiratory distress, Vol 179. New York: Marcel Dekker; 2003. pp. 75–114.
- 71.Hill JD, Ratliff JL, Parrott JC, Lamy M, Fallat RJ, Koeniger E, Yaeger EM, Whitmer G. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg 1976;71:64–71. [PubMed] [Google Scholar]
- 72.Barazzone C, Tacchini-Cottier F, Vesin C, Rochat AF, Piguet PF. Hyperoxia induces platelet activation and lung sequestration: an event dependent on tumor necrosis factor-alpha and CD11a. Am J Respir Cell Mol Biol 1996;15:107–114. [DOI] [PubMed] [Google Scholar]
- 73.Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431–2441. [DOI] [PubMed] [Google Scholar]
- 74.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008;358:261–273. [DOI] [PubMed] [Google Scholar]
- 75.George JN, Pickett EB, Saucerman S, McEver RP, Kunicki TJ, Kieffer N, Newman PJ. Platelet surface glycoproteins: studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest 1986;78:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez Diez F, Nieto ML, Fernandez-Gallardo S, Gijon MA, Sanchez Crespo M. Occupancy of platelet receptors for platelet-activating factor in patients with septicemia. J Clin Invest 1989;83:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Idell S, Maunder R, Fein AM, Switalska HI, Tuszynski GP, McLarty J, Niewiarowski S. Platelet-specific alpha-granule proteins and thrombospondin in bronchoalveolar lavage in the adult respiratory distress syndrome. Chest 1989;96:1125–1132. [DOI] [PubMed] [Google Scholar]
- 78.Cohen AB, Stevens MD, Miller EJ, Atkinson MA, Mullenbach G, Maunder RJ, Martin TR, Wiener-Kronish JP, Matthay MA. Neutrophil-activating peptide-2 in patients with pulmonary edema from congestive heart failure or ARDS. Am J Physiol 1993;264:L490–L495. [DOI] [PubMed] [Google Scholar]
- 79.Haynes JB, Hyers TM, Giclas PC, Franks JJ, Petty TL. Elevated fibrin(ogen) degradation products in the adult respiratory distress syndrome. Am Rev Respir Dis 1980;122:841–847. [DOI] [PubMed] [Google Scholar]
- 80.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest 2004;126:249–258. [DOI] [PubMed] [Google Scholar]
- 81.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest 2006;116:1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silliman CC, Bjornsen AJ, Wyman TH, Kelher M, Allard J, Bieber S, Voelkel NF. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–640. [DOI] [PubMed] [Google Scholar]
- 83.Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. The interaction of leukocytes with platelets in blood coagulation. Curr Opin Hematol 1995;2:47–54. [DOI] [PubMed] [Google Scholar]
- 84.Issekutz AC, Ripley M, Jackson JR. Role of neutrophils in the deposition of platelets during acute inflammation. Lab Invest 1983;49:716–724. [PubMed] [Google Scholar]
- 85.Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol 2007;36:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci USA 1998;95:5556–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets (new pathways to altered phenotype and function). Arteriosclero Thromb Vasc Biol 2008;28:s17–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 2006;113:2278–2284. [DOI] [PubMed] [Google Scholar]
- 89.Aird WC. The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clin Proc 2003;78:869–881. [DOI] [PubMed] [Google Scholar]
- 90.Maguire PB, Belton O, O'Donoghue N, Austini S. Platelet-endothalial interactions. In: Aird WC, editor. Endothelial biomedicine. Cambridge: Cambridge University Press; 2007. pp. 587–601.
- 91.Krishnamurti C, Kalayanarooj S, Cutting MA, Peat RA, Rothwell SW, Reid TJ, Green S, Nisalak A, Endy TP, Vaughn DW, et al. Mechanisms of hemorrhage in dengue without circulatory collapse. Am J Trop Med Hyg 2001;65:840–847. [DOI] [PubMed] [Google Scholar]
- 92.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, Satoh K, Kume S, Tigyi G, Igarashi Y, et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood 2000;96:3431–3438. [PubMed] [Google Scholar]
- 94.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999;99:301–312. [DOI] [PubMed] [Google Scholar]
- 95.Krump-Konvalinkova V, Yasuda S, Rubic T, Makarova N, Mages J, Erl W, Vosseler C, Kirkpatrick CJ, Tigyi G, Siess W. Stable knock-down of the sphingosine 1-phosphate receptor S1P1 influences multiple functions of human endothelial cells. Arterioscler Thromb Vasc Biol 2005;25:546–552. [DOI] [PubMed] [Google Scholar]
- 96.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 2005;105:3178–3184. [DOI] [PubMed] [Google Scholar]
- 97.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004;170:987–993. [DOI] [PubMed] [Google Scholar]
- 98.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251. [DOI] [PubMed] [Google Scholar]
- 99.Bhattacharya J. Lung injury: sphingosine-1-phosphate to the rescue. Am J Respir Crit Care Med 2004;170:928–929. [DOI] [PubMed] [Google Scholar]
- 100.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600. [DOI] [PubMed] [Google Scholar]
- 101.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001;104:1533–1537. [DOI] [PubMed] [Google Scholar]
- 102.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 2005;115:3378–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evangelista V, Pamuklar Z, Piccoli A, Manarini S, Dell'elba G, Pecce R, Martelli N, Federico L, Rojas M, Berton G, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood 2007;109:2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuebler WM. Selectins revisited: the emerging role of platelets in inflammatory lung disease. J Clin Invest 2006;116:3106–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lindemann SW, Weyrich AS, Zimmerman GA. Signaling to translational control pathways: diversity in gene regulation in inflammatory and vascular cells. Trends Cardiovasc Med 2005;15:9–17. [DOI] [PubMed] [Google Scholar]
- 106.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest 1996;97:1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dixon DA, Tolley ND, Bemis-Standoli K, Martinez ML, Weyrich AS, Morrow JD, Prescott SM, Zimmerman GA. Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Invest 2006;116:2727–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tvedten HW, Till GO, Ward PA. Mediators of lung injury in mice following systemic activation of complement. Am J Pathol 1985;119:92–100. [PMC free article] [PubMed] [Google Scholar]
- 109.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 2006;116:3211–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem 1997;272:28750–28756. [DOI] [PubMed] [Google Scholar]
- 111.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 1995;96:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tweardy DJ, Khoshnevis MR, Yu B, Mastrangelo MA, Hardison EG, Lopez JA. Essential role for platelets in organ injury and inflammation in resuscitated hemorrhagic shock. Shock 2006;26:386–390. [DOI] [PubMed] [Google Scholar]
- 113.Nieswandt B, Echtenacher B, Wachs FP, Schroder J, Gessner JE, Schmidt RE, Grau GE, Mannel DN. Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpIIb/IIIa antibody in mice. Blood 1999;94:684–693. [PubMed] [Google Scholar]
- 114.Snapper JR, Hinson JM Jr, Hutchison AA, Lefferts PL, Ogletree ML, Brigham KL. Effects of platelet depletion on the unanesthetized sheep's pulmonary response to endotoxemia. J Clin Invest 1984;74:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bredenberg CE, Taylor GA, Webb WR. The effect of thrombocytopenia on the pulmonary and systemic hemodynamics of canine endotoxin shock. Surgery 1980;87:59–68. [PubMed] [Google Scholar]
- 116.Downey GP, Dong Q, Kruger J, Dedhar S, Cherapanov V. Regulation of neutrophil activation in acute lung injury. Chest 1999; 116(1, Suppl)46S–54S. [PubMed] [Google Scholar]
- 117.Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med 2001;7:1123–1127. [DOI] [PubMed] [Google Scholar]
- 118.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003; 31(4, Suppl)S195–S199. [DOI] [PubMed] [Google Scholar]
- 119.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007;13:463–469. [DOI] [PubMed] [Google Scholar]
- 120.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 121.Urban C, Zychlinsky A. Netting bacteria in sepsis. Nat Med 2007;13:403–404. [DOI] [PubMed] [Google Scholar]
- 122.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 2006;16:401–407. [DOI] [PubMed] [Google Scholar]
- 123.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–2738. [DOI] [PubMed] [Google Scholar]
- 124.White KE, Ding Q, Moore BB, Peters-Golden M, Ware LB, Matthay MA, Olman MA. Prostaglandin E2 mediates IL-1beta-related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors. J Immunol 2008;180:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med 2006;203:1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Boer HC, Verseyden C, Ulfman LH, Zwaginga JJ, Bot I, Biessen EA, Rabelink TJ, van Zonneveld AJ. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler Thromb Vasc Biol 2006;26:1653–1659. [DOI] [PubMed] [Google Scholar]
- 127.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 2006;12:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stellos K, Gnerlich S, Kraemer B, Lindemann S, Gawaz M. Platelet interaction with progenitor cells: vascular regeneration or injury? Pharmacol Rep 2008;60:101–108. [PubMed] [Google Scholar]
- 129.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855–1863. [DOI] [PubMed] [Google Scholar]
- 130.Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest 2006;116:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]