Abstract
Reactive oxygen species have been known to be an important factor in the pathogenesis of hypertension. Bilirubin, one of the metabolites of heme degraded by heme oxygenase, is a potent anti-oxidant. We verified the effect of serum bilirubin level on the incidence of hypertension in normotensive subjects. We grouped 1,208 normotensive subjects by the criterion of the highest quintile value of serum bilirubin, 1.1 mg/dL. The incidence of hypertension was higher in group 1 with bilirubin less than 1.1 mg/dL than in group 2 with bilirubin 1.1 mg/dL or more (186/908 vs. 43/300, p=0.018). The relative risk for hypertension was 0.71 (95% confidence interval, 0.51-0.99), p=0.048 in group 2 compared to group 1 by Cox's proportional hazard model. Among the groups stratified by gender, smoking, and liver function status, the group 2 showed a lower risk of hypertension in females and in non-smokers. In conclusion, a mild increase within the physiological range of serum bilirubin concentration was negatively correlated with the incidence of hypertension. The effect of bilirubin on the development of hypertension was more evident in females and in non-smokers.
Keywords: Hypertension, Hyperbilirubinemia, Risk Factor
INTRODUCTION
Heme oxygenase (HO) is the rate limiting enzyme for the breakdown of heme to generate carbon monoxide, iron, and biliverdin. Biliverdin is rapidly converted to bilirubin by biliverdin reductase (1). Experimental evidence suggests that the induction of HO-1, the inducible isoform of HO, is an important endogenous mechanism for cytoprotection and the downstream products of heme degradation may mediate the beneficial effects, such as antioxidant, anti-inflammatory properties, etc (1). Induction of HO-1 has also been demonstrated to lower blood pressure in several animal models, including the spontaneous hypertensive rat (SHR) and experimental renovascular hypertension, as well as in angiotensin II-dependent hypertension (2-10). However, the mechanism by which HO-1 induction lowers blood pressure has not been fully verified. One possible mechanisms is decreasing vascular resistance by the HO-driven carbon monoxide (8, 9, 11). HO-1 also plays a crucial role in significant attenuation of angiotensin II-medicated tubulointerstitial injury and salt-resistant hypertension (6). An additional possible anti-hypertensive process linked to the induction of HO-1 is the antioxidant activity of heme degradation products. Reactive oxygen species (ROS) have been known to be an important factor in the pathogenesis of hypertension (12). The increased ROS production in the renal medulla is a key component of angiotensin II-dependent hypertension (13). Renal medullary superoxide production generated by angiotensin II infusion to the animal model is normalized with decreased blood pressure by pretreatment with HO-1 inducer (7). The major mechanism by which HO-1 induction reduces ROS is through the generation of bilirubin, a potent antioxidant (14). Increased levels of bilirubin can prevent the pressor actions of angiotensin II through the scavenging of superoxide anions in the vasculature (15) and also inhibit NADPH oxidase (16) and protein kinase C activity (17), both of which mediate angiotensin II-induced vascular injury (18).
In humans, the effects of mildly increased serum bilirubin levels have been reported to be a decreased risk for the development of coronary artery disease and atherosclerosis (19). However, few human studies have demonstrated the relationship between serum bilirubin level and the development of hypertension. In one study with a small subject sample, serum bilirubin level was lower in 34 untreated hypertensive subjects compared to 272 normotensive and 89 treated hypertensive subjects (20). The bilirubin level was not different between the normotensive and treated hypertensive subjects (20).
In the present study, we investigated the relationship between serum bilirubin level and the incidence of hypertension in normotensive subjects who had undergone repeated routine health check-ups. We also analyzed the data in subgroups stratified with possible important confounding factors affecting serum bilirubin levels, such as gender, hepatic functional status, and status of smoking (21).
MATERIALS AND METHODS
Study subjects
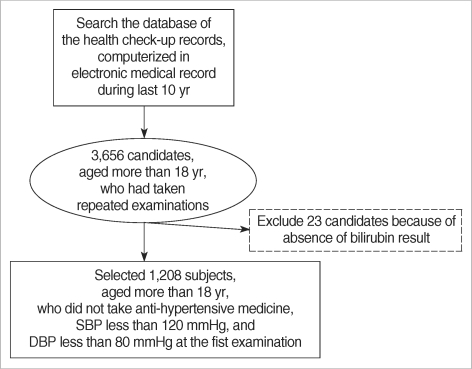
The institutional review board had approved this study. The 3, 668 study candidates aged 18 yr or more had undergone routine health check-ups twice or more during the 10 yr period from September 1995 to August 2005 in the outpatient clinic at the health promotion center of Seoul National University Hospital, Seoul, Korea. Among them, we selected 1, 208 subjects with systolic blood pressure less than 120 mmHg and diastolic blood pressure less than 80 mmHg who had not taken any anti-hypertensive medicine on the first health check-up (Fig. 1). The selected subjects had undergone health check-ups a mode of 3 times (range: 2-12 times) at an interval of 8 months (range 4-51 months) during 129 months.
Fig. 1.
Selection of study subjects.
Clinical data
The subjects filled up the questionnaires by themselves about their smoking habit, amount, intake habit, and history of medical diseases, such as hypertension and diabetes mellitus. Blood samples for laboratory tests were taken after 12 hr fasting. The following information was obtained from electronic medical records as well as the questionnaire items: height, weight, age, sex, history of diabetes mellitus, medication for diabetes mellitus, history of hypertension, antihypertensive medication, blood pressure, serum total bilirubin, alanine transaminase (ALT), aspartate aminotransferase (AST), anti-hepatitis B virus surface antigen, anti-hepatitis B virus surface antibody, anti-hepatitis C virus antibody, serum glucose, serum albumin, serum uric acid, serum cholesterol, serum triglyceride, serum high density lipoprotein (HDL) cholesterol, C-reactive protein, serum creatinine, and the findings of urinalysis by dipstick and microscopy. Medical history and blood pressure were also taken and physical examination was performed by physicians at the health promotion center. The blood pressure was measured once in the sitting position after 5 min resting using a mercury sphygmomanometer. The systolic pressure was measured when the first sound was heard at the beginning of phase I of Korotkoff sound. The diastolic pressure was measured from the moment the sound could not be heard at the end of phase IV of Korotkoff sound. In some case, such as subjects with aortic regurgitation, the muffling of phase IV Korotkoff sound continued at the lowest level. In that case, we took the diastolic blood pressure at the beginning of phase IV of Korotkoff sound.
We defined newly developed hypertension as systolic blood pressure 140 mmHg or more, diastolic blood pressure 90 mmHg or more, or newly taken anti-hypertensive medicine. We also defined the proteinuria as 2+ or more by the urinary dipstick test and hematuria as 5 or more RBCs in the spun urine sample observed by light microscopy at 400-times magnification. We defined liver dysfunction as ALT 40 U/L or more or AST 40 U/L or more.
Statistical analysis
The SPSS (SPSS version 12.0, Chicago, IL, U.S.A.) package was used for statistical analysis. The quintile values of serum bilirubin in the 1, 208 subjects were 0.6 mg/dL, 0.8 mg/dL, 1.0 mg/dL, and 1.1 mg/dL and we grouped the subjects according to the highest quintile value of serum bilirubin 1.1 mg/dL. Differences in proportions among the subject groups were compared by the Pearson's chi-square test. Group differences for continuous variables were assessed by the Student t-test. We defined the event period as the duration from the date of the first health check-up to the date when subjects had become hypertensive during follow-up health check-up (s). We compared the cumulative incidence of hypertension between the bilirubin groups by Log-rank test. To determine whether the bilirubin category was associated independently with the incidence of hypertension, we used Cox's proportional hazard model adjusted for serum albumin and univariate risk factors for the development of hypertension. We checked the violation of the proportional hazard assumption by partial residual plot against time for each covariate and log minus log plots (LML) for categorical covariates, such as bilirubin group. The average values of residuals for covariates included in Cox's model were zero and the LML for each categorical variable was parallel. Two-sided p values are reported, with 0.05 taken as the level of statistical significance. All data are shown as mean±standard deviation or frequency per observation.
RESULTS
Subject characteristics at initial examination
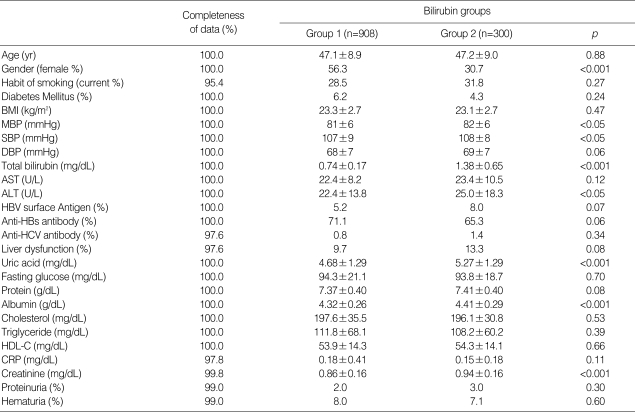
A total of 605 males and 603 females, aged 47.1±9.0 yr, were enrolled. Group 1 included 908 subjects and group 2 included 300 subjects. The frequency of females was higher in group 1 than in group 2. In group 1, the levels of mean blood pressure, systolic blood pressure, serum ALT, serum uric acid, serum albumin and serum creatinine were lower than in group 2 (Table 1). The differences of these clinical parameters between the two groups were mainly due to the gender discrepancy between the groups, as confirmed by the absence of these differences after the subjects were stratified by gender. The current smoking rate of the subjects was not different between the groups. There were no differences in the presence of hepatitis B virus surface antigenemia and anti-hepatitis C virus antibody. The frequency of liver dysfunction was slightly, but not significantly, higher in group 2 than in group 1.
Table 1.
The baseline characteristics of subjects
Group 1, subjects with bilirubin less than 1.1 mg/dL at initial examination; Group 2, subjects with bilirubin 1.1 mg/dL or more at initial examination; BMI, body mass index. MBP, mean arterial blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, Alanine transaminase; AST, Aspartate aminotransferase; HBV surface antigen, hepatitis B virus surface antigen; Anti-HBs antibody, anti-hepatitis B virus surface antigen antibody; Anti-HCV antibody, Anti-hepatitis C virus antibody; liver dysfunction, subjects with ALT 40 U/L or more or AST 40 U/L or more; HDL-C, high density lipoprotein cholesterol; proteinuria, urine protein 2+ or more by dipstick test; hematuria, urine RBC 5 or more/HPF by microscopic examination (×400).
The incidence of hypertension
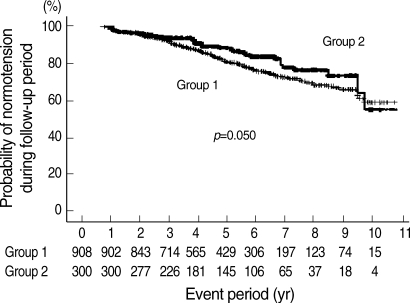
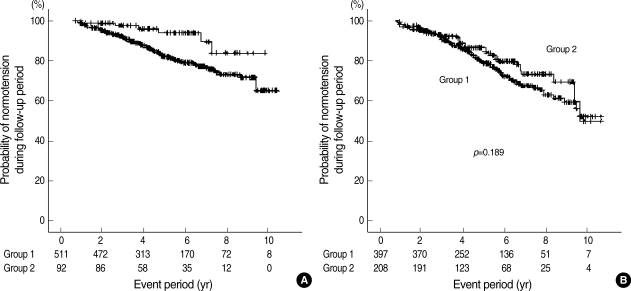
During health check-ups, hypertension was developed in 229 out of 1, 208 subjects (19.0%). The incidence of hypertension was significantly higher in group 1 than in group 2 (186/908 vs. 43/300, p=0.018). Kaplan-Meier analysis also showed that the cumulative incidence of hypertension was higher in group 1 than in group 2 (Fig. 2).
Fig. 2.
The probability of normotension in subjects. Group 1, subjects with initial bilirubin less than 1.1 mg/dL; Group 2, subjects with initial bilirubin 1.1 mg/dL or more. Number under the graph, subjects' number at the given period.
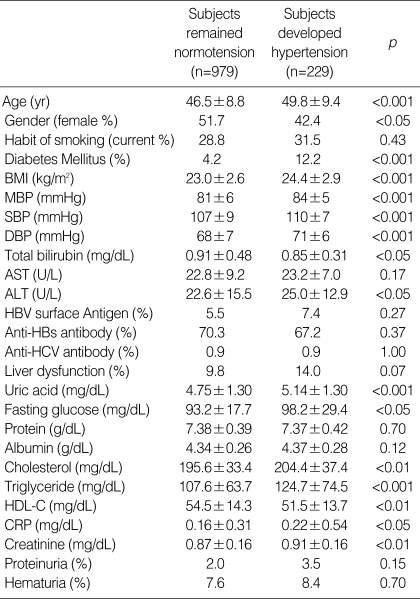
The subgroup which developed hypertension showed higher frequencies of male and diabetes mellitus. The initial values of age, mean arterial blood pressure, systolic blood pressure, diastolic blood pressure, serum creatinine, fasting serum glucose, serum uric acid, serum cholesterol, serum triglyceride, C-reactive protein (CRP), and AST were higher, while the values of HDL cholesterol, and serum bilirubin were lower in subjects who developed hypertension than in the normotensive subjects (Table 2). Biliurbin group 2 had a lower risk to develop hypertension than group 1 after partial or full adjustment for serum albumin and univariate factors. The relative risk (RR) for the development of hypertension was 29% lower in group 2 than in group 1 (Table 3). The other risk factors to the development of hypertension were older age (yr) (RR, 1.03 [95% confidence interval (CI), 1.01-1.04], p<0.001), presence of diabetes mellitus (RR, 1.99 [95% CI, 1.31-3.02], p=0.001), higher body mass index (BMI, kg/m2) (RR, 1.11 [95% CI, 1.06-1.16], p<0.001), and higher level of systolic blood pressure (mmHg) (RR, 1.05 [95% CI, 1.02-1.07], p<0.001).
Table 2.
The univariate risk factors to the development of hypertension during follow-up examination
Group 1; subjects with bilirubin less than 1.1 mg/dL at initial examination; Group 2; subjects with bilirubin 1.1 mg/dL or more at initial examination. BMI, body mass index; MBP, mean arterial blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, Alanine transaminase; AST, Aspartate aminotransferase; HBV surface antigen, hepatitis B virus surface antigen; Anti-HBs antibody, anti-hepatitis B virus surface antigen antibody; Anti-HCV antibody, Anti-hepatitis C virus antibody; liver dysfunction, subjects with ALT 40 U/L or more or AST 40 U/L or more; HDL-C, high density lipoprotein cholesterol; proteinuria, urine protein 2+ or more by dipstick test; hematuria, urine RBC 5 or more/HPF by microscopic examination (×400).
Table 3.
The Cox's hazard proportional analysis for the risk factors to the development of hypertension during follow-up examination
*Partially adjusted with age, gender, liver dysfunction, and serum albumin; †Fully adjusted with age, gender, diabetes mellitus, BMI, systolic blood pressure, diastolic blood pressure, serum uric acid, serum cholesterol, serum HDL cholesterol, serum triglyceride, CRP, serum creatinine, Bilirubin Group 1: subjects with bilirubin less than 1.1 mg/dL at initial examination, Bilirubin Group 2: subjects with bilirubin 1.1 mg/dL or more at initial examination.
Incidence of hypertension in the stratified subgroups
The difference of cumulative incidence of hypertension according to bilirubin level was significant in the female group but not in the male group (Fig. 3). In females, RR for the development of hypertension was 0.39 (95% CI, 0.17-0.91, p=0.028) in bilirubin group 2 compared to bilirubin group 1 by Cox's proportional hazard model adjusted for serum albumin and independent risk factors of age, body mass index, systolic blood pressure, history of diabetes mellitus, and serum uric acid level.
Fig. 3.
The probability of normotension according to gender. Group 1, subjects with initial bilirubin less than 1.1 mg/dL; Group 2, subjects with initial bilirubin 1.1 mg/dL or more. (A) Kaplan-Meier analysis in female subjects. (B) Kaplan-Meier analysis in male subjects. Number under the graph: subjects' number at the given period.
In the subjects with liver dysfunction, the bilirubin level was not associated with the incidence of hypertension, whereas in the subjects without liver dysfunction, the incidence of hypertension was lower in the bilirubin group 2 than in the bilirubin group 1 (35/260 vs. 162/820, p=0.022). The cumulative incidence of hypertension in subjects without liver dysfunction was also lower in group 2 than in group 1 but the bilirubin group was not an independent risk factor to hypertension by Cox's proportional hazard model.
The bilirubin level was not associated with the incidence of hypertension in current smokers, whereas in non-smokers, bilirubin group 2 had lower risk of hypertension than group 1 (Log rank test, p=0.023). In non-smokers, RR for hypertension in group 2 was 0.53 (95% CI, 0.34-0.82, p=0.005) adjusted with independent risk factors to hypertension compared to group 1.
DISCUSSION
The concentration of serum bilirubin is affected by gender, smoking status, fasting status, as well as diseased conditions. In a previous report, the individual changes in serum bilirubin concentration due to fasting averaged 0.18 mg/dL for women and 0.24 mg/dL for men (fasting values minus non-fasting values) (22). The serum bilirubin level is lower in females than in males (22, 23) and is lower in current smokers than in ex-smokers or non-smokers (21, 23). In this study, subjects were instructed to fast for at least 12 hr before blood sampling and we assumed that the serum bilirubin levels were measured after a similar fasting period among the subjects. We stratified the data according to the factors of gender, current smoking, and liver dysfunction status in order to eliminate the confounding factors which affect serum bilirubin concentration.
The subjects with higher bilirubin level showed a lower incidence of hypertension than did the subjects with lower bilirubin level, especially in females and in non-smokers.
In an animal hypertensive model using hyperbilirubinemic Gunn rats infused with angiotensin II, the rise in systolic blood pressure was markedly blunted compared to that in control rats (15). The oxidative stress was attenuated in the Gunn rat and in the aortic rings from angiotensin II-infused rats by bilirubin (15). Increased ROS has been demonstrated in the SHR (24), DOCA-salt hypertensive (25), and Dahl salt-sensitive rats (26), as well as in hypertension induced by angiotensin II (27). In humans, enhanced production of superoxide and hydrogen peroxide has been demonstrated in untreated individuals with mild to moderate hypertension. This abnormality can be reversed by the control of hypertension with beta blockers or ACE inhibitors (28). Thus, mild hyperbilirubinemia may have, at least in part, an anti-hypertensive role by the quenching of oxidative stress (15).
The bilirubin group was a risk factor to the development of hypertension only in the non-smokers. Bilirubin is an endogenous antioxidant and is destroyed by ROS (14). Smoking generates free radical species (29) which might consume the serum antioxidant, bilirubin, and enhance UDP-glucuronyltransferase activity (30) which is inversely related to serum bilirubin concentration. Smoking itself also increases blood pressure and aortic stiffness in humans (31). Therefore, the antioxidant effect of bilirubin can be decreased in the higher level of free radicals generated by current smoking and the potential anti-hypertensive capacity of bilirubin might be also reduced. In, this study, the rate of current smoking was much higher in males than in females, which is a possible explanation for why the relationship between bilirubin and hypertension incidence was evident only in females. The other possible explanation for the different effect of bilirubin on the development of hypertension between genders is the sex-difference of HO activity to stress. Trauma and hemorrhage doubled the hepatic HO-1 expression in female rats compared with male rats (32).
Subjects with liver dysfunction had more risk factors to develop hypertension, such as higher BMI, higher frequency of diabetes mellitus, and higher rate of hyperuricemia, compared to subjects with normal liver function (data not shown). The bilirubin effect on the development of hypertension may have been overwhelmed by those risk factors in subjects with liver dysfunction. In subjects with normal liver function, bilirubin effect on the hypertension was not consistent and dependent on the specific criterion of bilirubin. The criterion of 0.9 mg/dL was significant (data were not shown) but 1.1 mg/dL was not a risk factor to hypertension. So, there might be some other statistical or clinical confounding factors when we confine the group to the subjects with normal liver function.
A serum bilirubin level of 10 µM/L was suggested as a cutpoint for the discrimination of cardiovascular risk but other cut-points have been proposed in other studies (19). In this study, the serum bilirubin level assigned to discriminate the risk of hypertension was 1.1 mg/dL. It seemed that the appropriate bilirubin level to discriminate the risk of hypertension might be different in clinical situations because the bilirubin group with the criterion of 0.9 mg/dL in the non-smokers also the independent risk factor to the incidence of hypertension, which was lower than the criterion of 1.1 mg/dL in whole subjects (data were not shown). That implies a relatively lower level of bilirubin would be sufficient to protect the development of hypertension in subjects with a lower risk of hypertension.
This study suffered several limitations. Firstly, blood pressure was measured just once in the sitting position using a mercury sphygmomanometer. We asked subjects about their smoking habit, duration of smoking, and amount of smoking but could only get information on the smoking habit (current smoker or non-current smoker) at a reasonable rate among the subjects. We therefore could not analyze the effect of duration and smoking amount on the development of hypertension. Although the subjects were asked to fast for at least 12 hr before the blood tests, we did not confirm the exact fasting period before the measurement of serum bilirubin concentration. We used the unit of mg/dL for serum bilirubin concentration although the SI units (µM/L) are more informative and better able to discriminate serum bilirubin levels within the physiological range (19). We did not have information about age at menopause and estrogen replacement therapy in women which could affect on the development of hypertension. But, the mean age of female was not different between two bilirubin groups (p=0.072) and the number of females aged more than 47 yr, which is the mean age at menopause in Korean women (33), was not different between bilirubin groups, either (p=0.086). In younger subjects aged less than 50 yr, RR for the development of hypertension was 0.605 (95% CI, 0.372-0.984, p=0.043) in bilirubin group 2 compared to bilirubin group 1 and, in older subjects aged 50 yr or more, RR for the development of hypertension was not different between bilirubin groups (p=0.299). Although we could not assure whether the menopause and the estrogen replacement therapy were confounding factors to the relationship between bilirubin group and the incidence of hypertension in older subjects, the bilirubin effect on the hypertension was at least meaningful in younger subjects.
In conclusion, a mild increase within the physiological range of serum bilirubin concentration was negatively correlated with the incidence of hypertension in subjects with normal blood pressure. The effect of bilirubin on the development of hypertension was more evident in females and in non-smokers.
References
- 1.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 2.Goodman AI, Quan S, Yang L, Synghal A, Abraham NG. Functional expression of human heme oxygenase-1 gene in renal structure of spontaneously hypertensive rats. Exp Biol Med. 2003;228:454–458. doi: 10.1177/15353702-0322805-04. [DOI] [PubMed] [Google Scholar]
- 3.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 4.Ndisang JF, Zhao W, Wang R. Selective regulation of blood pressure by heme oxygenase-1 in hypertension. Hypertension. 2002;40:315–321. doi: 10.1161/01.hyp.0000028488.71068.16. [DOI] [PubMed] [Google Scholar]
- 5.Botros FT, Schwartzman ML, Stier CT, Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005;68:2745–2755. doi: 10.1111/j.1523-1755.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan A, Umezu M, Fukagawa M. Heme-oxygenase upregulation ameliorates angiotensin II-induced tubulointerstitial injury and salt-sensitive hypertension. Am J Nephrol. 2006;26:552–561. doi: 10.1159/000098001. [DOI] [PubMed] [Google Scholar]
- 7.Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1472–R1478. doi: 10.1152/ajpregu.00601.2006. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RA, Lavesa M, DeSeyn K, Scholer MJ, Nasjletti A. Heme oxygenase substrates acutely lower blood pressure in hypertensive rats. Am J Physiol. 1996;271:H1132–H1138. doi: 10.1152/ajpheart.1996.271.3.H1132. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension. 1995;25:166–169. doi: 10.1161/01.hyp.25.2.166. [DOI] [PubMed] [Google Scholar]
- 10.Wiesel P, Patel AP, Carvajal IM, Wang ZY, Pellacani A, Maemura K, DiFonzo N, Rennke HG, Layne MD, Yet SF, Lee ME, Perrella MA. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1 deficient mice. Circ Res. 2001;88:1088–1094. doi: 10.1161/hh1001.091521. [DOI] [PubMed] [Google Scholar]
- 11.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CG, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 12.Kitiyakara C, Wilcox CS. Antioxidants for hypertension. Curr Opin Nephrol Hypertens. 1998;7:531–538. doi: 10.1097/00041552-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 14.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1989;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 15.Pflueger A, Croatt AJ, Peterson TE, Smith LA, d'Uscio LV, Katusic ZS, Nath KA. The hyperbilirubinemic Gunn rats is resistant to the pressor effects of angiotensin II. Am J Physiol Renal Physiol. 2005;288:F552–F558. doi: 10.1152/ajprenal.00278.2004. [DOI] [PubMed] [Google Scholar]
- 16.Lanone S, Bloc S, Foresti R, Almolki A, Taille C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El-Benna J, Motterlini R, Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NAD (P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19:1890–1892. doi: 10.1096/fj.04-2368fje. [DOI] [PubMed] [Google Scholar]
- 17.Sano K, Nakamura H, Matsuo T. Mode of inhibitory action of bilirubin on protein kinase C. Pediatr Res. 1985;19:587–590. doi: 10.1203/00006450-198506000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vascular tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in Men: A meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 20.Papadakis JA, Ganotakis ES, Jagroop IA, Jagroop IA, Mikhailidis DP, Winder AF. Effect of hypertension and its treatment on lipid, lipoprotein(a), fibrinogen, and bilirubin levels in patients referred for dyslipidemia. Am J Hypertens. 1999;12:673–681. doi: 10.1016/s0895-7061(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 21.Van Hoydonck PG, Temme EH, Schouten EG. Serum bilirubin concentration in a Belgian population: the association with smoking status and type of cigarettes. Int J Epidemiol. 2001;30:1465–1472. doi: 10.1093/ije/30.6.1465. [DOI] [PubMed] [Google Scholar]
- 22.White GL, Jr, Nelson JA, Pedersen DM, Ash KO. Fasting and gender (and altitude?) influence reference intervals for serum bilirubin in healthy adults. Clin Chem. 1981;27:1140–1142. [PubMed] [Google Scholar]
- 23.Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827–835. doi: 10.1002/hep.20407. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- 25.Nicod L, Rodriguez S, Letang JM, Viollon-Abadie C, Jacqueson A, Berthelot A, Richert L. Antioxidant status, lipid peroxidation, mixed function oxidase and UDP-glucuronyl transferase activities in livers from control and DOCA-salt hypertensive male Sprague Dawley rats. Mol Cell Biochem. 2000;203:33–39. doi: 10.1023/a:1007041532523. [DOI] [PubMed] [Google Scholar]
- 26.Swei A, Lacy F, DeLano FA, Schmid-Schonbein GW. Oxidative stress in the Dahl hypertensive rat. Hypertension. 1997;30:1628–1633. doi: 10.1161/01.hyp.30.6.1628. [DOI] [PubMed] [Google Scholar]
- 27.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 28.Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- 29.Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Biochem J. 1991;277:133–138. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villard PH, Herber R, Seree EM, Attolini L, Magdalou J, Lacarelle B. Effect of cigarette smoke on UDP-glucuronyltransferase activity and cytochrome P450 content in liver, lung and kidney microsomes in mice. Pharmacol Toxicol. 1998;2:74–79. doi: 10.1111/j.1600-0773.1998.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 31.Rhee MY, Na SH, Kim YK, Lee MM, Kim HY. Acute effects of cigarettes smoking on arterial Stiffness and blood pressure in male smokers with hypertension. Am J Hypertens. 2007;20:637–641. doi: 10.1016/j.amjhyper.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Toth B, Yokoyama Y, Kuebler JF, Schwacha MG, Rue LW, 3rd, Bland KI, Chaudry IH. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch Surg. 2003;138:1375–1382. doi: 10.1001/archsurg.138.12.1375. [DOI] [PubMed] [Google Scholar]
- 33.Hong JS, Yi SW, Kang HC, Jee SH, Kang HG, Bayasgalan G, Ohrr H. Age at menopause and cause-specific mortality in South Korean Women: Kangwha Cohort Study. Maturitas. 2007;56:411–419. doi: 10.1016/j.maturitas.2006.11.004. [DOI] [PubMed] [Google Scholar]