Abstract
Intravenous immunoglobulin (IVIG) and/or plasmapheresis (PP) are effective in preventing antibody-mediated rejection (AMR) of kidney allografts, but AMR is still a problem. This study reports our experience in living donor renal transplantation in highly sensitized patients. Ten patients with positive crossmatch tests or high levels of panel-reactive antibody (PRA) were included. Eight patients were desensitized with pretransplant PP and low dose IVIG, and two were additionally treated with rituximab. Allograft function, number of acute rejection (AR) episodes, protocol biopsy findings, and the presence of donor-specific antibody (DSA) were evaluated. With PP/IVIG, six out of eight patients showed good graft function without AR episodes. Protocol biopsies revealed no evidence of tissue injury or C4d deposits. Of two patients with AR, one was successfully treated with PP/IVIG, but the other lost graft function due to de novo production of DSA. Thereafter, rituximab was added to PP/IVIG in two cases. Rituximab gradually decreased PRA levels and the percentage of peripheral CD20+ cells. DSA was undetectable and protocol biopsy showed no C4d deposits. The graft function was stable and there were no AR episodes. Conclusively, desensitization using PP/IVIG with or without rituximab increases the likelihood of successful living donor renal transplantation in sensitized recipients.
Keywords: Desensitization; Immunoglobulins, Intravenous; Plasmapheresis, Kidney Transplantation; Rituximab
INTRODUCTION
Several desensitization protocols have been developed to prevent antibody-mediated acute rejection (AMR) of kidney allografts, and this has increased the success rate of transplantation in sensitized recipients. However, protocols differ between centers and have different clinical outcomes, and comparisons have been difficult because of differences in patient characteristics, the assays used to define the presence and level of donor-specific antibody (DSA), and the assessment of outcomes (1).
Intravenous immunoglobulin (IVIG) and/or plasmapheresis (PP) are basic desensitization strategies (1-8), and anti-CD20 antibody is also included in some centers (2, 9-13). This study reports our experience of living donor renal transplantation in highly sensitized patients. The basic desensitization protocol included pretransplant PP and low dose IVIG, with the recent addition of anti-CD20 antibody to the basic protocol. Here we discuss the rationale of using PP and low dose IVIG and the clinical significance of anti-CD20 antibody in desensitization.
MATERIALS AND METHODS
Patients
A retrospective review was performed of 10 highly sensitized patients treated with PP/IVIG or PP/IVIG/rituximab prior to living donor renal transplantation between January 2003 and May 2007. Highly sensitized patients were defined as those who had previous or current positive crossmatch tests either by antihuman globulin-enhanced, complement-dependent cytotoxicity (AHG-CDC) or by flow cytometry (FCM), or patients who were retransplants and had PRA levels ≥50%.
Desensitization protocol
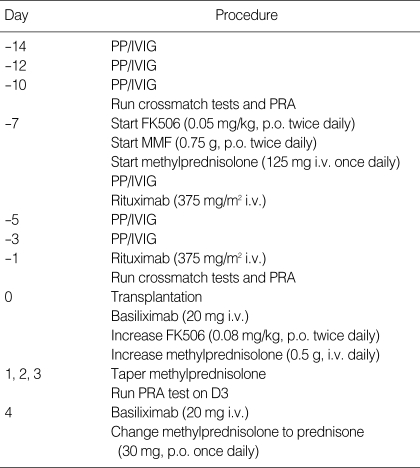
All 10 recipients underwent PP on transplant days -14, -12, -10, -7, -5, and -3. Following each PP, patients received 200 mg/kg per body weight IVIG (Table 1). Two recipients additionally received intravenous rituximab, an anti-CD20 antibody, at 375 mg/m2 body surface area on transplant days -7 and -1. Crossmatch tests and PRA tests were performed on transplant days -9 and -1. Follow-up PRA test was performed 3 days after transplantation. Additional tests were performed if clinically indicated.
Table 1.
Desensitization protocol
PP, plasmapheresis; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; PRA, panel-reactive antibody.
The goal of the desensitization protocol was to achieve a negative crossmatch test (cases 1-8) or a PRA level of <20% and no DSA (cases 9 and 10).
Characterization of alloantibody status and immunophenotyping of lymphocytes
Crossmatch tests were performed using the AHG-CDC assay and FCM. A positive crossmatch by FCM was defined as a displacement of the mean channel fluorescence (MCF) by more than 10 channels relative to a negative control and donor autologous control. Although we did not check median channel shift, we confirmed positive cases by relative median fluorescence (test MCF÷[recipient autologous MCF+donor autologous MCF+healthy autologous MCF]/3) ≥1.5 and test MCF greater than the negative MCF+3SD.
PRA levels were determined by enzyme-linked immunosorbent assays (ELISA) (LAT, One Lambda Inc., CA, U.S.A.). In PRA-positive cases, LAT1240 (LAT Class I and II) was used for the documentation of DSA. Subsequent crossmatch tests and PRA tests were performed after the third and sixth PP/IVIG in all patients (Table 1). In two patients who received rituximab, an antibody monitoring system (AMS, GTI Inc., WI, U.S.A.) was also used before and after desensitization. AMS is a solid-phase ELISA crossmatch test for detecting IgG antibody to the donor-specific solubilized HLA class I and class II antigens. We previously reported that AMS is useful as a supportive crossmatch test or as a monitoring test for detecting class I or II DSA (14). Eight recipients underwent transplantation after achieving a negative crossmatch by both AHG-CDC and FCM and no DSA. Two patients who received rituximab underwent transplantation after achieving PRA levels <20% and no DSA confirmed by PRA and AMS.
In patients who received rituximab, venous blood samples for lymphocyte immunophenotyping were collected in EDTA tubes. The percentages of CD4+, CD8+, and CD19+ lymphocytes were assessed by FCM before and after infusion of rituximab.
Immunosuppression
Maintenance immunosuppression consisting of tacrolimus (FK506), mycophenolate mofetil (MMF) and prednisone was started seven days prior to transplantation (Table 1). The initial dose of FK506 was 0.1 mg/kg per day by the oral route, which was increased to 0.16 mg/kg per day after transplantation. The target trough levels were 10-15 ng/mL in the first three months and 5-10 ng/mL thereafter. The initial dose of MMF was 1.5 g/day, and the dose was reduced in response to adverse effects such as diarrhea or leucopenia. Methylprednisolone was administered by intravenous infusion (125 mg/day) for seven days prior to transplant, increased to 1 g/day on transplant day 0 then tapered to an oral dose of 30 mg/day prednisone on posttransplant day 4. For induction therapy, basiliximab was administered intravenously (20 mg) on transplant days 0 and +4.
Renal allograft biopsies
A surveillance (protocol) biopsy was routinely performed on stable allografts on day 14 after transplantation. Stable graft function was defined as less than 20% increase in serum creatinine concentration in the three days before biopsy and no increase in the dose of immunosuppressive drugs. An indication biopsy was performed in cases of allografts that showed greater than 20% increase in serum creatinine concentration. An 18-gauge biopsy gun was used after ultrasonic localization of the allograft. The minimum criteria for tissue adequacy were six or more glomeruli and at least one arterial cross-section. Indirect immunofluorescence (IF) staining was performed using monoclonal antibodies against complement protein C4d (Biogenesis, Poole, U.K.; dilution 1:50) for detecting C4d deposition. C4d positivity was defined as diffuse (>50%) and linear staining of peritubular capillaries (PTC). Histopathological diagnosis was made according to the revised Banff 2001 criteria (15).
Clinical data and statistics
The following data were analyzed: 1) demographic and immunologic characteristics of patients, 2) effect of desensitization, 3) posttransplant allograft function and DSA, 4) histological findings of allograft biopsies, 5) rate of acute rejection (AR) and allograft loss, and 6) complications related to transplantation. Descriptive values were presented as mean±SD or median and range.
RESULTS
Patient demographics and immunologic status
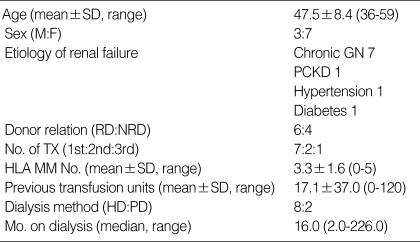
Table 2 shows the demographic characteristics of 10 highly sensitized patients who underwent desensitization during the study period. All patients were ABO-compatible. Three patients were retransplants, nine had previous transfusions, and five had more than three HLA mismatches. Four female recipients had previous pregnancy history.
Table 2.
Demographics
M, male; F, female; Chronic GN, chronic glomerulonephritis; PCKD, polycystic kidney disease; RD, related donor; NRD, nonrelated donor, TX, renal transplantation; HLA MM No., number of HLA mismatches; HD, hemodialysis; PD, peritoneal dialysis; Mo., months
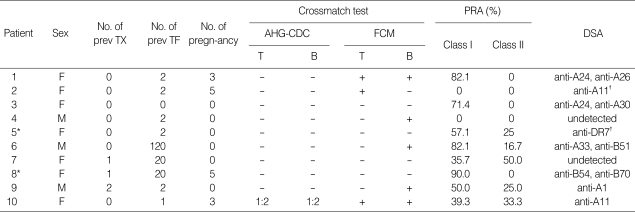
The results of crossmatch tests and PRA levels just before desensitization are listed in Table 3. Six patients were crossmatch-positive, and four patients were crossmatch-negative but had high PRA levels (≥50%). Of the crossmatch-negative patients, three had previous or current DSA detected by PRA and two were retransplants.
Table 3.
Immunologic characteristics before initiation of desensitization protocol
*Patients 5 and 8 were previously crossmatch-positive by FCM for both T cells and B cells; †Previous DSA detected by PRA.
M, male; F, female; Prev TX, previous transplantation; Prev TF, previous transfusion; AHG-CDC, antihuman globulin-enhanced complement-dependent cytotoxicity; FCM, flow-cytometry; PRA, panel-reactive antibody; DSA, donor-specific antibody.
Effect of desensitization
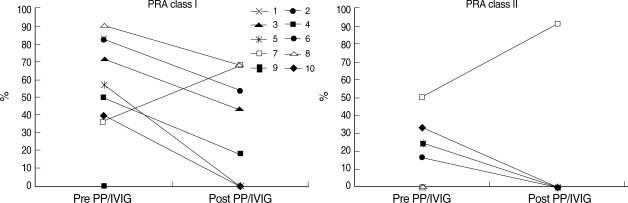
All 10 patients completed the desensitization protocol and were able to undergo transplantation. Of six patients who were crossmatch-positive, four patients became crossmatch-negative by both AHG-CDC and FCM after six PP/IVIG treatments. In three patients with negative crossmatch tests, previous or current DSA detected by PRA became undetectable after desensitization. In one patient, PRA levels increased after PP/IVIG, but the crossmatch test was negative and DSA was consistently undetectable by PRA. Of the eight patients who had pretransplant PRA levels ≥20%, seven showed a decrease in PRA levels. The mean decreases in class I and class II PRA levels were 61.5% and 100%, respectively (Fig. 1).
Fig. 1.
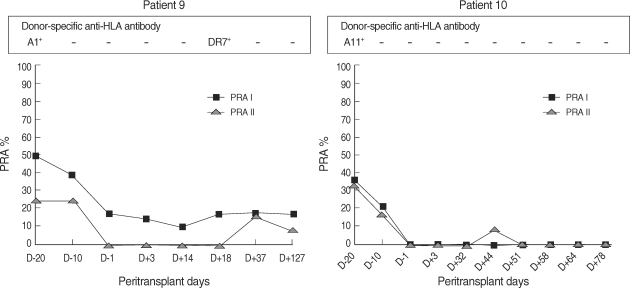
Change in PRA before and after PP/IVIG treatment. Of eight patients who had pretransplant PRA levels ≥20%, seven showed a decrease in PRA levels. The mean decreases in class I and class II PRA levels were 61.5% and 100%, respectively.
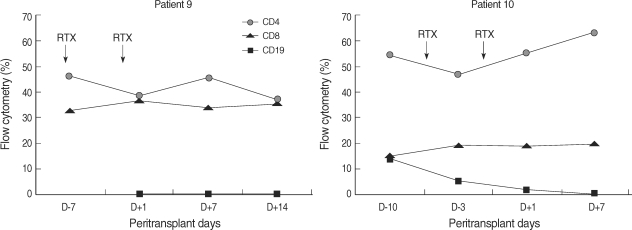
Two patients did not achieve negative crossmatch tests because they were treated with rituximab, which can interfere with standard crossmatch tests (16). Instead, their alloantibody status was monitored with PRA and AMS. In case 9, class I PRA level decreased by 64.2%, class II PRA level decreased by 100%, and DSA (anti-A1) became undetectable. In case 10, both class I and class II PRA levels decreased by 100%, and DSA (anti-A11 and anti-A33) became undetectable (Fig. 1, 2). In both patients, crossmatch by AMS was positive in T cells before desensitization, and became negative after desensitization. As shown in figure 3, peripheral CD19+ cells, which represent the pool of B cells, were selectively depleted after infusion of rituximab. In contrast, CD4+ or CD8+ cells were not affected.
Fig. 2.
Change in PRA and DSA in patients who received rituximab. In patient 9, the level of class I PRA decreased by 64.2%, the level of class II PRA decreased by 100%, and DSA (anti-A1) became undetectable. In patient 10, both class I and class II PRA levels decreased by 100%, and DSA (anti-A11, anti-A33) became undetectable. The reduction in PRA levels lasted for 2-4 months in both patients.
Fig. 3.
Change in peripheral blood CD19 cells in patients who received rituximab. After infusion of rituximab (RTX), CD19+ cells in the peripheral blood were selectively depleted, while CD4+ or CD8+ cells were not affected.
Posttransplant allograft function, DSA, and subsequent treatments
Seven patients showed immediate recovery of graft function (IGF), demonstrating active diuresis and rapid falls in serum creatinine concentration. Two patients showed slow recovery of graft function (SGF) without the need for dialysis, but did not reach normal serum creatinine concentration (1.2 mg/dL). One patient (case 8) showed IGF up to posttransplant day 6, when abrupt oliguria developed. Subsequent PP/IVIG and methylprednisolone therapy was needed in two patients: patient 5 was treated for seven days postoperatively, and patient 8 was treated for one day but lost graft function on posttransplant day 7. Posttransplant DSA monitoring revealed no reemergence of DSA except in one patient (case 8).
Histological findings of allograft biopsies
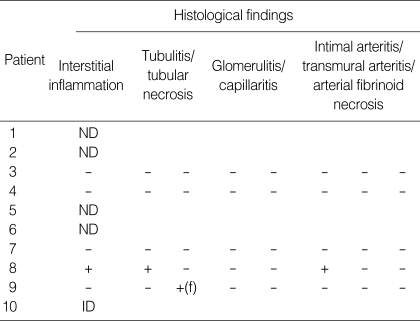
Of seven patients showing IGF, four underwent protocol biopsies on posttransplant day 14. The histological findings were nonspecific (Table 4), and no diffuse deposition of C4d was found along the PTC. An indication biopsy was performed in one patient (case 10) showing SGF on posttransplant day 10; however, the tissue specimen was inadequate for diagnosis. For the patient who underwent graft nephrectomy (case 8), histological analysis of the allograft showed acute cellular rejection grade IIB and thrombotic microangiopathy with no C4d deposits. In the other four patients, biopsies were not performed because of thrombocytopenia (n=2) or hematoma around the allograft (n=2).
Table 4.
Histological findings in allografts
ND, biopsy not done; f, focal; ID, inadequate for diagnosis.
Acute rejection and graft survival
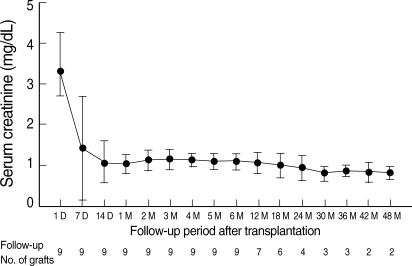
There were two AR episodes in the early posttransplantation period. One (patient 5) presented as a slow decline in urine output on posttransplant day 4 with no circulating DSA, and was crossmatch-negative either by AHG-CDC or FCM. The graft function improved with subsequent PP/IVIG and methylprednisolone therapy. An indication biopsy was not performed because of thrombocytopenia. The other (patient 8) presented as accelerated acute rejection on posttransplant day 6 with circulating DSA (anti-A2 and anti-B45). It resulted in graft failure despite subsequent PP/IVIG and methylprednisolone treatment. Graft nephrectomy was performed as signs of disseminated intravascular coagulation occurred before trial of anti-thymoglobulin or OKT3. Nine patients are currently dialysis-free after a median follow-up of 22.6 months (range: 6.9-52.0), and there have been no more AR episodes. The most recent serum creatinine concentration is 1.0±0.2 mg/dL (range: 0.7-1.4, Fig. 4). The patient survival rate is 100%.
Fig. 4.
Change in mean serum creatinine concentration in functioning allografts. Nine patients are currently dialysis free after a median follow-up of 22.6 months (range: 6.9-52.0), and there have been no more AR episodes. The latest serum creatinine concentration is 1.0±0.2 mg/dL (range: 0.7-1.4).
Non-rejection complications
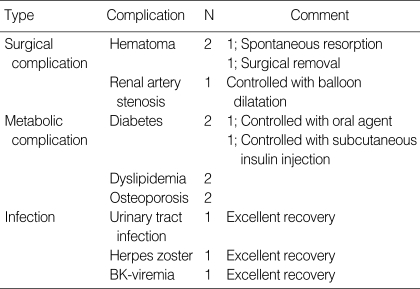
There was no procedure-related side effect of PP or infusion-related side effect of rituximab. The non-rejection complications associated with transplantation or immunosuppression are listed in Table 5. One patient developed BK-viremia and BK-viruria 6 months after transplantation. There was no graft dysfunction, and viremia was controlled after reducing the dose of FK506. No patient developed CMV infection.
Table 5.
Non-rejection complications directly related to transplantation and immunosuppression
DISCUSSION
The desensitization strategy at our center showed an excellent clinical outcome compared to other reports (3, 4, 8). The AR rate was 20% and graft loss rate was 10%. All functioning allografts have maintained stable graft function during follow-up. This finding demonstrates that the desensitization strategy is safe and effective in achieving successful transplantation in sensitized patients.
There are several explanations for the excellent outcomes in our center. First, we included patients with previously positive crossmatch tests or retransplants with PRA levels ≥50%, as well as currently crossmatch-positive patients. Second, our patients had low initial titers of DSA for T cells, and 30% of patients were crossmatch-positive only for B cells. Third, all patients were transplants from living donors, and 50% were haploidentical for HLA. Fourth, protocol biopsies and regular monitoring of PRA and DSA with AMS provided more precise information about the status of allografts. One may argue that patients with previously positive crossmatch tests (patients 5 and 8) and retransplants with PRA levels ≥50% (patient 7) may not need desensitization treatment before transplantation. However, these patients often show high alloreactivity after transplantation (17) and result in severe AMR and graft loss (18). Therefore we performed desensitization treatment. With regard to two patients with only B positive FCM crossmatch, we also performed desensitization treatment considering high risk of rejection and graft loss (19, 20).
There is no standardized protocol for desensitization strategy, but basic principle is elimination of preformed DSA, inhibition of production of de novo DSA and strong maintenance immunosuppression considering interaction between T and B cells. We used six treatments of PP based on previous reports that a minimum of four to a maximum of six cycles of pretransplant PP were sufficient to remove preformed DSA (1, 2, 7, 12). With respect to IVIG, there has been no randomized controlled trial comparing high dose IVIG and PP/low dose IVIG. Protocols using IVIG are usually divided into two groups, high dose IVIG (3-7 doses of 2 g/kg) (2, 3, 6, 8) and low dose IVIG (3-8 doses of 100 mg/kg) with PP (1, 2, 5, 7, 10, 12). We used low dose IVIG (6 doses of 200 mg/kg) for consecutive sessions, because of the cost-effectiveness of IVIG and the speculation that multiple doses of IVIG result in prolonged modulation of DSA. The elimination rate of DSA in our group was 100%, which shows that PP and low dose IVIG is effective in eliminating DSA. Maintenance immunosuppressuion using FK506 and MMF was started 1 week prior to transplantation to avoid overimmunosuppression (21). The high DSA-elimination rate may explain the high success rate and low AR rate in our patients.
We included rituximab after experiencing graft loss in a previously crossmatch-positive patient (case 8). The patient showed good graft function in the early posttransplant period, but lost graft function due to accelerated acute rejection on day 7. At that time, the crossmatch test was positive and DSA was documented by PRA. This finding provided the importance of de no production of DSA after transplantation, and led to the utilization of rituximab, a chimeric anti-CD20 monoclonal antibody, to prevent de novo production of alloantibodies by memory B cells. In our study, we used two doses of rituximab with one week interval (one week before and one day before transplantation), and each dose was 375 mg/m2. Until now, there is controversy regarding the time course and dose of rituximab for preventing AMR. The validity of using rituximab 1 week before transplantation is that action of rituximab on the peripheral CD20+ cells is rapid (Fig. 3) and the half-life of rituximab is 9-14 days in patients with end-stage renal disease (22). Single dose of rituximab may be sufficient to prevent the production of alloantibodies (23). However, recent reports show that single dose of rituximab is not enough to prevent AMR (24), and this unsatisfactory result may be related to the incomplete elimination of residual B cells in solid lymph organs (22, 24, 25). Therefore, it seems reasonable to use two doses of rituximab before transplantation.
There were two AR episodes in the early posttransplantation period. One presented as a slow decline in urine output on posttransplant day 4 with no circulating DSA, and was crossmatch-negative either by AHG-CDC or FCM. The graft function improved with subsequent PP/IVIG and methylprednisolone therapy (patient 5). An indication biopsy was not performed because of thrombocytopenia. The other presented as accelerated acute rejection on posttransplant day 6 with circulating DSA (anti-A2 and anti-B45). We treated this patient with PP/IVIG and methylprednisolone treatment but it failed to reverse AMR (patient 8). Graft nephrectomy was performed as signs of disseminated intravascular coagulation occurred before trial of anti-thymoglobulin or OKT3. This finding suggests that early posttransplant period (one week after transplantation) is important to determine successful transplantation in sensitized patients.
In addition to low AR rate, long-term graft and patient survival rates were excellent in our patients. Nine patients (90%) are currently dialysis-free after a median follow-up of 22.6 months (range: 6.9-52.0), and there have been no more AR episodes. The most recent serum creatinine concentration is 1.0±0.2 mg/dL (range: 0.7-1.4, Fig. 4), and the patient survival rate is 100%. This finding suggests that graft function can be maintained for a long time if sensitization treatment is successful, as shown in ABO-incompatible transplantation.
Detection of DSA is an important factor for determining desensitization treatment. We recommended using rituximab, but it may cause false positivity in cell-based crossmatch tests because of its action mechanisms such as complement activation and binding of Fc portion (16). Therefore, solid-phase crossmatch tests are needed in these patients. Indeed, two patients with rituximab treatment were crossmatch-positive both by AHG-CDC and FCM but negative by AMS, a solid phase ELISA-based crossmatch test. Therefore, we recommend setting up a solid-phase crossmatch before using rituximab (10, 16).
The use of protocol surveillance biopsies in our study allows a more precise assessment of allograft function and early detection of the histological changes indicating AMR. Gloor and colleagues (10) suggested that surveillance biopsies might be an important adjunct for monitoring allograft function in sensitized patients. Our transplant center has performed routine protocol biopsies for 14 years in patients with stable graft function on day 14 after transplantation. We previously reported that a protocol biopsy performed in the early posttransplantation period was useful for detecting subclinical rejection and predicting graft survival (26). To detect the presence of subclinical AMR, we performed C4d IF staining in protocol biopsies. Table 4 shows that all patients who had a protocol biopsy had minimal tissue injury and no deposits of C4d. Although the prognosis of C4d positivity in protocol biopsies with stable graft function is still undetermined, our results suggest that the desensitization was successful in preventing subclinical AMR in these patients.
There was no procedure-related side effect of PP or infusion-related side effect of rituximab. One patient developed BK-viremia and viruria 6 months after transplantation. There was no graft dysfunction, and it disappeared after reducing the dose of FK506. No patient developed CMV infection. Thrombocytopenia developed in two patients. But one patient already had thrombocytopenia before desensitization therapy, and the other developed thrombocytopenia during AR. Hematoma was observed in two patients, but it was related to the surgical procedure (simultaneous bilateral nephrectomy and friable vessels) rather than the complication of desensitization treatment. Therefore our desensitization protocol was tolerable and safe.
In summary, the desensitization protocol should both eliminate DSA and prevent de novo production of alloantibodies. It is clear that a desensitization strategy using PP/IVIG with or without rituximab increases the likelihood of successful living donor renal transplantation in highly sensitized recipients.
Footnotes
This work was supported by the Korea Science and Engineering Foundation (R13-2002-005-03001-0) through the Medical Science Research Center for Cell Death Disease Research Center at The Catholic University of Korea.
References
- 1.Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 2.Stegall MD, Gloor J, Winters JL, Moore SB, DeGoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346–351. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 3.Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, Hacen C, Duboust A, Bariety J. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg) Am J Transplant. 2002;2:758–760. doi: 10.1034/j.1600-6143.2002.20809.x. [DOI] [PubMed] [Google Scholar]
- 4.Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006;6:459–466. doi: 10.1111/j.1600-6143.2005.01214.x. [DOI] [PubMed] [Google Scholar]
- 5.Zachary AA, Montgomery RA, Ratner LE, Samaniego-Picota M, Haas M, Kopchaliiska D, Leffell MS. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003;76:1519–1525. doi: 10.1097/01.TP.0000090868.88895.E0. [DOI] [PubMed] [Google Scholar]
- 6.Vo AA, Toyoda M, Peng A, Bunnapradist S, Lukovsky M, Jordan SC. Effect of induction therapy protocols on transplant outcomes in crossmatch positive renal allograft recipients desensitized with IVIG. Am J Transplant. 2006;6:2384–2390. doi: 10.1111/j.1600-6143.2006.01472.x. [DOI] [PubMed] [Google Scholar]
- 7.Schweitzer EJ, Wilson JS, Fernandez-Vina M, Fox M, Gutierrez M, Wiland A, Hunter J, Farney A, Philosophe B, Colonna J, Jarrell BE, Bartlett ST. A high panel-reactive antibody rescue protocol for crossmatch-positive live donor kidney transplants. Transplantation. 2000;70:1531–1536. doi: 10.1097/00007890-200011270-00023. [DOI] [PubMed] [Google Scholar]
- 8.Jordan SC, Tyan D, Stablein D, Mcintosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 9.Magee CC, Mah H, Tinckam K, Wood I, Ji F, Powelson J. Successful living donor kidney transplantation across HLA and ABO incompatibilities. Nephrol Dial Transplant. 2007;22:602–604. doi: 10.1093/ndt/gfl696. [DOI] [PubMed] [Google Scholar]
- 10.Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, Larson TS, Griffen MD, Textor SC, Velosa JA, Schwab TR, Fix LA, Stegall MD. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003;3:1017–1023. doi: 10.1034/j.1600-6143.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 11.Sawada T, Fuchinoue S, Teraoka S. Successful A1-to-O ABO-incompatible kidney transplantation after a preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy, and double-filtration plasmapheresis. Transplantation. 2002;74:1207–1210. doi: 10.1097/00007890-200211150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, Simpkins CE, Montgomery RA. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315–1322. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 13.Tydén G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145–148. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang CW, Oh EJ, Lee SB, Moon IS, Kim DG, Choi BS, Park SC, Choi YJ, Park YJ, Han K. Detection of donor-specific anti-HLA class I and II antibodies using antibody monitoring system. Transplant Proc. 2006;38:2803–2806. doi: 10.1016/j.transproceed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria-an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 16.Book BK, Agarwal A, Milgrom AB, Bearden CM, Sidner RA, Higgins NG, Pescovitz MD. New crossmatch technique eliminates interference by humanized and chimeric monoclonal antibodies. Transplant Proc. 2005;37:640–642. doi: 10.1016/j.transproceed.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 17.Opelz G. Collaborative transplant study-10-year report. Transplat Proc. 1992;24:2342–2355. [PubMed] [Google Scholar]
- 18.Montgomery RA, Zachary AA. Transplanting patients with a positive donor-specific crossmatch: A single center's perspective. Pediatr Transplantation. 2004;8:535–542. doi: 10.1111/j.1399-3046.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 19.Kotb M, Russell WC, Hathaway DK, Gaber LW, Gaber AO. The use of positive B cell flow cytometry crossmatch in predicting rejection among renal transplant recipients. Clin Transplant. 1999;13:83–89. doi: 10.1034/j.1399-0012.1999.130104.x. [DOI] [PubMed] [Google Scholar]
- 20.Lazda VA. Identification of patients at risks for inferior renal allograft outcome by a strongly positive B cell flow cytometry crossmatch. Transplantation. 1994;57:964–969. doi: 10.1097/00007890-199403270-00033. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007;84:S4–S7. doi: 10.1097/01.tp.0000296008.08452.4c. [DOI] [PubMed] [Google Scholar]
- 22.Vieira CA, Agarwal A, Book BK, Sidner RA, Bearden CM, Gebel HM, Roggero AL, Fineberg NS, Taber T, Kraus MA, Pescovitz MD. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004;77:542–548. doi: 10.1097/01.tp.0000112934.12622.2b. [DOI] [PubMed] [Google Scholar]
- 23.Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007;84:S33–S36. doi: 10.1097/01.tp.0000296122.19026.0f. [DOI] [PubMed] [Google Scholar]
- 24.Matignon M, Tagnaouti M, Audard V, Dahan K, Lang P, Grimbert P. Failure of anti-CD20 monoclonal antibody therapy to prevent antibody-mediated rejection in three crossmatch-positive renal transplant recipients. Transplant Proc. 2007;39:2565–2567. doi: 10.1016/j.transproceed.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Beimler JH, Susal C, Zeier M. Desensitization strategies enabling successful renal transplantation in highly sensitized patients. Clin Transplant. 2006;20 Suppl 17:7–12. doi: 10.1111/j.1399-0012.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi BS, Shin MJ, Shin SJ, Kim YS, Choi YJ, Kim YS, Moon IS, Kim SY, Koh YB, Bang BK, Yang CW. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005;5:1354–1360. doi: 10.1111/j.1600-6143.2005.00830.x. [DOI] [PubMed] [Google Scholar]