Abstract
The activation of transcription factor NF-κB is controlled by two main pathways: the classical canonical (RelA/p65-p50)- and the alternative noncanonical (RelB/p52)–NF-κB pathways. RelB has been shown to play a protective role in RelA/p65-mediated proinflammatory cytokine release in immune–inflammatory lymphoid cells. Increased infiltration of macrophages and lymphoid cells occurs in lungs of patients with chronic obstructive pulmonary disease, leading to abnormal inflammation. We hypothesized that RelB, and its signaling pathway, is differentially regulated in macrophages and B cells and in lung cells, leading to differential regulation of proinflammatory cytokines in response to cigarette smoke (CS). CS exposure increased the levels of RelB and NF-κB–inducing kinase associated with recruitment of RelB on promoters of the IL-6 and macrophage inflammatory protein-2 genes in mouse lung. Treatment of macrophage cell line, MonoMac6, with CS extract showed activation of RelB. In contrast, RelB was degraded by a proteasome-dependent mechanism in B lymphocytes (human Ramos, mouse WEHI-231, and primary mouse spleen B cells), suggesting that RelB is differentially regulated in lung inflammatory and lymphoid cells in response to CS exposure. Transient transfection of dominant negative IκB-kinase-α and double mutants of NF-κB–inducing kinase partially attenuated the CS extract–mediated loss of RelB in B cells and normalized the increased RelB level in macrophages. Taken together, these data suggest that RelB is differentially regulated in response to CS exposure in macrophages, B cells, and in lung cells by IκB-kinase-α–dependent mechanism. Rapid degradation of RelB signals for RelA/p65 activation and loss of its protective ability to suppress the proinflammatory cytokine release in lymphoid B cells.
Keywords: chronic obstructive pulmonary disease, cigarette smoke, NF-κB, RelB, B cells
CLINICAL RELEVANCE
RelB is rapidly degraded in B cells, but not in macrophages and mouse lungs, in response to cigarette smoke exposure. RelB may be a potential target in controlling inflammation in lymphoid immune cells of patients with chronic obstructive pulmonary disease.
Cigarette smoke (CS) is a major etiologic factor in the pathogenesis of chronic obstructive pulmonary disease (COPD), which is characterized by a chronic inflammatory response in the lungs with a progressive and irreversible airflow limitation (1). We and others have shown that CS exposure resulted in lung inflammation with an increase in inflammatory cell influx that includes macrophages, neutrophils, CD8+ lymphocytes, and increased release of proinflammatory mediators (2–10). The numbers of neutrophils, macrophages, and lymphocytes have been shown to be increased in both airways and parenchyma of patients with COPD (11). Recently, it has been reported that the progression and severity of COPD is associated with increasing infiltration of airways by innate and adaptive inflammatory immune cells, such as polymorphonuclear leukocytes, macrophages, lymphocyte subtypes CD4+ and CD8+ T cells, and B lymphocytes, which are accumulated in absolute volume in the pool of inflammatory cells and form lymphoid follicles (12, 13). These lymphoid cells, such as CD4+ and CD8+ T cells and B cells, are involved in adaptive immune responses that are highly specific and exhibit a specific “memory” to previous insults. Furthermore, these cells are involved in airway obstruction of the small airways and are associated with a thickening of the airway wall in patients with COPD (12). Recently, Van der Strate and coworkers (13) have reported that B-cell follicles are detectable in lung sections of mice with airspace enlargement and in patients with emphysema, associated with increased release of proinflammatory mediators (IL-4, IL-6, keratinocyte chemoattractant [KC, IL-8], regulated on activation, normal T-cell expressed and secreted [RANTES], monocyte chemoattractant protein [MCP]-1, interferon-inducible protein [IP]-10, and IL-13), suggesting that proliferating B cells contribute to the inflammatory process in the aggregates of lymphoid follicles and/or development and perpetuation of emphysema. However, the presence of these B-cell follicles in CS-mediated, NF-κB–driven inflammatory process, specific antigen-driven reactions, or autoantibody production in the pathogenesis of COPD is not known.
The NF-κB family of transcription factors is essential for the control of immune and inflammatory responses as well as cell survival, proliferation and differentiation. The classical pathway requires IκB-kinase (IKK)-β activity, whereas the alternative pathway involves selective nuclear translocation of p52:RelB dimer upon NF-κB–inducing kinase (NIK)-mediated phosphorylation of IKK-α. We have recently shown that RelA/p65 subunit of NF-κB is activated in response to CS and oxidants, leading to increased release of inflammatory mediators that are involved in airway inflammation and pathogenesis of COPD (4, 14–19). The alternative NF-κB pathway activated by CD40 ligand, B-cell activating factor, IL-1β, and lymphotoxin-β is important in lymphoid organogenesis, B-lymphocyte differentiation, immune response, and antibody production (20–23). Furthermore, it has also been shown that RelB inhibits NF-κB–dependent proinflammatory mediator gene expression, and RelB is inducibly degraded upon activation of lymphoid cells (24, 25), whereas activation of RelB leads to proinflammatory cytokine release in nonlymphoid cells (21). Moreover, IKK-α plays a critical role in activation of the RelB/p52 pathway by processing p100 to form p52, which then associates predominantly with RelB in the cytoplasm (23, 26–28). The roles of RelB and its signaling pathway in response to environmental agents, particularly in response to CS in different immune–inflammatory and lymphoid cell types, are not known. We hypothesized that CS activates the alternative RelB/p52 pathway, leading to proinflammatory cytokine release in mouse lung, B-lymphocytes, and in human monocyte/macrophage (MonoMac6). Since IKK-α and NIK play critical roles in alternative NF-κB pathway, we studied the role of IKK-α and NIK in CS-mediated RelB activation in both lymphoid and nonlymphoid cells.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma Aldrich, Inc. (St. Louis, MO). Antibodies used include the following: β-actin (CP-01; Oncogene, San Diego, CA), NIK (A-12), RelB (C-19), NF-κBp52 (K-27), RelA/p65, and CD19 (SC-8417, SC-226, SC-298X, SC-372, and SC-8498; Santa Cruz Biotechnology Inc., Santa Cruz, CA), and IKK-α (05-536; Upstate, Charlottesville, VA).
Animals
Adult male C57BL/6J mice (8–10 wk old, 37 ± 1.5 g; Jackson Laboratory, Bar Harbor, ME) were housed in the Inhalation Core Facility of the University of Rochester. The Animal Research Committee of the University of Rochester approved all animal experimental procedures described here.
CS Exposure
Mice (6–8/group) were used for acute (3 d) CS exposure. The mice were placed in individual compartments of a wire cage, which was placed inside an aerated plastic box connected to the smoke source. The CS was generated from 2R4F research cigarettes (total particulate matter [TPM] concentration, 11.7 mg/cigarette; tar, 9.7 mg/cigarette; nicotine, 0.85 mg/cigarette; University of Kentucky, Lexington, KY). CS exposure was performed according to the Federal Trade Commission protocol (1 puff/min of 2-s duration and 35 ml volume) in an automatic Baumgartner-Jaeger CSM2082i CS machine (CH Technologies, Westwood, NJ). Mainstream CS was diluted with filtered air and directed into the exposure chamber. The smoke exposure (TPM per cubic meter of air, mg/m3) was monitored in real time with a MicroDust Pro-aerosol monitor (Casella CEL, Bedford, UK) and verified daily by gravimetric sampling. The smoke concentration was set at a nominal value of ∼ 300 mg/m3 TPM by adjusting the flow rate of the dilution air (4, 29–33). Sham control animals were exposed only to filtered air in the same manner for the same duration. Mice received two 1-hour exposures (1 h apart) per day for 3 days, and were killed 24 hours after the last exposure. The concentration of carbon monoxide in the CS-filled chamber was approximately 350 ppm. The dosimetry of carbon monoxide in CS was estimated by measuring blood carboxyhemoglobin levels. Mice tolerated CS without evidence of toxicity (carboxyhemoglobin levels ∼ 17%, and no body weight loss).
Tissue Harvest and Bronchoalveolar Lavage
Mice were injected with 100 mg/kg body weight of pentobarbiturate (Abbott laboratories, Abbott Park, IL) intraperitoneally, and then killed by exsanguination. The heart and lungs were removed en bloc, and the lungs were lavaged three times with 0.5 ml of 0.9% sodium chloride. The lavage fluid was centrifuged, and the cell-free supernatants were frozen at −80°C for luminex-based multiplex assay.
Analysis of Proinflammatory Mediators in Bronchoalveolar Lavage Fluid
Mouse bronchoalveolar lavage (BAL) fluid (150 μl) was analyzed for the cytokine levels using the sensitive mice Multi-Analyte Profile (version 1.6) screening by Luminex (Rules-Based Medicine, comarketed with Charles River Laboratories, Austin, TX). The assays permit simultaneous cytometric quantification of multiple chemokines/cytokines with minimal sample volume.
Immunohistochemistry
The expression and levels of RelB, RelA/p65, and CD19+ B cells (34) were measured in fixed mouse lung sections (4 μm thick) by immunohistochemical staining using specific antibodies (1:100 dilution), with the avidin–biotin–peroxidase complex method followed by hematoxylin counter staining. Preimmune serum was used as negative control. Appearance of dark brown color represents the presence of RelB, RelA/p65, and B cells in the lung sections.
B-Cell Isolation from Mouse Spleen
B lymphocytes were isolated from mouse spleen using a B-cell isolation kit (MACS, mouse B-cell isolation kit no. 130–090–862; Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. The spleens were removed from 6- to 8-week-old C57BL/6J mice, and splenic cells were dispersed by grinding between glass slides with MACS running buffer (0.5% FBS and 2 mM EDTA in PBS) to obtain a single-cell suspension. Cells were resuspended with MACS running buffer (40 μl) with a B-cell biotin antibody cocktail (10 μl; Miltenyi Biotech) per 1 × 107 cells, followed by removing erythrocyte lysis buffer (Sigma). After 15-minute incubation on ice, MACS antibiotin beads (20 μl/1 × 107 cells; Miltenyi Biotech) and MACS running buffer (30 μl/1 × 107 cells) were added to the cell suspension for magnetic labeling. Cell pellets were washed and then resuspended with MACS running buffer for sorting using an AutoMACS Separator (Miltenyi Biotech). Flow cytometry was used to determine the purity of enriched B cells (B220 versus CD3), which was > 98%.
Cell Culture
The MonoMac6 cell line, which was established from peripheral blood of a patient with monoblastic leukemia (35, 36), were grown in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 μg/ml penicillin, 100 U/ml streptomycin, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 1 μg/ml human holotransferrin, and 1 mM oxaloacetic acid. These cells do not require phorbol myristate acetate to differentiate into the macrophages, thus avoiding any stress to the cells. Human Burkitt B lymphoma cells (Ramos B cells), the line of which was established from the ascitic fluid of a 3-year-old boy with American-type Burkitt lymphoma (37), were grown in RPMI 1640 medium supplemented with 5 to 10% FBS, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, 10 mM HEPES, 100 μg/ml penicillin, 100 U/ml streptomycin and 50 μM 2-mercapthoethanol. Mouse immature B cells (WEHI-231) were grown in RPMI 1640 medium supplemented with 10% FBS, 100 μg/ml penicillin, 100 U/ml streptomycin, and 50 μM 2-mercapthoethanol (38). The cells were cultured at 37°C in a humidified atmosphere containing 7.5% CO2.
Preparation of Aqueous CS Extract
Research-grade cigarettes (1R3F) were obtained from the Kentucky Tobacco Research and Development Center at the University of Kentucky (Lexington, KY). Tar and nicotine contents of 1R3F were 15 mg/cigarette and 1.16 mg/cigarette, respectively. CS extract (CSE) (10%) was prepared by bubbling smoke from 1 cigarette into 10 ml of culture medium at a rate of 1 cigarette per 2 minutes, as described previously (39), with modifications (4, 15, 40, 41). The pH was adjusted to 7.4 in the CSE, which was sterile filtered through a 0.45-μm filter (25-mm Acrodisc; Pall, Ann Arbor, MI). The CSE preparation was standardized by monitoring the absorbance at 320 nm (optical density of 0.74 ± 0.05). The spectral variations observed between different CSE preparations at 320-nm wavelength were found to be within acceptable limits. CSE was freshly prepared for each experiment and diluted with culture medium containing 1% FBS immediately before use. Control medium was prepared by bubbling air through 10 ml of culture medium supplemented with 1% FBS, adjusting pH to 7.4, and sterile filtering as described for CSE preparation.
Treatments
MonoMac6, Ramos B, and WEHI-231 B cells were seeded at a density of less than 1 × 106 cells/well (total final volume = 2 ml), grown to ∼ 80–90% confluency in six-well plates containing RPMI 1640 medium with 10% FBS, washed in Ca2+- and Mg2+-free PBS, and then exposed to various treatments in media containing 1% serum. The cells were treated with CSE (0.5, 1.0, or 2.5%) for 1 hour at 37°C with 7.5% CO2. To investigate the involvement of proteolysis of RelB, Ramos B cells were pretreated with 25 μM calpain inhibitor I (ALLN, product no. 208750; Calbiochem, San Diego, CA) for 20 minutes. The pretreated cells were washed twice in PBS and then treated with CSE (0.5 and 1.0%) for 1 hour at 37°C with 7.5% CO2. Primary spleen B cells (7 × 107) were seeded in 100-mm culture dishes containing RPMI 1640 with 1% FBS, 100 μg/ml penicillin, and 100 U/ml streptomycin, and treated with CSE (0.1 and 0.5%) for 1 and 4 hours at 37°C with 7.5% CO2. All treatments were performed in duplicate. At the end of treatment, the cells were washed with cold, sterile Ca2+- and Mg2+-free PBS and lysed in RIPA buffer as whole lysate (Western blotting), and the lysates stored at −80°C.
Transfection
The plasmids for wild-type IKK-α, dominant negative IKK-α and NIK kinase mutant domain on lysine K429 and K430 (K429/A430) were obtained as previously described (2, 42). Transient transfection was performed with 1 μg of plasmids in the presence of Lipofectamine-2000 transfection reagent (product no. 11668–027; Invitrogen, Carlsbad, CA) in MonoMac6, Ramos B, and WEHI-231 cells. Transfection efficiency in the case of both the plasmids was greater than 80%. Following transfection, MonoMac6, Ramos, and WEHI-231 B cells were treated with CSE (0.5, 1.0, and/or 2.5%). Whole lysate was used in Western blotting analysis.
Cytoplasmic and Nuclear Protein Extraction
A total of 100 mg of lung tissue was mechanically homogenized in 0.5 ml buffer A (10 mM HEPES, pH 7.8, 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 M EDTA, 0.2 mM NaF, 0.2 mM Na orthovandate, 1% [vol/vol] NP-40, 0.4 mM PMSF, and 1 μg/ml leupeptin) on ice. The homogenate was centrifuged at 2,000 × g in a benchtop eppendorf centrifuge for 30 s at 4°C to remove cellular debris. The supernatant was then transferred to a 1.7 ml ice-cold microtube and further centrifuged for 30 s at 13,000 × g at 4°C. The supernatant was collected as a cytoplasmic extract. The pellet was resuspended in 200 μl of buffer C (50 mM HEPES, pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 M EDTA, 1 mM DTT, 10% [vol/vol] glycerol, 0.2 mM NaF, 0.2 mM Na orthovandate, and 0.6 mM PMSF) and placed on the rotor in the cold room for 30 minutes. After centrifugation at 13,000 × g in a micro-Eppendorf tube for 5 minutes, the supernatant was collected as the nuclear extract and kept frozen at −80°C for Western blotting.
Western Blotting
Lung tissue homogenate samples (cytoplasmic and nuclear proteins) were separated on 7.5–12% SDS-PAGE. MonoMac6, Ramos, WEHI-231, and primary mouse spleen B cells were harvested and lysed with 10% Igepal CA-630 lysis buffer supplemented with a protease inhibitor cocktail (leupeptin, aprotinin, pepstatin, and PMSF). Equal amounts of protein were subjected to electrophoresis on 7.5–12% PAGE gels, electroblotted onto nitrocellulose membranes (Amersham Bioscience, Piscataway, NJ), and then incubated overnight with primary antibodies at 4°C. The next day, membranes were washed and incubated for 1 hour at room temperature with the appropriate secondary antibody linked to horseradish peroxidase (Dako, Santa Barbara, CA). Bound complexes were detected with the use of the enhanced chemiluminescence method (Jackson Immunology Research, West Grove, PA).
Immunoprecipitation
A total of 100 μg of proteins in mouse lung tissue homogenate were immunoprecipitated with 1 μg of specific antibodies and 20 μl of protein A/G agarose beads (product no. SC-2003; Santa Cruz Biotechnology) in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25 mM EDTA, 5 mM NaF, 0.1% sodium deoxycholate, 1% Triton X-100, in PBS) overnight at 4°C. After immunoprecipitation, the precipitates were washed three times with 10 mM Tris, 1 mM EDTA, 150 mM NaCl, 1 mg/ml BSA, 1% Triton X-100, and protease inhibitor in PBS with spinning at 2,000 rpm for 1 minute at 4°C. The precipitants were resuspended in 50 μl of Laemmli sample buffer to a final concentration of 1× sample buffer, and heated at 95°C for 5 minutes. The collected supernatants (immunoprecipitants) were run on 7.5% SDS-PAGE.
Chromatin Immunoprecipitation
A total of 100 mg of lung tissue was homogenized in 1 mg/ml BSA with protease inhibitor cocktail in PBS, and cross-linked with 1% formaldehyde for 10 minutes, rinsed three times with PBS, and then 0.5 ml of 2.5 M glycine was added. After a brief centrifugation, cell pellets were resuspended in SDS-lysis buffer (50 mM Tris-HCl, 1% SDS, 5 mM EDTA, 5 mM Na-butyrate, protease inhibitors). Sonication of nuclear pellets containing chromatin was performed four times for 30 seconds and one time for 15 seconds, at a maximum speed, using Misonix-3000 Sonicator (Misonix Inc., Farmingdale, NY). Supernatants were collected and diluted (1:10) with buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl, pH 8.0, 5 mM NaButyrate, protease inhibitor), followed by preclearing the extract with 60 μl of protein A agarose/salmon sperm DNA (no. 16-157; Upstate) for 3 hours at 4°C (43). Immunoprecipitation was performed overnight at 4°C with 1 μg of specific antibodies, as mentioned above. After immunoprecipitation, 40 μl of protein A agarose/salmon sperm DNA was added and incubated for 2 hours and followed by brief centrifugation. Precipitates were washed sequentially with Paro buffer I (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl), Paro buffer II (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl), and Paro buffer III (0.25 M LiCl, 1% Igepal CA-630, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.1) for 5 minutes at 4°C. Precipitates were then washed again with Tris buffer twice for 5 minutes each. The antigen–antibody complexes were extracted twice with 50 μl elution buffer (0.6 μg/μl proteinase K, 1% SDS, 0.1 M NaHCO3). The eluted samples were heated at 65°C overnight to reverse formaldehyde crosslinking. The recovered DNA was purified with a QIAquick PCR purification kit (product no., 28106; Qiagen, Valencia, CA) (43). Samples of input DNA were also prepared in the same way as described above. PCR amplification was performed using a PTC-200 DNA engine (MJ Research, Waltham, MA) under the following conditions: 94°C for 180 seconds; 30–38 cycles at 94°C for 45 seconds, 60°C for 60 seconds, and 72°C for 60 seconds; and final elongation at 72°C for 10 minutes. PCR for the input reaction was performed using 100 ng of genomic DNA. Mouse primer sequences are described in Table 1, and PCR products were analyzed on a 1.5–2.0% agarose gel.
TABLE 1.
PRIMER SEQUENCES USED IN CHROMATIN IMMUNOPRECIPITATION ASSAY
| Gene | Primer | Sequence |
|---|---|---|
| MIP-2 | Sense | 5′-CAA CAG TGT ACT TAC GCA GAC G-3′ |
| Antisense | 5′-CTA GCT GCC TGC CTC ATT CTA C-3′ | |
| IL-6 | Sense | 5′-GAC ATG CTC AAG TGC TGA GTC AC-3′ |
| Antisense | 5′-AGA TTG CAC AAT GTG ACG TCG-3′ |
Definition of abbreviation: MIP = macrophage inflammatory protein.
Protein Assay
Protein levels were measured with a BCA kit (Pierce, Rockford, IL). Protein standards were obtained by diluting a stock solution of BSA. Linear regression was used to determine the actual protein concentration of the samples.
Statistical Analysis
Results are shown as means ± SEM. Statistical analysis of significance was calculated by one-way ANOVA followed by Fisher's protected least significant difference post hoc test for multigroup comparisons (StatView 5.0; SAS Institute, Cary, NC). Statistical significance is indicated in the figure legends.
RESULTS
CS Exposure Increased the Levels of RelB and RelA/p65 in Alveolar and Airway Epithelial Cells in Mouse Lung
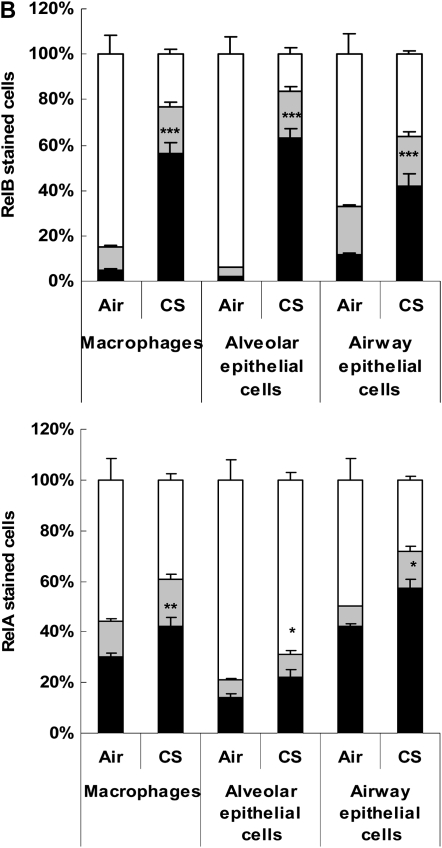
We studied the expression and localization of RelB and RelA/p65 in mouse lung sections by immunohistochemistry in mid-sagittal sections in response to air or CS exposure. RelB- and RelA/p65-positive cells with increased staining of RelB and RelA/p65 were detected in macrophages, type II alveolar, and airway epithelial cells in mouse lung tissue exposed to CS (Figures 1A and 1B). However, we were unable to detect B cells using the B-cell–specific surface marker CD19+ in lung sections of mice exposed to CS for acute (3-d) exposure (Figure 1A). Likewise, the expression of CD45R/B220, another marker of B cells, was also not detected in mouse lungs exposed to CS for 3 days. However, CD19- and CD45R/B220-positive cells were increased in mouse lung after 8 weeks of CS exposure compared with air exposure (data not shown). These observations suggest that CS exposure induced increased levels of RelB and RelA/p65 and increased numbers of B cells in mouse lungs.
Figure 1.
Cigarette smoke (CS) exposure increased the levels of RelB in alveolar macrophages and alveolar/airway epithelial cells, but not in CD19+ B cells. Mice were exposed to CS for 3 days, and were killed at 24 hours after the last CS exposure. (A) Representative photographs (400×) from immunostaining for serum (negative control), RelB, RelA, and CD19 in lung tissues from air- and CS-exposed mice. Appearance of dark brown color represents the presence of RelB and RelA, which were increased in mouse lung cells in response to CS exposure. Arrows indicate RelB- and RelA-positive macrophages, type II, and airway epithelial cells. However, the expression of CD19 (B cells) was not altered in lungs. Four slides of each sample of mouse lung tissue were used for immunohistochemistry. Alv, alveoli; E, epithelial cells; M, macrophages. (B) Immunostaining scores showing RelB and RelA per cell type in alveolar and airway regions of the lung. The assessment of immunostaining intensity was performed semiquantitatively in a blinded fashion. Solid bars, intense staining; shaded bars, moderate/weak staining; open bars, no staining. Results are means of five experiments ± SEM (n = 5). *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from respective air-exposed groups.
CS Exposure Increased the Levels of RelB and Its Interaction with NIK and p52 in Mouse Lung
We determined the levels of RelB and its interactions with NIK and p52 in lung tissue of mice exposed to CS for 3 days. The levels of RelB and NIK were significantly increased in both the nucleus and cytoplasm of cells in mouse lung tissue, whereas the level of p52 was increased only in the cytoplasm, but not in the nucleus in response to CS (Figures 2A and 2B). We next determined the interaction of RelB with NIK and p52 in mouse whole lung homogenates by immunoprecipitation with relevant antibodies. CS increased the level of RelB interaction with both NIK and p52 in mouse lungs (Figure 2B). These data showed that CS exposure increased the levels of both RelB and NIK in the nucleus, as well as the interactions of RelB with NIK and p52 in mouse lung.
Figure 2.
CS exposure increased the level of RelB and induced the interaction of RelB with NF-κB–inducing kinase (NIK) and p52 in mouse lung. Mice were exposed to CS for 3 days, and were killed at 24 hours after the last CS exposure. (A) The levels of RelB and NIK were significantly increased in both the nucleus and cytoplasm of cells in mouse lung tissue, whereas the level of p52 was increased only in the cytoplasm, but not in the nucleus, in response to CS. β-actin was used as a loading control from the experiments described previously (2). (B) The level of RelB, and its interaction with NIK and p52, in lungs was increased in response to CS (fold induction versus control). Nuc, nucleus; Cyt, cytoplasm. Data shown are mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from respective air-exposed mice (n = 4/group). Open bars, air; solid bars, CS.
CS Exposure Caused Recruitment of RelB on Proinflammatory Gene Promoters in Mouse Lung
It has been shown that increased levels of RelB are a prerequisite for the alternate pathway of NF-κB–dependent gene transcription in response to proinflammatory stimuli (20, 21, 23, 27, 44, 45). Therefore, we hypothesized that CS exposure would induce proinflammatory mediators by recruiting RelB on the promoters of proinflammatory genes. To test this hypothesis, chromatin immunoprecipitation (ChIP) assays were performed using antibodies against RelB in lungs of mice exposed to CS. As expected, the levels of RelB on the proinflammatory promoter sites of macrophage inflammatory protein (MIP)-2 and IL-6 were increased (Figures 3A and 3B). However, the regions that were noncoding for IL-6 and MIP-2 showed no change in gene expression, validating the specificity of the ChIP assays (S.-R. Yang and coworkers, unpublished data). These results demonstrate that RelB is recruited on the promoters of various proinflammatory genes, thereby exerting its effect on proinflammatory gene expression in mouse lung.
Figure 3.
CS exposure led to recruitment of RelB on promoters of IL-6 and MIP-2 genes in mouse lung. Mice were exposed to CS for 3 days and killed at 24 hours after the last CS exposure. (A) CS exposure caused recruitment of RelB on MIP-2 and IL-6 promoters. The nuclear extracts were immunoprecipitated with specific antibodies, and binding to the promoters of proinflammatory mediator genes was detected by PCR primers for IL-6 and MIP-2. Binding to the promoters was compared with PCR of the input DNA from the experiments described previously (2). IgG was used as a negative control. (B) The bands were measured by densitometry (fold induction versus control). Data shown are mean ± SEM. *P < 0.05, and ***P < 0.001, significantly different from respective air-exposed mice (n = 4/group).
CS Increased the Levels of NF-κB–Dependent Proinflammatory Mediators in Mouse Lung
We next determined whether or not CS induced the levels of RelB-dependent proinflammatory cytokines in mouse BAL fluid measured by Luminex-based multiplex assay. The proinflammatory mediators, including CD40, CD40 ligand (which are present on antigen-presenting cells, and are costimulatory molecules for proliferation and enhanced survival of T cells), eotaxin, and granulocyte chemotactic protein-2 (which are thought to be regulated by alternative NF-κB pathways) were significantly increased in BAL fluid in response to CS exposure (Figure 4). Previously, we have shown that the levels of MIP-2 and IL-6 were increased in BAL fluid 3 days after CS exposure (2). Taken together, these data suggest that CS induces the alternative pathway of NF-κB–dependent proinflammatory mediators in mouse lung.
Figure 4.
CS exposure increased the levels of RelB-dependent proinflammatory mediators in mouse lung. The levels of RelB-dependent proinflammatory cytokines were examined in bronchoalveolar lavage fluid of mice exposed to CS for 3 days (24 h after the last exposure) by Luminex-based multiplex assay. Data shown are mean ± SEM (n = 5–6/group). *P < 0.05, significant compared with air-exposed group.
CSE Increased the Levels of RelB, and IKK-α Is a Critical Regulator of RelB in MonoMac6
Macrophages play an important role in abnormal inflammatory responses observed in patients with COPD. Recently, we have shown that CS induces proinflammatory mediators by an NF-κB–dependent mechanism in macrophages (2, 4). We therefore determined whether RelB was also activated in response to CSE in MonoMac6 cells, leading to RelB-dependent proinflammatory cytokine release. Similar to the activation of RelA/p65 (2), we found that RelB was activated in response to CSE treatment associated with increased levels of its partner, p52 (Figures 5A and 5B). These data suggested that CS activates both the classical and the alternative NF-κB pathways in macrophages, leading to proinflammatory mediator release.
Figure 5.
CS extract (CSE) increased the levels of RelB and p52, and IκB-kinase (IKK)-α activated RelB in human monocyte/macrophages in response to CSE treatment. (A) MonoMac6 cells were transfected with dominant negative or wild-type IKK-α plasmids and treated with CSE (0.5, 1.0, and 2.5%) for 1 hour. Protein levels of RelB and p52 were then measured by Western blotting. CSE increased the levels of RelB and p52 rapidly in nontransfected cells, whereas the level of RelB was attenuated in cells transfected with dominant negative IKK-α plasmids. Transfection of wild-type IKK-α increased the levels of RelB and p52. β-actin was used as a loading control from the experiments described previously (2). (B) The bands were measured by densitometry (fold induction versus control). Data shown are mean ± SEM of four experiments. *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from respective controls; #P < 0.05, ##P < 0.01, and ###P < 0.001, significantly different from respective nontransfected groups.
It was recently shown that NIK activates the IKK-α homodimer in the IKK-s complex, which, in turn, results in activation of alternative NF-κB pathway, characterized by nuclear translocation of RelB (22, 44). Therefore, we determined whether NIK and IKK-α were involved in regulation of RelB in response to CSE in MonoMac6 cells. Macrophages were transfected with a dominant negative IKK-α or a double mutant of NIK (K429/A430), and treated with CSE (0.5, 1.0, and 2.5%) for 1 hour. The levels of RelB and p52 were increased in response to CSE in nontransfected cells, whereas the level of RelB was attenuated in both dominant negative IKK-α–transfected and double mutant of NIK (K429/A430, S.-R. Yang and coworkers, unpublished data)-transfected MonoMac6 cells. Transfection of wild-type IKK-α increased the levels of RelB and p52, suggesting that IKK-α and NIK are required for CS-mediated activation of alternative NF-κB pathway in macrophages (Figure 5A and 5B).
CSE Rapidly Degraded RelB in Ramos (Human), WEHI-231 (Mouse), and Spleen B Cells (Mouse), and the Loss of IKK-α Partially Restored RelB
Immune–inflammatory lymphoid cells appear to be important in the pathogenesis of COPD (12, 13), and RelB controls the alternate NF-κB pathways in these cells by forming a transcriptionally inactive complex with RelA/p65 (20, 21, 24, 25, 46). Hence, we determined the levels of RelB in response to CSE in B cells (human Ramos B-cells, mouse WEHI-231 B cells, and primary spleen B cells). In contrast to RelA/p65 activation in macrophages, we found that RelB was rapidly degraded in response to CSE treatments in B cells (Figures 6–8). Furthermore, the levels of p100 and p52 were also decreased in primary splenic B cells in response to CSE treatment, suggesting that CS exposure down-regulates the alternative NF-κB pathway in B cells (Figure 8).
Figure 6.
CSE rapidly degraded RelB, and loss of IKK-α partially restored RelB in human B cells. Ramos B cells were transfected with a dominant negative IKK-α plasmid and treated with CSE (0.5 and 1.0%) for 1 hour. Protein levels of RelB, IKK-α, NIK, and RelA/p65 were measured by Western blotting. The level of RelB was significantly decreased in response to CSE, whereas the levels of IKK-α, NIK, and RelA/p65 were significantly increased. However, the level of RelB was partially restored in cells transfected with dominant negative IKK-α plasmid. Furthermore, the level of RelB was partially attenuated, whereas the levels of NIK and RelA/p65 were normalized in cells transfected with wild-type IKK-α plasmid. (B) The bands were measured by densitometry (fold induction versus control). Data shown are mean ± SEM of four experiments. *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from respective controls; #P < 0.05, ##P < 0.01, and ###P < 0.001, significantly different from respective nontransfected groups.
Figure 8.
CSE down-regulates the alternative NF-κB pathway in mouse spleen B cells. Primary mouse spleen B cells were isolated using a B cell isolation kit and treated with CSE (0.1 and 0.5%) for 1 or 4 hours. Protein levels of RelB, p100, and p52 were measured by Western blotting. (A) CSE (0.1 and 0.5%) treatment significantly reduced the levels of RelB, p100, and p52 in primary spleen B cells at both 1- and 4-hour time points. β-actin was used as a loading control. (B) The bands were measured by densitometry (fold induction versus control). Data shown are mean ± SEM of three experiments. **P < 0.01 and ***P < 0.001, significantly different from respective controls.
Figure 7.
CSE rapidly degraded RelB, and loss of IKK-α and NIK restored RelB in mouse B cells. Mouse immature WEHI-231 B cells were transfected with dominant negative IKK-α and double mutant of NIK (K429/A430) plasmids and treated with CSE (0.5 and 1.0%) for 1 hour. Protein levels of NIK, IKK-α, RelB, and RelA/p65 were measured by Western blotting. CSE reduced the levels of RelB, and increased the levels of NIK and IKK-α in nontransfected cells, whereas the CSE-mediated reduction in level of RelB was partially attenuated when the cells were transfected with dominant negative IKK-α and completely restored in double mutant of NIK (K429/A430) plasmid transfected cells. The level of RelA/p65 was increased in nontransfected cells in response to CSE. However, the levels of RelA/p65 and NIK were attenuated in cells transfected with dominant negative IKK-α and double mutant of NIK (K429/A430) plasmids. β-actin was used as a loading control. (B) The bands were measured by densitometry (fold induction versus control). Data shown are mean ± SEM of four experiments. *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from respective controls; #P < 0.05, ##P < 0.01, and ###P < 0.001 significantly different from respective nontransfected groups.
We next determined whether CS-mediated degradation of RelB was associated with down-modulation of its signaling by NIK and IKK-α in B cells. Surprisingly, we found that CSE caused activation of both NIK and IKK-α, and that transient transfection with a dominant-negative IKK-α partially restored CSE-mediated loss of RelB, whereas transfection of double mutant of NIK (K429/A430) completely restored CSE-mediated loss of RelB in B cells (Figures 6 and 7). However, the levels of NIK and RelA/p65 were not changed in response to CSE in cells transfected with a dominant negative IKK-α plasmid compared with nontransfected cells. Furthermore, the levels of NIK and RelA/p65 were returned to basal levels in cells transfected with wild-type IKK-α. These data suggest that IKK-α activation may not be critical for CSE-mediated loss of RelB in B cells.
The Proteasome Inhibitor, Acetyl-L-Leucyl-L-Leucyl-Norleucinal, Prevented the Degradation of RelB in Response to CSE in B Cells
We hypothesized that CSE-mediated degradation of RelB in B cells is associated with proteasomal degradation. To determine the involvement of proteolysis of RelB, Ramos B cells were pretreated with a proteasome inhibitor (ALLN) and exposed to CSE and levels of RelB were determined. ALLN treatment prevented the degradation of RelB (Figures 9A and 9B). However, ALLN did not affect the levels of RelA/p65 (S.-R. Yang and coworkers, unpublished data). These data indicate that, in B cells, RelB is regulated by a proteasome-dependent degradation.
Figure 9.
CSE decreased the level of RelB via proteasome-mediated degradation in B cells. (A) CSE dose-dependently decreased the levels of RelB in B cells, and the proteasome inhibitor, ALLN, prevented the degradation of RelB by a proteasome-dependent mechanism. The cells were pretreated with the proteasome inhibitor, ALLN (25 μM), for 20 minutes before exposure to CSE (0.5 and 1.0%) for 1 hour. The level of RelB in whole cell lysates was then measured by immunoblotting. (B) The bands were measured by densitometry (fold induction versus control). Data shown are mean ± SEM of four experiments. ***P < 0.001, significantly different from respective controls.
DISCUSSION
The absolute volume of infiltrated inflammatory immune cells in lymphoid follicles in the small airways is associated with the progression of COPD (12). This suggests that recruitment of lymphoid cells (T and B lymphocytes) in the lung may occur in response to chronic CS exposure. This concept is supported by a recent study showing that B cells are increased in the lungs of patients with emphysema and in mouse lungs chronically exposed to CS (13). These observations were corroborated by our findings showing increased CD19- and CD45R/B220-positive cells (B cells) in mouse lungs exposed to CS for 8 weeks. However, 3 days of CS exposure did not show increases in the number of B cells. These studies suggest that chronic CS exposure recruits B cells into the lung. However, the functional role of B cells in the pathogenesis of COPD is not known. Here, we studied the alternative NF-κB signaling events that control the activation of RelB in B lymphocytes and macrophages in vitro and in mouse lung in vivo in response to CS exposure. We show that CS differentially regulates the level of RelB in lung structural cells, macrophages, and B lymphocytes, suggesting opposing effects of CS on RelB activation in a cell-specific manner. It is known that RelB activation is tissue- and cell-specific, especially in lymphoid organs and lymphoid cells, such as B and T cells and fibroblasts (20–22, 24, 25). In these cells, RelB acts as an inhibitor of transcription of proinflammatory genes, whereas it functions as proinflammatory in nonlymphoid cells (47). Here, we have expanded on these findings to study the expression of RelB in airway and alveolar epithelial cells and alveolar macrophages in response to CS. Immunohistochemical staining of mouse lung tissue sections demonstrated the localization of RelB in airway and alveolar epithelial cells, as well as in alveolar macrophages in response to CS exposure. Similarly, our data show increased levels of RelB in the lungs of mice exposed to CS. These new data support recent studies showing that TNF-α stimulation resulted in increased RelB levels in both the cytoplasm and the nucleus of mouse intestinal cells and macrophages, as well as in lymphoid cells (48, 49). Furthermore, TNF-α, IL-1β, and CD40L were induced in response to CS exposure in mouse lungs (2). Hence, CSE-induced TNF-α, IL-1β, and CD40L release may activate RelB pathways in mouse lung.
RelB-containing complexes can act as both activators and repressors of NF-κB–dependent gene transcription (25). For instance, the recruitment of RelB to the IL-12p40 promoter correlates with transcriptional down-regulation, whereas RelB up-regulates gene expression of certain proinflammatory mediators, such as CD40, CD40 ligand, eotaxin, granulocyte chemotactic protein-2, ELC/CCL19 (EBl1 ligand chemokine), macrophage-derived chemokine, RANTES, MIP-1α, MIP-1β, MIP-2, IP-10, MCP-1, KC/CINC (IL-8), IL-13, IL-1β, TNF-α, and IL-4 genes (13, 21, 50, 51). We have previously shown that CSE causes activation of certain proinflammatory cytokines in macrophages (MonoMac6 cells) and airway epithelial cells (2, 4, 41). Here, we show that RelB was recruited onto the promoters of certain proinflammatory cytokine genes in mouse lung tissues by CS, suggesting that these cytokines were up-regulated due to RelB and RelA/p65 activation in macrophages and epithelial cells. We observed that RelB was also activated in MonoMac6 cells exposed to CSE. This was associated with increased activation of p52, which forms active RelB:p52 complex. CS increased the level of RelB associated with its interaction with p52 and NIK in mouse lung, suggesting that this complex was active for gene transcription, which was confirmed by the ChIP assay.
The main inhibitor of RelB is p100, and generation of p52/RelB results from proteolytic cleavage of a unique pool of p100/RelB (44). The p100 functions as IκBα, inhibiting the RelB-mediated gene transcription. Furthermore, p100 is directly phosphorylated by NIK-IKK-α, and causes its processing into p52 in the cytoplasm (27, 44). RelB:p100 complex is inhibitory, whereas RelB or RelB:p52 can induce certain proinflammatory genes. Our observation of increased levels of p52 in the cytoplasm implies that p100 is cleaved by IKK-α signaling in mouse lungs in response to CS. However, the level of p52 in the nucleus was not detected, suggesting that RelB directly binds on the promoters of certain proinflammatory genes, as shown by the ChIP assay.
It is interesting to note that RelB is differentially regulated by CS in mouse lung tissue, macrophages, and B lymphocytes. Surprisingly, RelB was rapidly degraded in B cells in response to CSE exposure. A question can be posed regarding the signaling mechanism whereby RelB is differentially regulated in different cells, and what the significance is for these opposing effects. Previously, it has been shown that NIK and IKK-α act as activators of RelB:p52–NF-κB–controlled gene transcription and lymphoid cell proliferation (52, 53). Furthermore, it is known that IKK-α regulates the late differentiation of B cells by intrinsic NIK-IKK-α signaling (22). Our new data show that RelB is associated with NIK in CS-exposed mouse lung tissue. We further determined whether or not NIK and IKK-α were involved in the regulation of RelB in response to CSE in MonoMac6 cells. CSE-mediated increased levels of RelB were reduced when the cells were transfected with a dominant negative IKK-α or a double mutant of NIK (K429/A430). Transfection of wild-type IKK-α increased RelB levels, suggesting that NIK–IKK-α signaling was required for CS-mediated activation of RelB in macrophages. We then used a similar approach of gain and loss of NIK and IKK-α, and determined whether or not CS-mediated degradation of RelB was associated with down-modulation of its signaling by NIK and IKK-α in B cells. Surprisingly, we found that CSE caused activation of both NIK and IKK-α, and transient transfection with a dominant negative IKK-α or a double mutant of NIK (K429/A430) partially restored CSE-mediated loss of RelB in B cells. These data suggest that, apart from NIK-IKK-α activation, there is another signaling mechanism for CSE-mediated regulation of RelB in B cells.
In contrast to lung cells and macrophages, the level of RelB was decreased in B cells, suggesting that its degradation was regulated by a proteasome-dependent mechanism. It is known that RelB is degraded by rapid phosphorylation at amino acids Thr84 and Ser52, followed by cleaving N-terminal amino acids and complete degradation in the proteasome (24). Our data show that the proteasome inhibitor, ALLN, prevented the degradation of RelB in response to CSE in B cells. These results agree with the concept that RelB is an essential regulator required for suppression of NF-κB function and modulation of chemokine expression in activated B and T cells and fibroblasts (21). This contention is supported by the observations that the disruption of the RelB locus results in impaired cellular immunity and severe pathology associated with dysfunction of the hematopoietic system and inflammatory response in lungs of RelB−/− mice (54). Furthermore, Xia and coworkers (21) have also demonstrated that RelB is an important regulator of chemokine expression in mouse fibroblast and lymphoid cells, thereby playing a key role in the resolution of acute inflammation by inhibiting RelA/p65.
RelB is known to dampen RelA/p65 activity (20, 25). RelB forms transcriptonally inactive complexes with RelA/p65, so that RelA/p65 is unable to bind to κB sites in fibroblasts (25). Moreover, serine-276 domain of RelA/p65 seems to be a critical phosphorylation site for TNF-α–induced RelA/RelB complex formation. It is possible that down-regulated alternative NF-κB pathway (RelB degradation and decreased levels of p100 and p52) would lead to RelA/p65 activation, as seen in lymphoid cells in response to CSE. Overexpression of RelB suppresses LPS-induced NF-κB activity and proinflammatory mediator release in fibroblasts (20). It is also known that RelB modulate IκB-α stability and suppresses the production of certain cytokines in fibroblasts (20). RelB exerts its transcriptional suppressor function through the stabilization of IκB-α protein. Activation of RelA/p65, along with recruitment of IKK-α on proinflammatory gene promoters, would lead to acetylation of histone proteins in response to CS (2). Hence, our data imply that RelB degradation would lead to chromatin remodeling and modification of RelA/p65, which would influence excessive expression of proinflammatory cytokines in response to CS. RelA/p65 is translocated into the nucleus, leading to NF-κB activation in response to stimuli. However, the level of RelA/p65 was increased in whole lysates of B cells in response to CS exposure, which is consistent with our previous studies (4, 14). The mechanism underlying this finding is not known, but it may be associated with a positive autocrine regulation by RelA/p65 in response to CS. Although RelB was differentially regulated in mouse lungs, macrophages, and B cells by CS, the direct role of RelB in CS-mediated lung inflammatory response is not clear. The use of RelB-deficient mice and the cells deficient in RelB may elucidate the role of RelB in CS-mediated lung inflammatory–immune and injurious responses.
The degradation of RelB in response to CSE in B cells will have consequences on altering acquired immunity, as tobacco smoke–reactive agents can react with components of extracellular matrix proteins to form new, highly immunogenic protein adducts, such as aldehyde-modified proteins. Immune complex formation may occur, eliciting an inflammatory response, altering antibody production (perhaps by inducing autoantibody production), and subsequently harbor bacteria and viruses or vice-versa in lymphoid follicles in lungs of patients with COPD. In this way, B cells may start producing autoantibodies in response to CS, which appears to occur in patients with COPD (55, 56). The proinflammatory and antiinflammatory (RelA versus RelB) pathways are differentially modified in B cells in response to CS, and this alteration may be key in signaling autoantibody production in COPD.
In summary, CS regulates RelB differentially in a cell-specific manner, and NIK-IKK-α is involved in RelB regulation. In structural cells of the lungs and macrophages, RelB was increased due to the activation of NIK-IKK-α, and RelB was recruited onto proinflammatory gene promoters. In contrast, RelB was degraded rapidly by proteolysis in B lymphocytes, independent of NIK-IKK-α activation in response to CSE. Our findings not only provide novel concepts of cell type–specific differential regulation of RelB in response to CS, but also a new understanding that the alternative NF-κB pathway might be considered as a critical target for COPD therapies, particularly in lymphoid cells.
Acknowledgments
The authors thank Dr. Aruna Kode, Samuel Caito, David Adenuga, and Ryan Henry for their technical assistance. They also thank Dr. Paul Kirkham (Imperial College, UK) for useful discussion on B cell immunology in chronic obstructive pulmonary disease.
This work was supported by National Institutes of Health grants R01-HL085613 (I.R.), HL088325 (P.J.S.), and DE011390 (R.P.P.), and National Institute of Environmental Health Sciences Center grant ES-01247.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0207OC on August 7, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Burchfiel CM, Marcus EB, Curb JD, Maclean CJ, Vollmer WM, Johnson LR, Fong KO, Rodriguez BL, Masaki KH, Buist AS. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med 1995;151:1778–1785. [DOI] [PubMed] [Google Scholar]
- 2.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edrisinghe I, Caito S, Adenuga D, Henry R, Fromm G, et al. IKKα causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 2008;38:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- 4.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 2006;291:L46–L57. [DOI] [PubMed] [Google Scholar]
- 5.Szulakowski P, Crowther AJ, Jimenez LA, Donaldson K, Mayer R, Leonard TB, MacNee W, Drost EM. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:41–50. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–1976. [DOI] [PubMed] [Google Scholar]
- 7.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 2004;31:633–642. [DOI] [PubMed] [Google Scholar]
- 8.Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, et al. Increased expression of nuclear factor-κB in bronchial biopsies from smokers and patients with COPD. Eur Respir J 2002;20:556–563. [DOI] [PubMed] [Google Scholar]
- 9.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 1999;159:473–479. [DOI] [PubMed] [Google Scholar]
- 10.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996;153:530–534. [DOI] [PubMed] [Google Scholar]
- 11.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:726–733. [DOI] [PubMed] [Google Scholar]
- 12.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 13.van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, Hylkema MN, van den Berg A, Timens W, Kerstjens HA. Cigarette smoke–induced emphysema: a role for the B cell? Am J Respir Crit Care Med 2006;173:751–758. [DOI] [PubMed] [Google Scholar]
- 14.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke–induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 2007;292:L567–L576. [DOI] [PubMed] [Google Scholar]
- 16.Rahman I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem Pharmacol 2002;64:935–942. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–1071. [DOI] [PubMed] [Google Scholar]
- 18.Murphy TF. The role of bacteria in airway inflammation in exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis 2006;19:225–230. [DOI] [PubMed] [Google Scholar]
- 19.Yagi O, Aoshiba K, Nagai A. Activation of nuclear factor-κB in airway epithelial cells in patients with chronic obstructive pulmonary disease. Respiration 2006;73:610–616. [DOI] [PubMed] [Google Scholar]
- 20.Xia Y, Chen S, Wang Y, Mackman N, Ku G, Lo D, Feng L. RelB modulation of IκBα stability as a mechanism of transcription suppression of interleukin-1α (IL-1α), IL-1β, and tumor necrosis factor α in fibroblasts. Mol Cell Biol 1999;19:7688–7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- 22.Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKα-dependent signals. Proc Natl Acad Sci USA 2007;104:6359–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beinke S, Ley SC. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem J 2004;382:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marienfeld R, Berberich-Siebelt F, Berberich I, Denk A, Serfling E, Neumann M. Signal-specific and phosphorylation-dependent RelB degradation: a potential mechanism of NF-κB control. Oncogene 2001;20:8142–8147. [DOI] [PubMed] [Google Scholar]
- 25.Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M. RelB forms transcriptionally inactive complexes with RelA/p65. J Biol Chem 2003;278:19852–19860. [DOI] [PubMed] [Google Scholar]
- 26.Solan NJ, Miyoshi H, Carmona EM, Bren GD, Paya CV. RelB cellular regulation and transcriptional activity are regulated by p100. J Biol Chem 2002;277:1405–1418. [DOI] [PubMed] [Google Scholar]
- 27.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J 2004;23:4202–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusco AJ, Savinova OV, Talwar R, Kearns JD, Hoffmann A, Ghosh G. Stabilization of RelB requires multidomain interactions with p100/p52. J Biol Chem 2008;283:12324–12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D'Armiento JM. Superoxide dismutase expression attenuates cigarette smoke– or elastase-generated emphysema in mice. Am J Respir Crit Care Med 2006;173:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke–induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 2005;289:L322–L328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor–deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-κB component RelB. Am J Pathol 2007;170:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke–induced lung inflammation and emphysema in mice. Am J Pathol 2008;172:1222–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke–mediated inflammatory and oxidative responses are strain dependent in mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L1174–L1186. [DOI] [PubMed] [Google Scholar]
- 34.Zhou LJ, Ord DC, Omori SA, Tedder TF. Structure of the genes encoding the CD19 antigen of human and mouse B lymphocytes. Immunogenetics 1992;35:102–111. [DOI] [PubMed] [Google Scholar]
- 35.Moesby L, Jensen S, Hansen EW, Christensen JD. A comparative study of Mono Mac 6 cells, isolated mononuclear cells and Limulus amoebocyte lysate assay in pyrogen testing. Int J Pharm 1999;191:141–149. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler-Heitbrock HW, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer 1988;41:456–461. [DOI] [PubMed] [Google Scholar]
- 37.Klein G, Giovanella B, Westman A, Stehlin JS, Mumford D. An EBV-genome–negative cell line established from an American Burkitt lymphoma; receptor characteristics: EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology 1975;5:319–334. [DOI] [PubMed] [Google Scholar]
- 38.McCabe MJ Jr, Laiosa MD, Li L, Menard SL, Mattingly RR, Rosenspire AJ. Low and nontoxic inorganic mercury burdens attenuate BCR-mediated signal transduction. Toxicol Sci 2007;99:512–521. [DOI] [PubMed] [Google Scholar]
- 39.Carp H, Janoff A. Possible mechanisms of emphysema in smokers: in vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 1978;118:617–621. [DOI] [PubMed] [Google Scholar]
- 40.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18:1897–1899. [DOI] [PubMed] [Google Scholar]
- 41.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 2006;7:132–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kweon SM, Wang B, Rixter D, Lim JH, Koga T, Ishinaga H, Chen LF, Jono H, Xu H, Li JD. Synergistic activation of NF-κB by nontypeable H. influenzae and S. pneumoniae is mediated by CK2, IKKβ-IκBα, and p38 MAPK. Biochem Biophys Res Commun 2006;351:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulger M, Schubeler D, Bender MA, Hamilton J, Farrell CM, Hardison RC, Groudine M. A complex chromatin landscape revealed by patterns of nuclease sensitivity and histone modification within the mouse β-globin locus. Mol Cell Biol 2003;23:5234–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dejardin E. The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol 2006;72:1161–1179. [DOI] [PubMed] [Google Scholar]
- 45.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-κB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev 2006;17:281–293. [DOI] [PubMed] [Google Scholar]
- 46.Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE. Induction of RelB participates in endotoxin tolerance. J Immunol 2006;177:4080–4085. [DOI] [PubMed] [Google Scholar]
- 47.Lernbecher T, Kistler B, Wirth T. Two distinct mechanisms contribute to the constitutive activation of RelB in lymphoid cells. EMBO J 1994;13:4060–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J 2003;22:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bren GD, Solan NJ, Miyoshi H, Pennington KN, Pobst LJ, Paya CV. Transcription of the RelB gene is regulated by NF-κB. Oncogene 2001;20:7722–7733. [DOI] [PubMed] [Google Scholar]
- 50.Saccani S, Pantano S, Natoli G. Modulation of NF-κB activity by exchange of dimers. Mol Cell 2003;11:1563–1574. [DOI] [PubMed] [Google Scholar]
- 51.Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor–mediated induction of chemokines. Biochem Biophys Res Commun 2007;363:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider G, Saur D, Siveke JT, Fritsch R, Greten FR, Schmid RM. IKKα controls p52/RelB at the skp2 gene promoter to regulate G1- to S-phase progression. EMBO J 2006;25:3801–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park GY, Wang X, Hu N, Pedchenko TV, Blackwell TS, Christman JW. NIK is involved in nucleosomal regulation by enhancing histone H3 phosphorylation by IKKα. J Biol Chem 2006;281:18684–18690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κ B/Rel family. Cell 1995;80:331–340. [DOI] [PubMed] [Google Scholar]
- 55.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 2007;13:567–569. [DOI] [PubMed] [Google Scholar]
- 56.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, Zhang Y, Sciurba FC, Duncan SR. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]