Abstract
It is generally accepted that one-year post-transplant proteinuria over 0.5 gm per day has a negative impact on renal graft survival. In this study, the effects of minimal proteinuria less than 0.5 g/day were analyzed in 272 renal recipients who had survived for one year with a functioning graft. Recipients were classified by one-year post-transplant proteinuria: no proteinuria group (<0.2 g/day), minimal proteinuria group (0.2-0.5 g/day), and overt proteinuria group (≥0.5 g/day). Recipients were followed up for 87.1±21 months after transplantation and 38 (13.9%) lost their graft during follow-up. Fifteen percent of patients had minimal proteinuria and 7.8% had overt proteinuria. Five-year graft survival in the minimal proteinuria group was 83.0%, and that in the overt proteinuria group was 70%, in contrast to 97.1% in the no proteinuria group (p=0.01 for trend). In a multivariate analysis, the minimal proteinuria group (relative risk [RR], 4.90; 95% confidence interval [CI], 2.09-11.46) and the overt proteinuria group (RR, 8.75; 95% CI, 3.29-23.29) had higher risks of graft failure than the no proteinuria group. Even minimal proteinuria at one year after transplantation was strongly associated with poor graft outcome. Therefore, it appears logical to consider a low level of proteinuria as a risk factor for graft survival in renal recipients.
Keywords: Graft Survival, Kidney Transplantation, Proteinuria
INTRODUCTION
Despite improvements in short-term renal allograft survival, the rate of chronic graft loss after the first year has remained unchanged (1). The leading causes of late allograft loss are patient death and chronic allograft nephropathy (2). Chronic allograft nephropathy is clinically characterized by a slow but steady decline in allograft function. It is a highly complex pathophysiological process, which involves alloantigen and non-alloantigen dependent factors. Examples of the latter include hypertension, hyperlipidemia, obesity, metabolic syndrome, and proteinuria (2).
Proteinuria is a cardinal manifestation of kidney disease and is associated with a poor outcome in most renal parenchymal disorders. It is also generally accepted that one-year post-transplant proteinuria over 0.5 gm per day predicts the development of chronic allograft nephropathy and poor graft outcome (3-5). Therefore, the administration of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) is recommended in kidney transplant recipients with proteinuria exceeding 0.5 g/day (6, 7).
Low levels of proteinuria of less than 0.5 g/day, including microalbuminuria, have been found to be associated with an increased risk of chronic kidney disease and cardiovascular mortality (4, 8) and this has emerged as a useful target for therapy in subjects with hypertension or diabetes (9). Thus, we investigated whether proteinuria of less than 0.5 g/day one year after transplantation is associated with long-term graft survival.
MATERIALS AND METHODS
Study design and patients
We reviewed all adult recipients who had undergone renal transplantation in the Samsung Medical Centre between January 1995 and December 1999. During this period, 291 primary kidney transplantations were performed. Of these, 19 transplantations were excluded due to death (4), graft loss (11), or being lost to follow-up (4) within one year. Therefore, 272 primary kidney transplant recipients were enrolled in this study. One-hundred seventy-seven (65.1%) transplants were cadaveric donor transplants and 95 (34.9%) transplants were living donor transplants.
Baseline pre-transplant and one-year post-transplant evaluations
All the patients received a baseline and one-year post-transplant evaluation according to a standard protocol. Baseline variables included age, sex, donor age, donor sex, donor type, duration of pre-transplant maintenance dialysis, and number of human leukocyte antigen (HLA) mismatches. The presence or absence of delayed graft function (DGF), and acute rejection within one year were also recorded. One-year post-transplant variables included systolic and diastolic blood pressure, post-transplant diabetic mellitus (PTDM), body mass index (BMI), serum creatinine levels, amount of urinary protein excreted, and medications (anti-hypertensive drugs, including ACEI and ARB, and immunosuppressants).
Immunosuppressive regimen
Our institution's immunosuppressive regimen consisted of calcineurin inhibitor, prednisolone, and azathioprine from January 1995 to March 1998 or mycophenolate mofetil thereafter. Prednisolone at 1 mg/kg per day was commenced on the day of transplantation and was gradually tapered to a daily maintenance dose of 5 mg at six months after transplantation. The initial dose of cyclosporine was designed to achieve 150-250 ng/mL trough levels for the first 12 months and 100-150 ng/mL thereafter. Azathioprine was commenced at 2.5 mg/kg per day or mycophenolate mofetil was commenced at 1.5 g/day and adjusted subsequently according to the white blood cell count or significant gastrointestinal side effects.
Definitions and measurements
DGF was defined as the failure of a renal allograft to function immediately after transplantation and the need for dialysis within one week. Acute rejection was defined when biopsy-proven cases received anti-rejection therapy. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or the use of any anti-hypertensive drug. Blood pressure was measured in the sitting position after five minutes resting. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). PTDM was defined by the symptoms of diabetes plus a random glucose value of ≥200 mg/dL, a fasting plasma glucose value of ≥126 mg/dL, or a two-hour plasma glucose value of ≥200 mg/dL after a 75 g glucose load (10). Eight-hour fasting blood samples were used to determine glucose and creatinine levels. Renal allograft function was assessed by the estimated glomerular filtration rate (GFR), calculated with the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation (11, 12). Twenty-four-hour urine samples were obtained for the determination of urinary protein excretion. Urinary protein concentrations were measured using the pyrogallol red-molybdate complex method, which has a lower detection limit of 1.0 mg/L. Recipients were classified into one of three groups according to their levels of one year post-transplant proteinuria: less than 0.2 g/day as the no proteinuria group; 0.2-0.5 g/day as the minimal proteinuria group; and more than 0.5 g/day as the overt proteinuria group. 'Graft loss' refers to a return to dialysis or a second transplantation. Graft survival was examined without censoring for death.
Statistical analyses
Statistical analyses were performed using SPSS version 12.0. Parametric parameters are expressed as means±standard deviations, whereas nonparametric parameters are expressed as medians (interquartile ranges). To compare means, Student's t-test or one-way analysis of variance (ANOVA) was used. When one-way ANOVA was performed, we used Turkey-B test as post hoc test. The chi square test or Fischer's exact test was used to evaluate the distributions of categorical variables. Graft survival rates were computed using the Kaplan-Meier method, and compared using the log-rank test. Cox proportional hazard model was used to identify parameters associated with graft loss during follow up. All p values reported were considered significant at the 0.05 level.
RESULTS
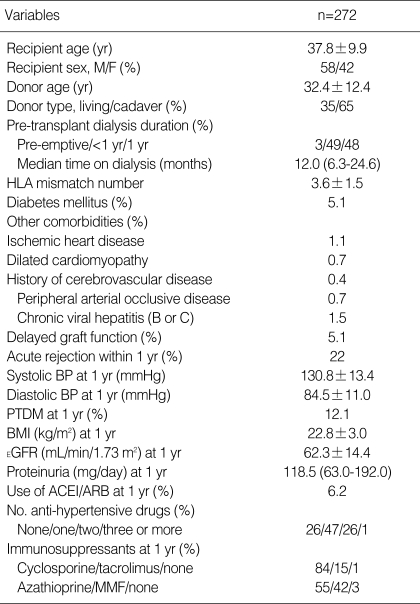
As outlined in Table 1, 177 (65.1%) transplants were cadaveric donor transplants and 95 (34.9%) were living donor transplants. Recipient age and donor age were 37.8±9.9 yr and 32.4±12.4 yr, respectively. One-hundred and fifty-nine (58.4%) patients were male. The mean number of HLA mismatches was 3.6±1.5. Diabetes mellitus was present in 5.1% of patients at the time of transplantation. DGF and acute rejection within one year occurred in 5.0% and 21.3% of recipients, respectively. Hypertension was detected at one year after transplantation in 219 (80.9%) recipients, and mean systolic and diastolic blood pressures were 130.8±13.4 mmHg and 84.5±11.0 mmHg, respectively. PTDM was present in 33 (12.1%) recipients, and mean estimated GFR was 62.3±14.4 mL/min/1.73 m2. Seventeen (6.2%) recipients were treated with ARB or ACEI. Cyclosporine was used in 84.2% of recipients and tacrolimus in 15.4%; mycophenolate mofetil was used in 41.5% and azathioprine in 55.1%.
Table 1.
Demographic and transplant characteristics of the study population
Data are expressed as means±SD, except proteinuria and time on dialysis (expressed as median and interquartile range).
HLA, human leukocyte antigen; BP, blood pressure; PTDM, post-transplant diabetic mellitus; BMI, body mass index; EGFR, estimated glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; MMF, mycophenolate mofetil.
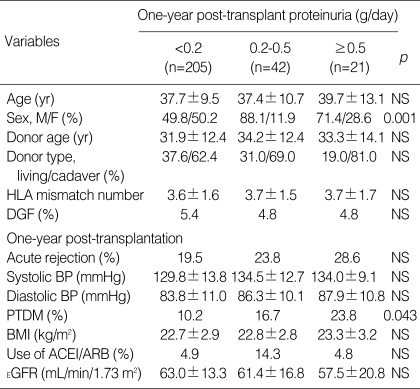
Urinary protein excretion levels were not available for four patients. Of the remaining 268 patients, 15.7% showed one-year post-transplant proteinuria of 0.2-0.5 g/day, and 7.8% had proteinuria of more than 0.5 g/day (Table 2). The minimal and overt proteinuria groups showed a male predominance. Recipient age, donor age, donor type, number of HLA mismatches, DGF, acute rejection, blood pressure, and BMI did not differ between the three groups. Estimated GFR at the first year was also similar among the three groups. PTDM was more common in the overt proteinuria group than in the minimal proteinuria or the no proteinuria group (23.8%, 16.7%, and 10.2%, p=0.043 for trend).
Table 2.
Baseline and one-year post-transplant characteristics according to the amount of urinary protein excretion
Data are expressed as means±SD.
HLA, human leukocyte antigen; DGF, delayed graft function; BP, blood pressure; PTDM, post-transplant diabetic mellitus; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, aniotensin-receptor blocker; EGFR, estimated glomerular filtration rate; NS, not significant.
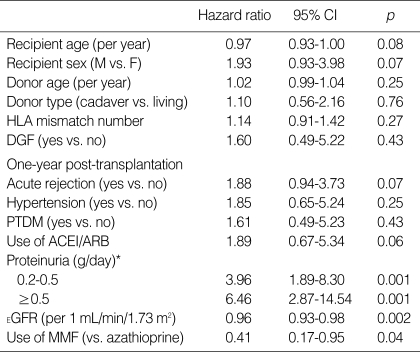
Recipients were followed up for a mean period of 87.1 (13-130) months after transplantation and 38 (13.9%) recipients lost their graft during follow-up. Univariate analysis of graft loss (Table 3) identified the following significant predictors, the presence of proteinuria, estimated GFR at the first year, and the use of mycophenolate mofetil. The minimal proteinuria group (hazard ratio [HR], 3.96; 95% confidence interval [CI], 1.89-8.30) and the overt proteinuria group (HR, 6.46; 95% CI, 2.87-14.54) were at higher risk of graft loss than the no proteinuria group. Each 1 mL/min/1.73 m2 increment in the estimated GFR one year after transplantation decreased the risk of graft loss (HR, 0.96; 95% CI, 0.93-0.98). The use of mycophenolate mofetil was associated with a lower risk of graft loss than the use of azathioprine (HR, 0.41; 95% CI, 0.17-0.95).
Table 3.
Risk factors for graft loss by univariate analysis
*, The reference category was proteinuria of <0.2 g/day.
HLA, human leukocyte antigen; DGF, delayed graft function; PTDM, post-transplant diabetic mellitus; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; EGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; CI, confidence interval.
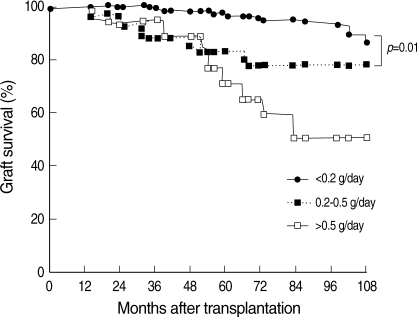
Overall five-year graft survival rate was 92% and patients in the no proteinuria group (five-year graft survival, 97%) presented with better graft survival than those in the other two groups. The five-year graft survival in the minimal proteinuria group was 83%, and that in the overt proteinuria group was 70%. There was no significant difference between the minimal and overt proteinuria groups (p=0.272).
Fig. 1 shows the effect of one-year post-transplant proteinuria on long-term graft survival. The minimal proteinuria group had poor graft survival than the no proteinuria group (five-year graft survival, 83% and 97%, respectively).
Fig. 1.
Effect of one-year post-transplant proteinuria on long-term graft survival. The minimal proteinuria group showed poor graft survival than the no proteinuria group. Log-rank test: p<0.01.
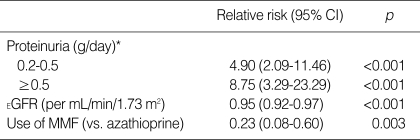
The results of multivariate analysis of graft loss (Table 4) indicated that one-year post-transplant proteinuria was a strong predictor of graft loss. The overt proteinuria group had a 8.75-fold higher risk of graft loss (95% CI, 3.29-23.29, p<0.001), and even the minimal proteinuria group had a 4.90-fold higher risk of graft loss than the no proteinuria group after adjustment for the use of mycophenolate mofetil and estimated GFR at the first year in a continuous fashion (95% CI, 2.09-11.46, p<0.001).
Table 4.
Multivariate-adjusted relative risks of graft loss
This model also includes sex, age, donor age, donor type, HLA mismatch number, delayed graft function, acute rejection within one year, systolic blood pressure, post-transplant diabetic mellitus, and use of ACEI/ARB.
*, The reference category was proteinuria of ≤0.2 g/day.
CI, confidence interval; EGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil.
During the follow-up period, three patients died. One patient in the minimal proteinuria group died of small bowel infarction and two in the no proteinuria group died of hepatic failure and anal cancer.
DISCUSSION
The results of this study indicate that even a low level of proteinuria (0.2-0.5 g/day) one year after transplantation is an independent predictor of renal allograft loss. Several studies have implied that proteinuria is a marker of poor long-term allograft outcomes (3, 5, 13, 14). A large, single-centre, retrospective study by Roodnat et al. found that proteinuria (mean 1 g/day) increased both the risk of graft failure and the risk of patient death. Fernandez-Fresnedo et al. (3) showed that persistent proteinuria of more than 0.5 g/day is an independent risk factor for poor allograft outcome and cardiovascular mortality (4, 5). On the basis of these findings, the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative Guidelines, concluded that the use of ACEI or ARB in patients with a spot urine total protein/creatinine ratio of more than 0.5 g/g may have a beneficial effect on graft survival and mortality (6).
Recently, Halimi et al. brought up the risk of graft loss at a lower degree of proteinuria level (15, 16). Primarily, microalbuminuria has been predominantly important not only as a clinical predictor of progressive renal function but also as a target of treatment in diabetic and hypertensive kidney disease for many years (8, 17). It has been suggested that glomerular hypertension and hyperfiltration are key factors in mediating progressive renal damage, because they have been shown to predict the development of microalbuminuria in diabetic and hypertensive kidneys (18, 19). Because renal allograft recipients have a single kidney, it is presumed that they are exposed to hyperfiltration before any clinical sign of chronic allograft nephropathy. Therefore, as seen from our results, it was predictable that proteinuria of 0.2-0.5 g/day is a risk factor for graft loss.
Mechanisms by which proteinuria may contribute to declines in renal function have been recently postulated. Leakage of protein conferred to proximal tubular epithelial cells has been shown to promote progression of renal disease by stimulating cytokines and oxidative stress associated interstitial fibrosis (20). It has also been argued that the allograft is particularly vulnerable to the adverse effect of proteinuria by expressing more major histocompatibility complex II antigen, rendering them potentially susceptible to immune reaction (21).
In our study, three patients died. This low mortality rate might have been due to the relative youthfulness of the recipients and the low proportion of diabetes, and other co morbidities, because cardiovascular disease is the major cause of death in renal transplant recipients (22). Proteinuria is generally accepted as an independent predictor of cardiovascular disease in renal transplantation (4). However, the lower limit of proteinuria related to patient survival has not been determined. We could not assess the association between a low level of proteinuria and patient survival because of the low mortality rate.
Proteinuria of more than 0.5 g/day was found in 7.8% of our study subjects, which is lower than that previously reported (3, 5, 15). Several studies have suggested that post-transplantation proteinuria is the consequence of many factors, including pre-transplant renal lesions, ischemia-reperfusion injury, and immunological aggression (15). Thus, the low prevalence of proteinuria in this study might be attributable to donor and recipient ages and the low incidence of DGF and acute rejection.
Some limitations of our study should be noted. First, our study participants were not representative general renal recipients. Our subjects were relatively young, had a low prevalence of diabetes and other comorbidities. And more than 50% of them spent less than 1 yr on dialysis prior to transplantation. Although these characteristics limit the generalizability of our finding, it suggests that even a small amount of proteinuria should be worth paying attention in kidney transplants with a low risk profile. Second, we used a single 24-hr urine sample to evaluate proteinuria. The disadvantages of 24-hr urine samples are their inconvenience and potential inaccuracy. However, the patients were trained in the proper method for collection in this study, and 24-hr urine collection is still recommended for the definitive measurement of urinary protein excretion in renal recipients (23). Third, it was unclear whether the origin of the proteinuria was glomerular or tubular. These two forms of proteinuria are induced by different mechanisms and thus may have different prognostic significance. An analysis of the correlation between proteinuria and allograft pathology should be conducted to clarify the role of proteinuria (2). Fourth, few patients received ACEI or ARB, so the effects of both drugs could not be evaluated. The use of ACEI and ARB is becoming increasingly common in treating hypertension in renal recipients, but it has been discouraged in the early post-transplantation period, because it may compromise effective allograft blood flow in renal artery stenosis or in recipients with calcineurin-induced vasoconstriction.
In conclusion, we have demonstrated that minimal proteinuria one year after transplantation is associated with poor allograft outcomes as in recent studies. This association has an additive value in terms of being able to apply to the patient groups with low risk profile. Therefore, it seems logical to consider minimal proteinuria as a predictor for graft survival in renal transplant recipients. These findings provide a rationale for prospective studies that aim to verify whether antiproteinuric therapy can prolong graft survival in recipients with minimal proteinuria.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 3.Roodnat JI, Mulder PG, Rischen-Vos J, van Riemsdijk IC, van Gelder T, Zietse R, IJzermans JN, Weimar W. Proteinuria after renal transplantation affects not only graft survival but also patient survival. Transplantation. 2001;72:438–444. doi: 10.1097/00007890-200108150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Fresnedo G, Escallada R, Rodrigo E, De Francisco AL, Cotorruelo JG, Sanz De Castro S, Zubimendi JA, Ruiz JC, Arias M. The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation. 2002;73:1345–1348. doi: 10.1097/00007890-200204270-00028. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Fresnedo G, Plaza JJ, Sánchez-Plumed J, Sanz-Guajardo A, Palomar-Fontanet R, Arias M. Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant. 2004;19(Suppl 3):iii47–iii51. doi: 10.1093/ndt/gfh1015. [DOI] [PubMed] [Google Scholar]
- 6.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–S290. [PubMed] [Google Scholar]
- 7.Heinze G, Mitterbauer C, Regele H, Kramar R, Winkelmayer WC, Curhan GC, Oberbauer R. Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J Am Soc Nephrol. 2006;17:889–899. doi: 10.1681/ASN.2005090955. [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment? Kidney Int Suppl. 2004:S2–S6. doi: 10.1111/j.1523-1755.2004.09201.x. [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Remuzzi G. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int. 2003;63:2254–2261. doi: 10.1046/j.1523-1755.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 10.Sulanc E, Lane JT, Puumala SE, Groggel GC, Wrenshall LE, Stevens RB. New-onset diabetes after kidney transplantation: an application of 2003 International Guidelines. Transplantation. 2005;80:945–952. doi: 10.1097/01.tp.0000176482.63122.03. [DOI] [PubMed] [Google Scholar]
- 11.Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, Gherardi G, Gotti E, Segoloni G, Salvadori M, Rigotti P, Valente U, Donati D, Sandrini S, Sparacino V, Remuzzi G, Perico N. Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant. 2004;4:1826–1835. doi: 10.1111/j.1600-6143.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 12.Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, Hall PM. Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant. 2006;6:100–108. doi: 10.1111/j.1600-6143.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 13.Massy ZA, Guijarro C, Wiederkehr MR, Ma JZ, Kasiske BL. Chronic renal allograft rejection: immunologic and nonimmunologic risk factors. Kidney Int. 1996;49:518–524. doi: 10.1038/ki.1996.74. [DOI] [PubMed] [Google Scholar]
- 14.Hohage H, Kleyer U, Bruckner D, August C, Zidek W, Spieker C. Influence of proteinuria on long-term transplant survival in kidney transplant recipients. Nephron. 1997;75:160–165. doi: 10.1159/000189525. [DOI] [PubMed] [Google Scholar]
- 15.Halimi JM, Laouad I, Buchler M, Al-Najjar A, Chatelet V, Houssaini TS, Nivet H, Lebranchu Y. Early low-grade proteinuria: causes, short-term evolution and long-term consequences in renal transplantation. Am J Transplant. 2005;5:2281–2288. doi: 10.1111/j.1600-6143.2005.01020.x. [DOI] [PubMed] [Google Scholar]
- 16.Halimi JM, Buchler M, Al-Najjar A, Laouad I, Chatelet V, Marliere JF, Nivet H, Lebranchu Y. Urinary albumin excretion and the risk of graft loss and death in proteinuric and non-proteinuric renal transplant recipients. Am J Transplant. 2007;7:618–625. doi: 10.1111/j.1600-6143.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 17.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 18.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–1507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 19.Palatini P, Mormino P, Dorigatti F, Santonastaso M, Mos L, De Toni R, Winnicki M, Dal Follo M, Biasion T, Garavelli G, Pessina AC. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 2006;70:578–584. doi: 10.1038/sj.ki.5001603. [DOI] [PubMed] [Google Scholar]
- 20.Gross ML, Hanke W, Koch A, Ziebart H, Amann K, Ritz E. Intraperitoneal protein injection in the axolotl: the amphibian kidney as a novel model to study tubulointerstitial activation. Kidney Int. 2002;62:51–59. doi: 10.1046/j.1523-1755.2002.00402.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkranz AR, Mayer G. Proteinuria in the transplanted patient. Nephrol Dial Transplant. 2000;15:1290–1292. doi: 10.1093/ndt/15.9.1290. [DOI] [PubMed] [Google Scholar]
- 22.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7:158–165. doi: 10.1681/ASN.V71158. [DOI] [PubMed] [Google Scholar]
- 23.Kasiske BL, Vazquez MA, Harmon WE, Brown RS, Danovitch GM, Gaston RS, Roth D, Scandling JD, Singer GG. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11(Suppl 15):S1–S86. [PubMed] [Google Scholar]