Abstract
Corticosteroids are the most common therapeutic approach for control of tissue inflammation. Combination IL-2/IL-4 is known to induce T-cell steroid resistance. This can be reversed with IFN-γ; however, the mechanism by which this occurs is unknown. In the current study, we found that treatment of peripheral blood mononuclear cells with combination IL-2/IL-4 for 48 hours, but not with IL-2 or IL-4 alone, abrogated dexamethasone (DEX)-induced glucocorticoid receptor (GCR)-α nuclear translocation in both CD4+ and CD8+ T cells. The presence of IL-4 significantly down-regulated IFN-γ production by IL-2–stimulated cells. Importantly, addition of IFN-γ to the IL-2/IL-4 combination restored GCRα nuclear translocation in response to DEX. Furthermore, DEX-induced mitogen-activated protein kinase (MAPK) phosphatase-1 induction, used as a readout for corticosteroid-induced transactivation, was significantly greater (P < 0.05) in media and IL-2/IL-4/IFN-γ–treated conditions compared with IL-2/IL-4–treated cells. The combination of IL-2/IL-4 induced p38 MAPK activation in CD3+ cells (30.5 ± 5.7% cells expressed phospho-p38 MAPK versus no phospho-p38 MAPK expression after media treatment). The presence of the p38 MAPK inhibitor, SB203580, or IFN-γ inhibited p38 MAPK phosphorylation and enhanced GCRα nuclear translocation in response to DEX. These data indicate that combination IL-2/IL-4 inhibits GCRα nuclear translocation in human T cells, and this effect is reversed by IFN-γ via inhibition of p38 MAPK activation.
Keywords: T cells, cytokines, glucocorticoid receptor, steroid resistance, p38 MAPK
Corticosteroids are the most commonly used antiinflammatory treatment of allergic and autoimmune disorders (1, 2). Unfortunately, some patients fail to respond to corticosteroid therapy (3). Corticosteroid resistance, associated with persistent tissue inflammation, has been well documented in subsets of systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, and steroid-resistant (SR) asthma (1, 4). Interestingly, these patients have persistent activation of their T cells, but they develop severe corticosteroid-induced toxicity of their nonimmune tissues (e.g., osteoporosis, cataracts). Because there is only a single glucocorticoid receptor (GCR) gene, it suggests that steroid resistance is acquired in most patients (4, 5). T cells of SR patients with asthma, as compared with steroid-sensitive (SS) patients with asthma, have also been found to maintain an activated phenotype, despite high-dose corticosteroid therapy, and have significantly higher levels of IL-2 and IL-4 gene expression in their airways, suggesting that local cytokine secretion could be inducing steroid resistance (6). Indeed, the Kam and colleagues (7) found that combination IL-2/IL-4 induced steroid resistance in normal human peripheral blood mononuclear cells (PBMCs). This resulted in a significant reduction of T-cell inhibition by steroids. The mechanism, however, by which combination IL-2/IL-4 induces SR T cells is not understood.
On a cellular level, corticosteroids interact with a GCR, which acts as a ligand-dependent transcription factor. Its transcriptional activation, DNA binding, and ligand binding are localized to specific domains (1, 2). The GCR is localized in the cytoplasm of cells where it forms a heterocomplex with chaperone and stabilizing proteins. Upon hormone binding, the GCR undergoes conformational changes that allow the hormone-bound receptor to translocate to the nucleus and bind to glucocorticoid response elements (GREs) in regulatory regions of the target genes (1). In the current study, we examined whether IL-2/IL-4 alters GCRα nuclear translocation in response to steroids in PBMC and defined the conditions that restore steroid sensitivity.
MATERIALS AND METHODS
Subjects
This study was approved by the Institutional Review Board at National Jewish Health (Denver, CO); 16 normal, healthy adult donors with no history of atopic or respiratory diseases were enrolled.
PBMC Purification and Culture
Human PBMCs were isolated by Ficoll–Hypaque density gradient centrifugation of heparinized venous blood, resuspended in RPMI 1,640 (Mediatech, Inc., Herndon, VA) containing 10% steroid-free, charcoal-filtered, heat-inactivated FCS, 40 μM L-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 20 mM HEPES (Mediatech, Inc.) and cultured with 50 U/ml of IL-2/IL-4 in the presence and absence of 100 U/ml of IFN-γ (all cytokines from R&D Systems, Minneapolis, MN) or 1 μM SB203580 (specific p38 mitogen-activated protein kinase [MAPK] inhibitor) (Calbiochem, San Diego, CA), or remained cultured in media alone for 48 hours. Cell viability was assessed by the trypan blue dye exclusion test. There was a slight decrease (10–15%) in cell viability when PBMCs were cultured with IL-2/IL-4 plus IFN-γ or p38 MAPK inhibitor as compared with all other culture conditions. However, this did not alter relative frequencies of CD4 and CD8 lymphocytes in culture.
GGRα Nuclear Translocation
GCRα intracellular shuttling in response to 10−6 M dexamethasone (DEX) (Sigma Chemical Co., St. Louis, MO) treatment was analyzed by immunofluorescent staining. In these experiments, after 48 hours of culture with or without cytokines (see above), lymphocytes were seeded at 1 × 106 cells/ml on poly-D-lysine–coated cover slips. Cells were then treated with 10−6 M DEX or cultured in media alone for 1 hour, fixed in 4% paraformaldehyde in PBS for 10 minutes room temperature (RT), permeabilized for 15 minutes at RT in permeabilization solution (PBS containing 0.1% [vol/vol] Tween 20, 0.1% [wt/vol] bovine serum albumin [Sigma], and 0.01% [wt/vol] saponin [Sigma]), blocked with a commercial blocking solution (Superblock; Scytek, Logan, UT) for 15 minutes at RT. The cells were then incubated with an affinity-purified polyclonal antibody to GCRα (Affinity Bioreagents, Golden, CO) diluted in permeabilization solution (1:250) overnight at 4°C, washed, and then incubated with donkey anti-rabbit IgG, F(ab′)2-cy3–conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) (1:200). The nucleus was then counterstained with 300 nm 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma) for 1 hour at RT, washed, and mounted on slides. Purified nonimmune rabbit IgG (Southern Biotechnology Associates, Inc., Birmingham, AL) was used as an isotype control. The slides were analyzed by fluorescent microscopy (Leica, Wetzlar, Germany) with Slidebook imaging analysis software (Intelligent Imaging Innovations, Denver, CO), and the data are presented as GCR (cy3) nuclear:cytoplasm ratio, as described by us previously (8); 60–100 cells were analyzed per each slide. The observer was blinded to the treatment group for the immunofluorescent microscopic analysis.
An alternative technique was also used to quantify bulk GCRα cellular shuttling (i.e., TransAM GR transcription assay kit [Active Motive, Carlsbad, CA]) according to manufacturer's instructions. In brief, the cells were cultured and stimulated with DEX, as described above. Nuclear and cytoplasmic cellular extracts were prepared with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL). A total of 5 μg of nuclear extracts from untreated and DEX-treated cells was added to the plate coated with GRE consensus sequence (5′-GGTACAnnnTGTTCT-3′), incubated for 1 hour at RT with mild agitation, and washed three times. GCR contained in nuclear extracts bound specifically to the oligonucleotide, and was detected with an antibody directed against GCR, recognized by secondary anti-rabbit horseradish peroxidase–conjugated antibody after addition of the developing solution. Absorbance was read on a spectrophotometer at 450 nm with a reference wavelength of 655 nm. Nuclear extract from DEX-treated HeLa cells was used as a positive control. To validate the specificity of the GCR–GRE interaction, competitor GRE consensus sequence oligonucleotide (40 pmol/well) was added to the wells before the addition of nuclear extracts to prevent GCR binding to the probe immobilized to the plate. GCR activation was expressed as optical density at 450 nm (OD450) in all nuclear extracts.
To assess steroid responses of CD4+ and CD8+ T cells after 48 hours of culture with IL-2/IL-4 versus media, PBMCs were cultured as described above for 48 hours, then treated with 10−6 M DEX, or remained untreated for additional 1 hour. After the incubation, CD4+ and CD8+ cells were sorted by negative selections using magnetic bead human CD4+ T cell isolation kit II and human CD8+ T cell isolation kit II (Miltenyi Biotec, Auburn, CA), respectively. The purity of the enriched cells was evaluated by flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ), and was always over 90% positive. Nuclear extracts were prepared from purified CD4+ and CD8+ cells using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce); 8 μg of each extract was analyzed for nuclear GCR by TransAM GR transcription assay kit, CA) as described above. A total of 5 × 106 CD4+ and CD8+ cells was analyzed for each condition studied; cells from four different donors were tested.
Western Blotting
GCRα nuclear translocation was analyzed in cell extracts from PBMCs treated for 48 hours with IL-2/IL-4 versus IL-2/IL-4/IFN-γ versus media. Nuclear and cytoplasmic extracts from cells were prepared with NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce). Western blotting was performed as previously described (8). Membranes were blotted with anti-GCR (H300; Santa Cruz Biotechnology, Santa Cruz, CA). To control the quality of nuclear and cytoplasmic protein preparation, the membranes were probed with anti–TATA binding protein (TBP) and anti-actin antibodies (Sigma) as nuclear and cytoplasmic proteins, respectively.
Assessment of GCR Phosphorylation
To analyze GCR phosphorylation status, human PBMCs (n = 3) were cultured and treated with 10−6 M DEX for 3 hours, as described above for GCR nuclear translocation. The cells were fixed on coverslips with 4% paraformaldehyde in PBS, rinsed with Tris-buffered saline, permeabilized for 15 minutes at RT in permeabilization solution (Tris-buffered saline containing 0.1% [vol/vol], Tween 20, 0.1% [wt/vol] BSA [Sigma] and 0.01% [wt/vol] saponin), and blocked with a Superblock solution supplemented with 10% goat serum (Jackson ImmunoResearch Laboratories) for 1 hour at RT. The cells were then incubated with antibodies to phosphor-S211, phosphor-S226 of GCR, or rabbit IgG (Abcam, Cambridge, MA) in permeabilization solution (1:100) overnight at 4°C, washed, and then incubated with goat anti-rabbit IgG, F(ab′)2-cy3–conjugated secondary antibody (Jackson ImmunoResearch Laboratories) (1:200) and DAPI for nuclear counterstaining for 1 hour at RT, washed, and mounted on slides. The slides were analyzed as described above. The mean fluorescence intensity (MFI) of cy3 staining (phosphor-GCR) in cells was assessed by the analysis software within the computer-generated masks for nuclear (DAPI-staining) regions of the cells.
p-p38 MAPK Intracellular Staining
For these experiments, cells were fixed with 4% paraformaldehyde in PBS for 10 minutes at RT, washed, permeabilized for 15 minutes at RT in permeabilization solution (PBS containing 0.1% [vol/vol] Tween 20, 0.1% [wt/vol] BSA [Sigma] and 0.01% [wt/vol] saponin [Sigma]), blocked with Superblock commercial blocking solution for 15 minutes at RT. The cells were then incubated with FITC anti-CD3 and AlexaFluor 647 mouse anti-p-p38 MAPK (pT180/pY182) antibodies (BD Pharmingen, San Diego, CA) diluted in permeabilization solution for 1 hour at 4°C. AlexaFluor 647–conjugated mouse IgG1 (BD Pharmingen) was used as an isotype control. The cells were analyzed by flow cytometry; FlowJo v. 4.0.1 (Tree Star Inc., San Carlos, CA) was used for data analysis. The data were expressed as percent of CD3+ cells that expressed p-p38 MAPK.
Human Phospho-MAPK Array
The relative level of phosphorylation of MAPKs and other serine/threonine kinases was determined and analyzed in parallel in cell lysates from IL-2/IL-4– versus IL-2/IL-4/IFN-γ–stimulated cells using human phospho-MAPK array kit (R&D Systems) following manufacturer's instructions.
Real-Tme PCR
Mitogen-induced MAPK phosphatase (MKP)-1 induction by steroids was assessed by real-time PCR. At the end of the 48-hour incubation with cytokines, the cells were treated with 10−6 M DEX for an additional 4 hours or remained untreated, and total RNA was extracted according to the manufacturer's guidelines (Qiagen, Valencia, CA), transcribed into cDNA, and analyzed by real-time PCR using the dual-labeled fluorigenic probe method on an ABI Prism 7,000 sequence detector (Applied Biosystems, Foster City, CA), as previously described (9). Primers and probes for human MKP-1 and 18s RNA were purchased from Applied Biosystems. Standard curves for MKP-1 and 18s RNA were generated using the fluorescent data from 10-fold serial dilutions of total RNA of the highest-expression sample. Quantities of MKP-1 in test samples were normalized to the corresponding 18s RNA levels in each sample. The data were expressed as MKP-1 fold induction by DEX as compared with untreated cells.
Statistical Methods
Data were expressed as means (± SEM). The paired t test was used to compare functional responses of pre– and post–DEX-treated cells from the same donors (hence, paired). The test was also used to compare data gathered from the media-treated versus cytokine-treated cells from the same donors. Before testing, paired difference distributions were examined for outliers, which can indicate violation to the normality assumption of the parametric t test. No outliers were apparent. Multiple group comparisons were performed with a one-way ANOVA. A P value of less than 0.05 was considered statistically significant. All reported P values were based on two-sided tests.
RESULTS
Impaired GCRα Nuclear Translocation in IL-2/IL-4–Treated Human Lymphocytes
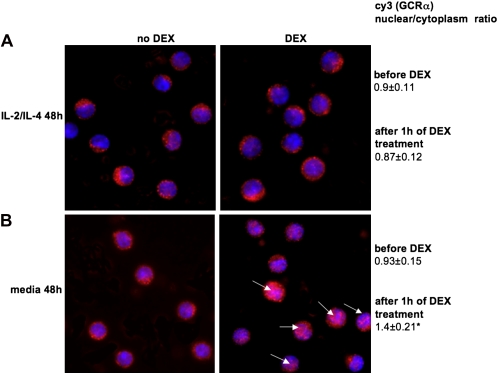
To investigate the mechanism by which IL-2/IL-4 induces steroid resistance, we initially compared the ability of GCRα to translocate in PBMCs stimulated with either IL-2, IL-4, or a IL-2/IL-4 combination versus media alone for 48 hours. The nonadherent cell fraction was collected and then treated with 10−6 M DEX for 1 hour at 37°C. This cell fraction consisted mainly of CD3+ lymphocytes (76.0 ± 2.8% for media-cultured cells and 74.3 ± 3.2% for IL-2/IL-4–cultured cells; n = 6); there was no difference in the CD4+:CD8+ ratio between IL-2/IL-4 versus media cultured conditions (1.71 ± 0.09 versus 1.64 ± 0.13, respectively; n = 6). In these experiments, the nuclei were defined by staining with the blue-fluorescent DNA dye, DAPI. GCRα localization was examined by indirect immunofluorescence using anti-GCRα rabbit polyclonal antibody detected by cy3-conjugated secondary antibody (red) (Figure 1). In the absence of DEX, GCRα was located in the cell cytoplasm of lymphocytes. This was associated with a cy3 (GCRα) nuclear:cytoplasmic ratio lower than 1 (0.9 ± 0.11 in IL-2/IL-4–treated group and 0.93 ± 0.15 in the media-treated group, respectively). The cells, grown in media alone, translocated GCRα to the nucleus in response to DEX. This corresponded to a cy3 (GCRα) nuclear:cytoplasmic ratio greater than 1. However, after IL-2/IL-4 treatment, GCRα failed to translocate, and was still localized in the cytoplasm of the cells (Figure 1) (cy3 [GCRα] nuclear:cytoplasmic ratio, 0.87 ± 0.12 in IL-2/IL-4–treated group as compared with 1.4 ± 0.21 in media-treated group; P < 0.01).
Figure 1.
Treatment with IL-2/IL-4 combination inhibits glucocorticoid receptor (GCR)-α nuclear translocation in human peripheral blood mononuclear cells (PBMCs). (A) Pretreatment of human PBMCs with the IL-2/IL-4 for 48 hours inhibited GCRα nuclear translocation in response to steroids (×630 magnification; 4′,6′-diamidino-2-phenylindole [DAPI] [blue]; cell nuclei, cy3 [red]; GCRα). (B) The 48-hour media–treated cells showed normal translocation of GCRα in response to steroids. Each panel is representative of six independent experiments; 100 cells were analyzed in each experiment. *P < 0.01 as compared with dexamethasone (DEX)-treated IL-2/IL-4–cultured cells. Arrows indicate the cells that show nuclear localization of the GCRα after DEX treatment.
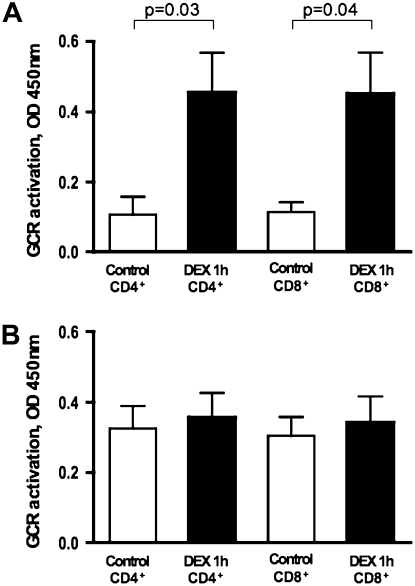
To evaluate whether the inhibition of GCRα nuclear translocation in response to steroids was equally affected in CD4+ and CD8+ cells after IL-2/IL-4 treatment, GCR was measured in nuclear extracts from purified CD4+ and CD8+ cells that were sorted from 48-hour–cultured PBMCs. GCR was measured based on its interaction with a GRE consensus motif immobilized to the plate (TransAM GR transcription factor assay). After DEX treatment, both CD4+ and CD8+ cells showed a significant increase in the amount of nuclear GCR when PBMCs were cultured in media only for 48 hours (Figure 2A). However, when cells were cultured in the presence of IL-2/IL-4 for 48 hours, addition of DEX did not alter the amount of nuclear GCR in the cells (Figure 2B).
Figure 2.
CD4+ and CD8+ cells are not responsive to DEX after treatment with IL-2/IL-4 for 48 hours. CD4+ and CD8+ T cells were purified by negative selection from PBMC cultures. GCR was measured in cellular nuclear extracts from media- (A) versus IL-2/IL-4 (B)–treated cells ± DEX (1 h) based on its interaction with glucocorticoid response elements (GRE) consensus motif immobilized to the plate (TransAM GR transcription factor assay) (n = 4). Data are presented as means ± SEM.
Of interest, neither IL-2 nor IL-4 alone was able to alter GCRα nuclear translocation. GCRα translocated normally in cells that were cultured with IL-2 or IL-4 only for 48 hours (Figure 3A), but remained in the cytoplasm of the cells pretreated with IL-2/IL-4 for 48 hours (Figure 3A) (cy3 [GCRα] nuclear:cytoplasmic ratio after DEX treatment, 1.36 ± 0.11 in IL-2–treated group, 1.41 ± 0.18 in IL-4–treated group, as compared with 0.85 ± 0.19 in IL-2/IL-4–treated group; P < 0.01). These data suggest that the resistance of IL-2/IL-4–stimulated cells to DEX is due to a failure of GCRα to translocate from cytoplasm to nucleus, and that a combination of both cytokines is necessary to abrogate GCR function.
Figure 3.
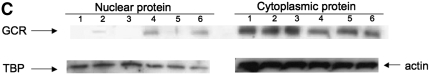
Effects of cytokines on steroid-induced GCRα nuclear translocation (×630 magnification; DAPI [blue]; cell nuclei, cy3 [red]; GCRα). (A) GCRα nuclear translocation in response to DEX occurs normally in IL-2– or IL-4–treated human PBMCs cultured for 48 hours, but is impaired after IL-2/IL-4 48-hour pretreatment. (B) IFN-γ is required for normal GCRα nuclear translocation in response to steroids. Human PBMCs were cultured with 50 U/ml IL-2/IL-4 with 100 U/ml IFN-γ for 48 hours, or cultured with 50 U/ml IL-2 with 1 μg/ml anti–IFN-γ blocking antibody for 48 hours before DEX treatment. Arrows indicate the cells that show nuclear localization of the GCRα after DEX treatment. Each panel is representative of six independent experiments; *P < 0.01. (C) Inhibition of GCRα nuclear translocation in cellular extracts from PBMCs treated with IL-2/IL-4 versus IL-2/IL-4/IFN-γ versus media for 48 hours. Cells were treated with IL-2/IL-4, IL-2/IL-4/IFN-γ, or media alone for 48 hours, and then treated with 10−6 M DEX, or remained in the culture media for an additional hour. The loading of nuclear and cytoplasmic proteins was controlled with blotting for TATA binding protein (TBP; nuclear protein) and actin (cytoplasmic protein). A representative experiment out of three independent experiments performed is provided. Lane 1, IL-2/IL-4 48, hours; lane 2, IL-2/IL-4, 48 hours, DEX, 1 hour; lane 3, media, 48 hours; lane 4, media, 48 hours, DEX, 1 hour; lane 5, IL-2/IL-4/IFN-γ, 48 hours; lane 6, IL-2/IL-4/IFN-γ, 48 hours, DEX, 1 hour.
IFN-γ Reverses GCRα Intracellular Shuttling in Response to Steroids
IFN-γ has been previously shown to restore sensitivity to steroids by affecting GCR binding affinity to steroids (7), but the mechanisms have not been previously reported. IL-4 is known to inhibit IFN-γ production (10). Therefore, we further examined the relationship between IFN-γ and IL-4 in modulating steroid responsiveness. We compared levels of IFN-γ in culture supernatants from cells of four different donors grown with IL-2 alone versus IL-2/IL-4–treated cells. Incubation with an IL-2/IL-4 combination significantly reduced the production of IFN-γ to 36.2 ± 5.6% of what was induced by IL-2 alone (the amounts of IFN-γ in cell culture supernatants were 1397 ± 369 pg/ml versus 526 ± 156 pg/ml for IL-2 versus IL-2/IL-4 cultures, respectively; P < 0.05; n = 4).
The addition of IFN-γ also restored GCRα nuclear translocation in the presence of combination IL-2/IL-4 (Figure 3B) as compared with IL-2/IL-4–treated cells (Figure 3A). There was no influence of IFN-γ on cell viability (data not shown). The importance of IFN-γ was further supported by the observation that, when IL-2–stimulated cells were grown in the presence of 1 μg/ml anti-IFN-γ blocking antibody and then treated with DEX, the majority of cells did not translocate their GCR in response to DEX (Figure 3B).
GCRα nuclear translocation was also analyzed by Western blot in cell extracts from PBMCs treated with IL-2/IL-4 versus IL-2/IL-4/IFN-γ versus media for 48 hours and confirmed inhibition of GCRα nuclear translocation in IL-2/IL-4–treated cells; this was restored in the presence of IFN-γ (Figure 3C).
Activation of P38 MAPK by IL-2/IL-4 Interferes with Functional Responses to Steroids
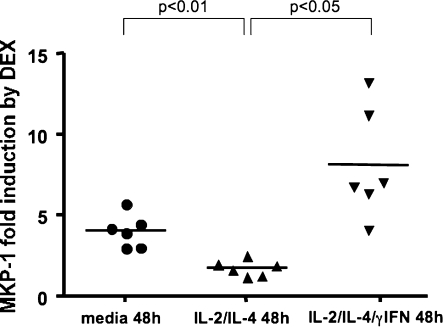
MKP-1 induction represents one of the earliest functional markers of steroid response (11, 12). Steroids induce a rapid increase in expression of MKP-1 mRNA, and functional responsiveness to steroids correlates with in vitro MKP-1 induction by DEX. To analyze functional responses to steroids, human PBMCs were cultured in media alone, or in the presence of either combination IL-2/IL-4 or IL-2/IL-4/IFN-γ, for 48 hours, then treated with 10−6 M DEX for 4 hours or remained untreated. MKP-1 induction by DEX was evaluated by real-time PCR and expressed as MKP-1 fold induction in the DEX-treated group versus the untreated group. Significant induction of MKP-1 was found in media-treated cells. In contrast, low MKP-1 induction by steroids was present in cells from the IL-2/IL-4–treated group. Addition of IFN-γ to the IL-2/IL-4 combination resulted in significant enhancement of MKP-1 production by the cells treated with DEX (MKP-1 fold activation after 4 h of 10−6 M DEX as compared with 4 h of media was 1.72 ± 0.25 [IL-2/IL-4] versus 3.34 ± 0.30 [media], P < 0.01; 8.15 ± 1.18 [IL-2/IL-4/IFN-γ], P < 0.05, as compared with IL-2/IL-4–treated cells, respectively) (Figure 4).
Figure 4.
IL-2/IL-4 combination inhibits mitogen-induced mitogen-activated protein kinase (MAPK) phosphatase (MKP)-1 mRNA induction by DEX. At the end of the 48-hour incubation with cytokines, the cells were treated with 10−6 M DEX for an additional 4 hours, or remained untreated. MKP-1 mRNA induction by DEX in cell isolates as compared with medium-treated cells was analyzed by real-time PCR.
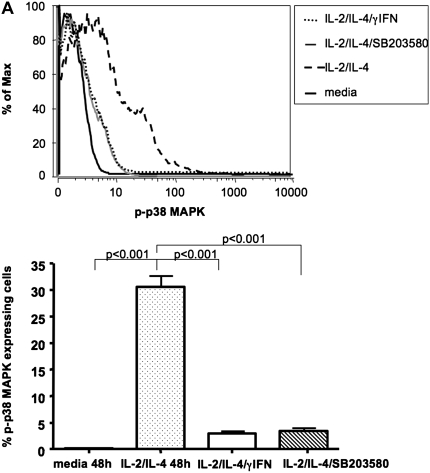
We also evaluated the presence of activated p38 MAPK after 48 hours of culture, as described above. No p-p38 MAPK was found in media-treated cells, whereas 30.5 ± 5.7% CD3+ gated cells treated with IL-2/IL-4 contained activated p38 MAPK. Culturing cells in the presence of combination IL-2/IL-4/IFN-γ or the specific p38 MAPK inhibitor, SB203580, resulted in significant inhibition of p38 MAPK activation (P < 0.001) (Figure 5A).
Figure 5.
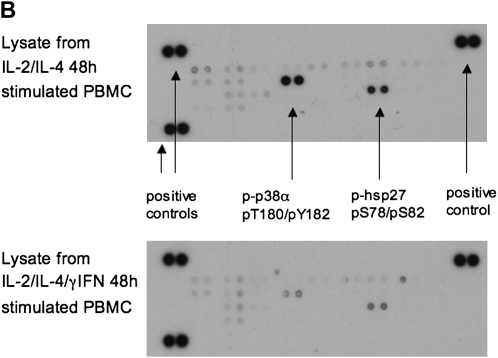
Treatment of PBMCs with combination IL-2/IL-4 induced p38 MAPK activation. (A) Presence of specific p38 MAPK inhibitor, SB203580, or IFN-γ inhibited p38 MAPK phosphorylation in CD3+ cells, as shown by p-p38 MAPK staining of IL-2/IL-4–stimulated human PBMCs analyzed by flow cytometry. Results are representative of six independent experiments. p38 MAPK activation was analyzed in the gate of CD3+ cells. Data are presented as means ± SEM. (B) Human phospho-MAPK array profiler of cell lysates from IL-2/IL-4– versus IL-2/IL-4/IFN-γ–stimulated human PBMCs. Shown is a representative blot of three independent experiments.
Using a human phospho-MAPK array profiler assay, p38α MAPK (T180/Y182) was the only activated MAPK present after 48 hours of IL-2/IL-4 treatment (no phosphorylated [p] extracellular signal–regulated kinase-1 or -2, p-Jun-N-terminal kinase [JNK]-1, p-JNK2, p-JNK3, p38β, p38γ, p38δ MAPK, ribosomal protein S6 kinase, p90 (RSK)1, RSK2, mitogen- and stress-activated kinase (MSK)1, MSK2, Akt1, Akt2, Akt3, glycogen synthase kinase-3α/β or -3β, or p70 S6 kinases were detected); this was further confirmed by phosphorylation of heat shock protein 27—the downstream phosphorylation substrate for the activated p38 MAPK (13). Phosphorylation of both molecules was significantly diminished when cells were cultured with IL-2/IL-4/IFN-γ for 48 hours (Figure 5B).
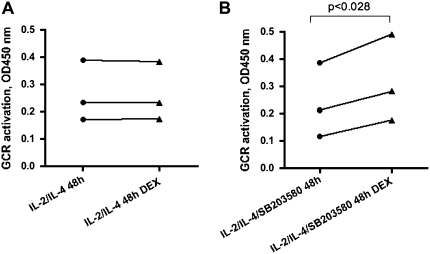
To directly assess the effect of p38 MAPK activation on GCR translocation, human PBMCs were stimulated with IL-2/IL-4 with or without a nontoxic 1 μM concentration (as previously defined by us) of the p38 MAPK–specific inhibitor, SB203580 (14), for 48 hours and then additionally treated with 10−6 M DEX for 1 hour or remained untreated. GCR was measured in nuclear extracts from these cells based on its interaction with a GRE consensus motif immobilized to the plate (TransAM GR transcription factor assay). As expected, addition of DEX did not change the amount of nuclear GCR when cells were cultured with IL-2/IL-4 for 48 hours (Figure 6A). However, when DEX was added to the cells that were cultured in the presence of p38 MAPK inhibitor, there was a significant increase in nuclear GCR (Figure 6B).
Figure 6.
Inhibition of p38 MAPK activation by SB203580 specific inhibitor (1 μM) in IL-2/IL-4–treated cells results in nuclear translocation of GCR in response to DEX. GCR was measured in nuclear extracts from IL-2/IL-4– versus IL-2/IL-4/SB203580–treated cells ± DEX (1 h) based on its interaction with GRE consensus motif immobilized to the plate (TransAM GR transcription factor assay) (n = 3).
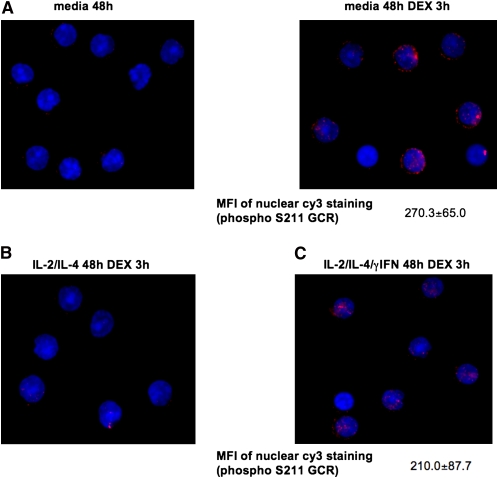
IL-2/IL-4 Treatment Inhibits DEX-Induced GCR Phosphorylation for S211
It has been shown that DEX treatment induces GCR phosphorylation, and this modifies GCR transcriptional activity (15). To investigate whether cytokine treatment modifies GCR phosphorylation status, human PBMCs were cultured with IL-2/IL-4, IL-2/IL-4/IFN-γ, or media alone for 48 hours, followed by 3 hours of DEX treatment. The expression of phosphor-S211 and phosphor-S226 GCR was assessed by immunostaining. In the absence of DEX, no phosphorylation of S211 was noted in cells from all experimental groups. DEX treatment induced S211 phosphorylation of GCR in media-cultured cells (MFI 270.3 ± 65.0; n = 3) (Figure 7A). In contrast, no DEX-induced phosphorylation of S211 in GCR was observed for the cells that were cultured in the presence of IL-2/IL-4 (Figure 7B). However, when cells were cultured in the presence of IL-2/IL-4/IFN-γ, DEX-induced S211 GCR phosphorylation was restored (MFI 210.0 ± 87.7; n = 3) (Figure 7C). No changes in S226 GCR phosphorylation was noted between three experimental groups studied, and GCR phosphorylated for S226 remained cytoplasmic after DEX treatment (data not shown).
Figure 7.
IL-2/IL-4 treatment inhibits DEX-induced S211 GCR phosphorylation in human lymphocytes (×630 magnification; DAPI (blue); cell nuclei, cy3 [red]; phospho S211 of GCR). DEX treatment (3 h) induces phosphorylation of S211 of GCR in media- (A) and IL-2/IL-4γ/IFN–treated cells (C), but S211 GCR phosphorylation is impaired in IL-2/IL-4–treated cells (B). Each panel is representative of three independent experiments.
DISCUSSION
The current study demonstrates that the cytokine environment may modify steroid-induced GCRα nuclear translocation in human lymphocytes. We found that pretreatment of human CD4+ or CD8+ T cells with the IL-2/IL-4 combination results in the loss of steroid responsiveness due to the inhibition of DEX-induced GCRα nuclear translocation. However, neither IL-2 nor IL-4 alone inhibited GCR translocation. After IL-2/IL-4 treatment (according to the GRE binding assay), a significantly higher number of nuclear GCRs was present in both CD4 and CD8 T-cell populations as compared with media-treated cells. This observation goes alone with previously published data by Kam and colleagues (7) and Sher and colleagues (16) that reported increased GCR expression per cell after IL-2/IL-4 preincubation, thus explaining higher baseline GRE binding in cells after IL-2/IL-4 pretreatment.
The current study extended previous work on cytokine regulation of GCR binding affinity (7) by demonstrating the lack of GCR nuclear translocation in response to steroids after exposure to cytokines, which is likely more important in accounting for lack of GC response. We found that addition of IFN-γ is required to sustain steroid sensitivity and reverses IL-2/IL-4–induced inhibition of the GCRα translocation. In addition, we demonstrated that reduced functional responses to DEX (MKP-1 induction and GCRα nuclear translocation), after treatment with IL-2/IL-4, were associated with selective activation of p38 MAPK that could be abrogated by addition of IFN-γ. This observation was supported by experiments demonstrating that addition of the p38 MAPK–specific inhibitor, SB203580, to IL-2/IL-4–treated cells enhanced GCR nuclear translocation in response to DEX. Taken together, our data suggest that IFN-γ reverses steroid response via inhibition of p38 MAPK pathway.
Many steroid hormone receptors, including the GCR, are phosphoproteins (15). Importantly, steroid hormone receptor phosphorylation modulates their function (15, 17). The GCR is thought to be a substrate for several kinases and phosphatases, and has been shown to be polyphosphorylated on serine residues in the N-terminal region of the protein (15, 17–20). Although the precise role of each specific phosphorylation event remains unclear, mutations of multiple phosphorylation sites have been shown to have a major impact on the ability of GCR to translocate to the nucleus, receptor stability, and protein half-life and signaling (15). GCR can be phosphorylated at three major serine sites within the N-terminal region of the receptor (S203, S211, and S226 using the human numbering scheme) (15). Using a model system, it has been determined that, in the absence of hormone, the level of GCR phosphorylation at Ser211 was low compared with phosphorylation at Ser203 (20). Phosphorylation of both residues increased upon treatment with the GCR agonist, dexamethasone. Biochemical fractionation studies and immunostaining after hormone treatment indicated that the Ser203-phosphorylated form of the receptor was predominantly cytoplasmic, whereas Ser211-phosphorylated GCR was found in the nucleus (20). Using a battery of agonists and antagonists, it was found that the transcriptional activity of GCR correlated with the amount of phosphorylation at Ser211, suggesting that Ser211 phosphorylation can serve as a biomarker for activated GCR in vivo (20).
Our experimental data suggest that cytokine environment may modify GCR phosphorylation status (IL-2/IL-4 pretreatment inhibits DEX-induced S211 phosphorylation, and IL-2/IL-4/IFN-γ restores it) and, hence, may control GCR translocation in response to steroid treatment and GCR transcriptional activity. A recent study using selective serine/threonine protein phosphatase inhibitors (okadaic acid and calyculin) suggested that GCR ligand-binding domain–linked phosphatases, such as PP1 and PP2A, may be responsible for dephosphorylation of S211 (21). Inhibition of phosphatase activity by okadaic acid results in GCR hyperphosphorylation, a redistribution of the receptor from the nucleus to the cytoplasm, and the inability of GCR to re-enter the nucleus (22). It is tempting to suggest that IL-2/IL-4 pretreatment and p38 MAPK activation may affect expression and/or activity of these phosphatases, thus resulting in inhibition of DEX-induced S211 phosphorylation of GCR and lack of GCR nuclear translocation in response to hormone.
Work by Dr. Garabedian's group (23) has shown that activation of p38 MAPK in the HeLa cell line impairs GCR activation of the mouse mammary tumor virus (MMTV)-Luc reporter plasmid. Using a “domain swap” technique, they found that p38 MAPK targets the C-terminal ligand binding domain (LBD)/activation function (AF)-2 domain of the GCR (amino acids 525–795). Their data suggest that p38 MAPK activation has an indirect effect on GCR function, as they mutated the only potential p38 MAPK phosphorylation site within this region (Ala547–Thr547); however, despite this point mutation, GCR activation of the reporter plasmid remained impaired in the presence of constitutively active p38 MAPK (23). There were several potential mechanisms suggested by which p38 MAPK could impair GCR function: (1) phosphorylation of the AF-2/LBD domain of GCR by kinase(s) lying downstream in the p38 activation pathway could be responsible for the p38 inhibitory effect; (2) direct or indirect phosphorylation of a nonreceptor protein associated with GCR, perhaps coactivators of p160 family; (3) p38 activation might cause cytoplasmic sequestration of the GCR (e.g., [a] by locking the interaction with heat shock protein 90 bound to LBD, [b] activation of transrepressor, or [c] inducing other transcriptional factors that sequester critical GCR coactivators) (23). Such modifications can affect GCR recognition by nuclear transport machinery. The latter is yet to be determined.
Our data support a previously published observation by Irusen and colleagues (24), who reported that treatment of cells with IL-2/IL-4 combination results in p38 MAPK activation. The authors showed that reduced GCR ligand-binding affinity induced by combination IL-2/IL-4 can be blocked with the specific p38 MAPK inhibitor, suggesting that p38 MAPK inhibitors may potentially reverse steroid insensitivity. Our current study further expands this work by showing that IFN-γ could be a potential inhibitor of cytokine-induced p38 MAPK activation, and that IFN-γ is critical to maintain corticosteroid sensitivity. We also found that, after treatment of IL-2–stimulated cells with anti–IFN-γ blocking antibody, the majority of cells did not translocate their GCR in response to DEX. In contrast, IFN-γ reverses IL-2/IL-4–induced inhibition of GCRα translocation in response to steroids. Immunostaining and ELISA-based GCR activation assays used in this study allowed us to characterize GCR nuclear translocation on the total population of cells. Hence, our results are reflective of total lymphocyte behavior. Work recently published by our research group (8) demonstrated that, when mouse T cells were treated with IL-2, the GCR no longer translocated to the nucleus after DEX treatment. Immunoprecipitation experiments revealed that interactions between the GCR and phosphorylated signal transducer and activator of transcription-5 are important in contributing to the failure of GCR to translocate to the nucleus and suppress proliferation. In these experiments, we showed that IL-2–induced steroid resistance could be blocked by an inhibitor of p38 MAPK as well (8).
In the current study, we demonstrate that the IL-2/IL-4 combination treatment of PBMCs induces GC resistance that can be reversed with IFNγ treatment or p38 inhibition. However, the mechanism for integration of these pathways is not clearly understood and requires further investigation. Although it is true that other cell types and cytokines are involved in steroid resistance, our previous data (6, 7, 16, 25) established that the combination IL-2/IL-4 is important in T-cell steroid resistance, and this was the focus of the current study. Importantly, our data suggest active cross-talk between the cytokine environment and cellular steroid responses via GCR. This should be taken into consideration while studying in vivo allergic inflammatory models of steroid responses.
Our current studies demonstrate a potential role for p38 MAPK pathways in the inhibition of GCR translocation and induction of T-cell steroid resistance. Successful development of MAPK inhibitors may have important implications for the treatment of glucocorticoid resistance, and for therapy of inflammatory disease in general.
Acknowledgments
The authors thank Maureen Sandoval for her help in preparing this manuscript.
This work was supported by National Institutes of Health grants HL36577, AR41256, HL37260, and AI70140.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, National Heart, Lung, and Blood Institute, or the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0327OC on September 5, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol 2003;111:3–22. [DOI] [PubMed] [Google Scholar]
- 2.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005;353:1711–1723. [DOI] [PubMed] [Google Scholar]
- 3.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull 2000;56:1054–1070. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE [serial on the Internet]. 2005. [accessed 15 December 2008];pe48. Available from: http://stke.sciencemag.org/cgi/reprint/sigtrans; 2005/304/pe49.pdf [DOI] [PubMed]
- 5.Nimmagadda SR, Szefler SJ, Spahn JD, Surs W, Leung DY. Allergen exposure decreases glucocorticoid receptor binding affinity and steroid responsiveness in atopic asthmatics. Am J Respir Crit Care Med 1997;155:87–93. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, Hamid Q. Dysregulation of interleukin 4, interleukin 5, and interferon γ gene expression in steroid-resistant asthma. J Exp Med 1995;181:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor–binding affinity and T cell response to glucocorticoids. J Immunol 1993;151:3460–3466. [PubMed] [Google Scholar]
- 8.Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2–induced glucocorticoid resistance. J Immunol 2002;169:5934–5940. [DOI] [PubMed] [Google Scholar]
- 9.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003;171:3262–3269. [DOI] [PubMed] [Google Scholar]
- 10.Paludan SR. Interleukin-4 and interferon-γ: the quintessence of a mutual antagonistic relationship. Scand J Immunol 1998;48:459–468. [DOI] [PubMed] [Google Scholar]
- 11.Li LB, Leung DY, Hall CF, Goleva E. Divergent expression and function of glucocorticoid receptor β in human monocytes and T cells. J Leukoc Biol 2006;79:818–827. [DOI] [PubMed] [Google Scholar]
- 12.Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY. Increased glucocorticoid receptor β alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 2006;173:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross S, Chen T, Yu V, Tudor Y, Zhang D, Liu L, Tamayo N, Dominguez C, Powers D. High-content screening analysis of the p38 pathway: profiling of structurally related p38α kinase inhibitors using cell-based assays. Assay Drug Dev Technol 2006;4:397–409. [DOI] [PubMed] [Google Scholar]
- 14.Li LB, Goleva E, Hall CF, Ou LS, Leung DY. Superantigen-induced corticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal–regulated kinase (MEK–ERK) pathway. J Allergy Clin Immunol 2004;114:1059–1069. [DOI] [PubMed] [Google Scholar]
- 15.Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci 2004;1024:86–101. [DOI] [PubMed] [Google Scholar]
- 16.Sher ER, Leung DY, Surs W, Kam JC, Zieg G, Kamada AK, Szefler SJ. Steroid-resistant asthma: cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest 1994;93:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 2005;70:407–417. [DOI] [PubMed] [Google Scholar]
- 18.Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol 1997;17:3947–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogatsky I, Waase CL, Garabedian MJ. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3): species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem 1998;273:14315–14321. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem 2002;277:26573–26580. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal–associated protein phosphatase. Mol Endocrinol 2007;21:625–634. [DOI] [PubMed] [Google Scholar]
- 22.DeFranco DB, Qi M, Borror KC, Garabedian MJ, Brautigan DL. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol 1991;5:1215–1228. [DOI] [PubMed] [Google Scholar]
- 23.Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem 2004;279:43708–43715. [DOI] [PubMed] [Google Scholar]
- 24.Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase–induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol 2002;109:649–657. [DOI] [PubMed] [Google Scholar]
- 25.Spahn JD, Leung DY, Surs W, Harbeck RJ, Nimmagadda S, Szefler SJ. Reduced glucocorticoid binding affinity in asthma is related to ongoing allergic inflammation. Am J Respir Crit Care Med 1995;151:1709–1714. [DOI] [PubMed] [Google Scholar]