Abstract
In contrast to the extensive analysis of the regulation of Cyclin B protein levels during developmental progression through meiosis in oogenesis, little is known about Cyclin A. Repression of cyclin A translation early in prophase I in Drosophila is important to maintain the oocyte in meiosis, and this has been shown to be mediated by deadenylation of the mRNA and inhibition by the Bruno repressor. We find that at oocyte maturation as meiosis resumes, Cyclin A protein reappears, coincident with polyadenylation of the mRNA and loss of Bruno repressor. Cyclin A is multiphosphorylated in a pattern consistent with autophosphorylation, and this form accumulates aberrantly in metaphase I if the Cortex form of the Anaphase Promoting Complex/Cyclosome is inactive. The PAN GU (PNG) kinase positively promotes translation of Cyclin A, beginning in oogenesis, an earlier onset than previously recognized. After egg activation and the completion of meiosis, PNG promotes further polyadenylation of cyclin A mRNA and appears to antagonize repression of translation by the PUMILIO inhibitor. Epistasis studies with png; apc mutants indicate that PNG acts solely to promote translation, rather than having a parallel function to inhibit degradation. These studies reveal multiple levels of posttranscriptional regulation of Cyclin A protein by translational and proteolytic control during oocyte maturation and the onset of embryogenesis.
Keywords: APC/C, Drosophila, oocyte maturation, PNG kinase, translation
Animal development requires precise regulation of the meiotic cell cycle and restart of the embryonic cell cycle. During oogenesis the meiotic cell cycle is arrested at 2 points. The primary arrest point, in prophase I, permits differentiation of the oocyte. The secondary arrest point, at metaphase I in insects and metaphase II in most vertebrates, allows coordination between the completion of meiosis and fertilization. In organisms that undergo rapid embryogenesis such as insects, marine invertebrates, and amphibians, a modified embryonic cell cycle is used in which DNA replication oscillates with mitosis without gap phases, growth, or transcription.
Ooctye maturation causes exit from the primary meiotic arrest and progression into meiotic divisions. It is driven by Maturation Promoting Factor (MPF), the Cyclin B/CDK1 kinase complex. The regulation of the meiotic cell cycle at maturation is best understood in Xenopus, where control of translation by polyadenylation leads to the translation of both Cyclin B to produce MPF and the Mos kinase, which is crucial in maintaining the secondary meiotic arrest (1). In Drosophila oogenesis, some mRNAs become polyadenylated at maturation, dependent on the GLD2 cytoplasmic poly(A) polymerase (2). The cyclin B mRNA is polyadenylated initially at oocyte maturation, coinciding with appearance of the protein in meiosis (3, 4). Cyclin B is required for female fertility in Drosophila (5), and Cyclin B/CDK1 activity is likely to promote oocyte maturation and the release of the prophase I arrest. In both CDK1 and twine mutants, lacking the meiotic form of the Cdc25 phosphatase, oocyte maturation is delayed (6, 7). POLO, a known activator of Cdc25, also activates exit from prophase I arrest and is kept inactive during the arrest by the Mtrm inhibitor (6). The Drosophila endosulfine gene (dendos) too is needed for oocyte maturation, via its effect on levels of Twine and POLO proteins (7).
In Drosophila, egg activation, which occurs as the oocyte moves into the uterus, releases the metaphase I secondary arrest but in addition triggers additional polyadenylation, renewed translation, hardening of the vitelline envelope and cytoskeletal rearrangements (8). Levels of Cyclin B protein also are translationally activated in Drosophila during egg activation and during the embryonic cell cycles. The cyclin B mRNA poly(A) tail is further extended during activation, and this appears to be needed for translation of normal levels of Cyclin B protein during the embryonic divisions (4). Cyclin B protein is targeted for destruction on exit from meiosis by a meiosis-specific form of the Anaphase Promoting Complex/Cyclosome, APC/CCort, and possibly by APC/CFzy(Cdc20) as well (9, 10). Renewed translation of Cyclin B after the completion of meiosis is dependent on the PNG kinase complex, which acts by both poly(A) dependent and independent mechanisms (4). In the absence of PNG function meiosis is completed, but the meiotic products persist in an interphase state, undergoing DNA replication but not mitosis to produce giant, polyploid nuclei (11, 12).
Less is understood about the roles and regulation of Cyclin A during metazoan meiosis. Cyclin A2 is expressed in mouse oocytes, but its function is not yet defined (13). In Drosophila oogenesis Cyclin A levels must be controlled precisely for oocyte differentiation and for the onset of meiosis. The mitotic divisions early in oogenesis that produce the oocyte and its sister nurse cells are dependent on Cyclin A (14). Although these cells are connected by cytoplasmic bridges, solely the oocyte goes through meiosis, while the nurse cells become polyploid by the endo cycle. Cyclin A protein levels are controlled posttranscriptionally early in oogenesis by deadenylation of the mRNA (15) and during the prophase I arrest by repression of translation by Bruno (16). After oocyte maturation Cyclin A is degraded by metaphase I by targeting by APC/CCort (10). This degradation is also dependent on Cks30A (17). The levels of Cyclin A protein are reduced in png mutants, although Cyclin B appears to be the critical target responsible for the embryonic cell cycle defects in these mutants (18, 19).
Here, we exploit the ability to isolate sufficient quantities of oocytes at specific stages of the meiotic cell cycle to analyze the control of Cyclin A protein during Drosophila oogenesis and early embryogenesis. We find that it is regulated by multiple posttranscriptional controls correlated with oocyte maturation, completion of the meiotic cell cycle and restart of the embryonic cell cycles.
Results
Modified Cyclin A Protein Appears at Maturation.
To analyze the state of Cyclin A protein during progression of the meiotic cell cycle we hand dissected Drosophila egg chambers, prepared protein extracts, and examined the levels of Cyclin A protein on immunoblots. Drosophila egg chambers have distinct morphologies that identify successive stages of meiosis in the oocyte. The prophase I arrest occurs in stages 6–12. Oocyte maturation occurs in stages 12–13, with the nuclear envelope breaking down in stage 13, the meiotic spindle assembling, and prometaphase I occurring (20, 21). A stable metaphase I secondary arrest is achieved by stage 14. This secondary arrest is released as the oocyte traverses the uterus and is activated, regardless of whether it is fertilized, so in both unfertilized eggs and fertilized embryos meiosis is completed (22, 23). Fertilized embryos resume the cell cycle with a modified rapid S–M cycle.
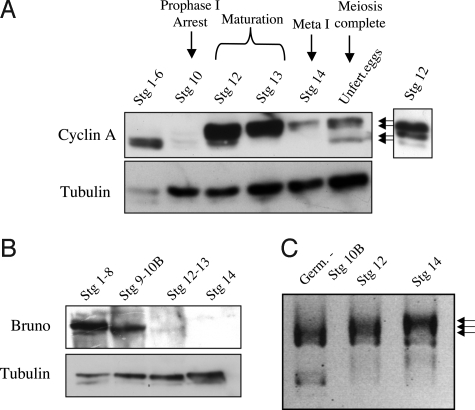
Consistent with the results in ref. 16, we found that the levels of Cyclin A protein are markedly lower during prophase I arrest than in earlier stages (Fig. 1A, stages 1–6 versus stage 10). Strikingly, upon oocyte maturation, levels of Cyclin A protein dramatically increase, and the protein is present in multiple forms with reduced electrophoretic mobility (Fig. 1A, stage 12). Levels of Cyclin A protein are low in metaphase I arrested oocytes (Fig. 1A, stage 14), but the high mobility form of the protein becomes detectable again after the completion of meiosis (Fig. 1A, Unfertilized eggs). These results suggest that Cyclin A protein becomes translated and posttranslationally modified at oocyte maturation.
Fig. 1.
Developmental regulation of Cyclin A protein. (A) Cyclin A levels and forms in staged egg chambers detected by immunoblot. Cyclin A protein is nearly undetectable during the prophase I arrest (stage 10) but abundant at maturation (stage 12). Two fast migrating forms are present premeiotically in stages 1–6, whereas from maturation through metaphase I 2 slower migrating forms appear (arrows). A shorter exposure shows the forms present in stage 12. All 4 forms of Cyclin A are present in unfertilized eggs. Tubulin serves as a loading control. (B) Immunoblot of Bruno protein. Bruno protein levels markedly decline upon oocyte maturation (stages 12–13) and are undetectable at metaphase I (stage 14). Tubulin serves as a loading control. (C) PAT assay of poly(A) tail length on cyclin A mRNA. Polyadenylation occurs at maturation (stage 12, middle arrow) and the poly(A) tail is further lengthened by metaphase I (stage 14, top arrow). The length of the poly(A) tail in each lane is demarcated by the top of the smear.
Although the oocyte is stockpiled with cyclin A mRNA, it is blocked from translation during prophase I arrest by the Bruno translational repressor, shown by Sugimura and Lilly to bind to the 3′UTR of the mRNA (16). Failure of this repression leads to loss of the prophase I arrest, and Bruno protein was undetectable at metaphase I (16). We examined protein levels of Bruno during oocyte progression through meiosis to test the hypothesis that loss of this translational repressor permits Cyclin A translation to facilitate entry into meiosis I. Although Bruno protein is abundant in early egg chambers and prophase I-arrested egg chambers (Fig. 1B, stages 1–8 and 9–10B), the levels abruptly decline at oocyte maturation (Fig. 1B, stages 12–13) and are undetectable at the metaphase I arrest (Fig. 1B, stage 14). These observations are consistent with renewed translation of Cyclin A at maturation after loss of the Bruno repressor protein.
Maternal mRNAs also can be masked for translation by a short poly(A) tail. Oocyte maturation in Xenopus is triggered by polyadenylation of cyclin B mRNA, leading to translation of Cyclin B and active MPF (cyclin B/CDK1) (1). In Drosophila oogenesis, poly(A) tail lengthening occurs both at oocyte maturation and activation (3). Lengthening of the poly(A) tail of cyclin B mRNA at each of these meiotic transitions is correlated with renewed appearance of the protein (3, 4), and polyadenylation of the mRNA for the meiosis-specific APC/C activator Cortex by the GLD2 cytoplasmic poly(A) polymerase has been shown to be required for its translation at maturation (2). Given these precedents, we tested whether polyadenylation could be an additional mechanism besides loss of Bruno contributing to the increase of Cyclin A protein at maturation. We isolated RNA from staged egg chambers and used PAT assays to measure the length of the poly(A) tail on cyclin A mRNA. We found that the poly(A) tail is lengthened at maturation at stage 12 and further lengthened during progression to metaphase I (Fig. 1C). Thus, in addition to loss of repression by Bruno, polyadenylation may also contribute to the reappearance of Cyclin A protein as oocytes exit the prophase I arrest and resume the meiotic cell cycle.
Cyclin A Protein Is Multiply-Phosphorylated During Meiotic Progression.
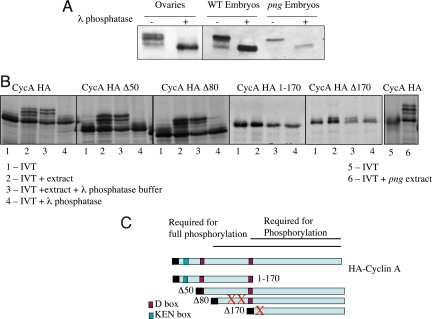
Cyclin A protein has been shown to exist in phosphorylated forms in the Drosophila embryo, with the most highly phosphorylated forms correlated with active Cyclin A/CDK1 kinase (24). Furthermore, Cyclin A recently has been demonstrated to undergo autophosphorylation (25). We observed 4 forms of Cyclin A present during oogenesis: 2 fast migrating, premeiotic forms, 4 forms in prometaphase I, the slowest migrating form in metaphase I, and all 4 forms after the completion of meiosis (Fig. 1A). These 4 forms match the forms present in early Drosophila embryos (Fig. 2A). Treatment of protein extracts made from total ovaries or early embryos with lambda phosphatase demonstrated that indeed the altered mobility is due to phosphorylation, because as assayed by immunoblots, phosphatase treatment converted all of the Cyclin A protein to the faster migrating form (Fig. 2A). We confirmed the previous observation that the PNG kinase is not required for the modification of Cyclin A (Fig. 2A) (18).
Fig. 2.
Phosphorylation of Cyclin A. (A) Protein extracts from wild-type ovaries, embryos or embryos produced by png1058 mutant females were incubated in λ phosphatase buffer, with or without λ phosphatase and immunoblotted for Cyclin A. In all extracts treatment with λ phosphatase collapses the forms of Cyclin A to the fastest mobility form, indicating the 3 slower mobility forms are due to phosphorylation. (B) In vitro phosphorylation assay for Cyclin A. Full length and truncations of HA-Cyclin A were translated in vitro, labeled with 35S methionine (lane 1 for each construct). The translated protein was incubated with embryonic extracts and the mobility forms tested (lane 2). These mobility shifts were shown to be the consequence of phosphorylation by incubation with λ phosphatase (lane 4) compared with phosphatase buffer alone (lane 3). The extracts produce the same phosphorylation shifts observed in vivo during oogenesis and embryogenesis. Lanes 5 and 6 demonstrate that png mutant extracts are capable of promoting phosporylation. (C) Diagram of the Cyclin A deletions assayed for phosphorylation (26). The C terminus of the protein is required for phosphorylation, amino acids 1–170 are required for 2 of the phosphorylations, but amino acids 1–80 are dispensable for phosphorylation. The autophosphorylation sites for cyclin A are T145, S154, and S180, shown by red X's (25).
We developed an in vitro phosphorylation assay for Cyclin A and applied it to map the domains of the protein needed for phosphorylation. 35S methionine-labeled Cyclin A protein was synthesized by a coupled in vitro transcription/translation system and incubated with a Drosophila embryonic extract. Addition of the embryonic extract led to the altered electrophoretic mobility of Cyclin A, shown to be the consequence of phosphorylation by phosphatase treatment (Fig. 2B, far left). This in vitro system permitted us to exploit deletion derivatives of Cyclin A to delineate the domains of the protein required for phosphorylation (26). The C-terminal half of the protein was essential for phosphorylation, and all of the protein except the N-terminal 80 aa was needed for full phosphorylation (Fig. 2 B and C). Interestingly, the C terminus contains the cyclin box and is responsible for interaction between Cyclin A and CDK1. Two of the 3 principal autophosphorylation sites in Cyclin A map to the N-terminal 170 aa (T145 and S154), but a third is at S180 (25). Thus, it is striking that a single phosphoform is observed in the form of Cyclin A deleted for the N-terminal 170 aa. These mapping observations are consistent with both the meiotic and embryonic forms of Cyclin A resulting from autophosphorylation. As in vivo, in vitro PNG kinase activity is not required for Cyclin A phosphorylation (Fig. 2B, far right).
Effect of cort, apc, and png Mutants on Cyclin A Phosphoforms.
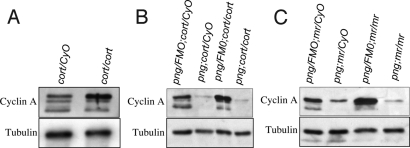
The Cortex form of the APC/C is needed for degradation of Cyclin A in meiosis and levels of Cyclin A are decreased by metaphase I (9, 10). We compared the abundance of the forms of Cyclin A in cort mutants and in mutants for morula, which encodes the APC2 subunit (27), to determine whether particular forms of Cyclin A persist when APC/C is compromised. In fertilized embryos from cortQW55/RH65 or mr1/2 mutant females the most highly phosphorylated form of Cyclin A was specifically enriched (Fig. 3A). Similar results were obtained for metaphase I arrested oocytes and unfertilized eggs from these mutants. These results indicate either that the most phosphorylated form of Cyclin A is targeted for destruction by APC/CCort or that the increased levels of Cyclin A resulting from mutation of APC/CCort lead to increased autophosphorylation of Cyclin A/CDK1.
Fig. 3.
Effects of cort, mr, and png mutants on the phospho forms of Cyclin A. Protein extracts were prepared from unfertilized eggs or embryos from mothers of the indicated genotypes and immunoblots done for Cyclin A. (A) Mutation of cort leads to the accumulation specifically of the most phosphorylated form of Cyclin A. The immunoblot shown was prepared from extracts from 0–1-h embryos, but unfertilized eggs and mature oocytes give the same result. (B) png, cort epistasis test. Protein extracts prepared from unfertilized eggs from png, cort heterozygous mothers, mothers homozygous for one and heterozygous for the other, or double homozygous mutant mothers were immunoblotted for Cyclin A. In contrast to cort mutants, png mutants have low levels of Cyclin A protein, and it is the most phosphorylated form. The levels of Cyclin A protein are not restored in png, cort double mutants. The same result is obtained in embryos. (C) Epistasis tests between png and mr also do not show restoration of Cyclin A levels; png is epistatic to mr.
We showed that the PNG kinase complex is required for normal levels of Cyclin A protein in unfertilized eggs and during the embryonic divisions (18). Given the posttranscriptional control of these cycles, PNG could affect Cyclin A levels either by promoting translation or inhibiting degradation. The dependence of Cyclin A degradation in meiosis on APC/CCort permitted us to do an epistasis experiment to determine whether PNG function contributes to Cyclin A protein levels by inhibiting its proteolysis. If this were the case, the levels of Cyclin A protein would be restored in a png, cort double mutant in which degradation is blocked by the cort mutation. We examined Cyclin A levels in unfertilized eggs from png1058/3318, cortQW55/RH65 mutant females and found that the low levels of Cyclin A protein observed in png mutants were not restored by mutation of cort (Fig. 3B). The same result was obtained with embryos from the double mutant mothers. These results show that png does not affect Cyclin A protein levels by inhibiting APC/CCort.
To test whether PNG could act by inhibiting the other form of the APC/C present during these developmental stages, APC/CFzy (9, 10), we examined double mutants with png1058/3318 and the female-sterile alleles of the APC2 gene, mr1/2. Although Cyclin A levels were high in the mr unfertilized eggs, they were present at low levels in the unfertilized eggs from png; mr double mutant mothers, comparable to the single png mutant (Fig. 3C). Cyclin A levels also were low in embryos from double mutant mothers. Thus, PNG does not appear to affect Cyclin A levels by inhibiting either form of APC/C.
PNG Kinase Complex Promotes the Translation of Cyclin A.
The PNG kinase complex has been shown to promote the translation of Cyclin B after the completion of meiosis by antagonizing (directly or indirectly) the translational repressor PUMILIO (4). The png, cort epistasis experiment raised the possibility that the PNG complex also promotes the translation of Cyclin A.
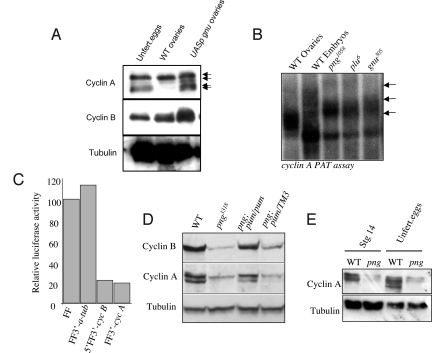
Activity of the PNG kinase requires its association with the activating subunits GNU and PLU (28). PNG kinase can be activated prematurely in oogenesis by induction of GNU (29), and this leads to increased levels of Cyclin B protein (4). We found that induction of GNU during oogenesis also resulted in increased levels of Cyclin A protein, but strikingly of the unphosphorylated form (Fig. 4A). Given that this form of Cyclin A appears in a PNG-dependent manner in unfertilized eggs and embryos after the completion of meiosis (Figs. 2A and 4A), it appears that the Cyclin A protein translated at maturation becomes phosphorylated and degraded by APC/Ccort and that PNG promotes translation of new Cyclin A after meiosis, which is in the unphosphorylated form.
Fig. 4.
PNG overcomes repression of Cyclin A translation. (A) Premature activation of PNG activity by induction of GNU (from a nanos GAL4 driver and a UASp gnu-GFP) results in increased levels of Cyclin A in ovaries enriched for late stage egg chambers. Strikingly, the unphosphorylated form of Cyclin A appears (arrows), whereas normally it is not seen until after the completion of meiosis (as in extracts from unfertilized eggs). The immunoblots were probed also with antibodies against Cyclin B to confirm that it too is increased by induction of GNU, and against Tubulin as a loading control. (B) PAT assay on cyclin A mRNA isolated from wild-type ovaries or embryos laid by wild type, png, plu or gnu mutant females. The cyclin A poly(A) tail is lengthened at egg activation (top arrow, compare wild type embryo to ovary, bottom arrow). The poly(A) tail does not fully lengthen in the absence of PNG kinase activity (middle arrow, png, plu and gnu mutants). (C) PNG is needed to overcome inhibition of Cyclin A translation conferred by the 3′UTR. mRNAs were transcribed in vitro from the luciferase reporter gene alone or flanked by the α-tubulin 3′UTR, the 5′ and 3′ UTRs of cyclin B, or the 3′UTR of cyclin A. These were injected into wild-type or png1058 mutant embryos, and the level of luciferase activity assayed. The data are presented as a ratio of luciferase activity obtained from png mutant embryos relative to wild type. (D) Double mutants between png and the translational repressor pum show an increase in Cyclin A protein levels compared with png mutants and the reappearance of the unphosphorylated form. Protein extracts were prepared from embryos laid by mothers of the indicated genotype and immunoblots bound to antibodies against Cyclin A, Cyclin B, or Tubulin. Only 2 forms of Cyclin A are distinguishable under the electrophoresis conditions used in this experiment. (E) Cyclin A protein levels are decreased in png mutant eggs and in stage 14 oocytes, suggesting the PNG kinase complex functions before egg activation to promote Cyclin A levels.
PNG promotes Cyclin B translation by facilitating additional polyadenylation of the mRNA at egg activation and by a poly(A)-independent mechanism (4). We isolated RNA from embryos from wild type, png, plu and gnu mutant mothers to test whether PNG activity was needed for cyclin A polyadenylation. PAT assays showed that although some polyadenylation of cyclin A mRNA occurred in png, plu, or gnu mutants, the length of the poly(A) tail was shorter than wild type in these mutant embryos (Fig. 4B). Given the relationship between poly(A) tail length and efficiency of translation (1), these results are consistent with compromised translation of Cyclin A in PNG kinase mutants. This experiment also shows that cyclin A mRNA is present in png mutant embryos.
We used a luciferase translation reporter and embryo injection assay as an additional test for whether PNG affects Cyclin A translation (4). A firefly luciferase mRNA is translated efficiently after injection into embryos from null png1058 mutant mothers (Fig. 4C). Addition of the 3′UTR from α-tubulin did not affect translation of the reporter mRNA, but in contrast addition of the 3′UTR from cyclin A resulted in inhibition of translation in png mutants (Fig. 4C). The levels of inhibition were comparable to those seen with a reporter with the 5′ and 3′ UTRs of cyclin B (Fig. 4C). These results indicate that, as for cyclin B, the 3′UTR of cyclin A contains a target for inhibition of translation, and functional PNG kinase is necessary to overcome this inhibition.
PNG acts antagonistically to the PUMILIO translational repressor, because mutation of pum dramatically suppresses the png mutant phenotype of absence of mitosis in the embryonic cell cycle (4). This is associated with an increase in the levels of Cyclin B protein (4). We analyzed whether loss of pum could also restore Cyclin A protein levels in png mutants and observed this to be the case (Fig. 4D). This result suggests that PNG promotes the translation of both Cyclin A and B by overcoming inhibition by PUMILIO.
Phenotypically the onset of the cell cycle defects resulting from loss of PNG kinase activity is after the completion of meiosis. The meiotic products in the egg go through a transient interphase state, then in the first mitotic phase of development, the chromosomes of the polar body meiotic products condense (30). If PNG kinase activity is compromised, these meiotic products do not leave interphase and unregulated DNA replication occurs (12, 11). Consistent with this, it has been demonstrated that activation of PNG kinase after meiosis can at least partially rescue the cell cycle and maternal mRNA destablization functions of PNG by restoring Cyclin B and SMG translation (31, 32). In analyzing Cyclin A protein in oogenesis, we made the unexpected observation that levels already are reduced in stage 14 oocytes in png1058 mutant mothers (Fig. 4E). Thus, PNG begins to promote Cyclin A protein accumulation as early as metaphase I, indicating that PNG kinase is active during oogenesis.
Discussion
Developmental Control of Cyclin A Translation.
These studies provide support for 2 mechanisms regulating translation of cyclin A mRNA during oogenesis and early embryogenesis, by polyadenylation and translational repressors (Fig. 5). The poly(A) tail of cyclin A mRNA is short during prophase I arrest, and its lengthening correlates with appearance of the protein at oocyte maturation. It remains to be determined how this polyadenylation is controlled, but a likely possibility is that it may involve the GLD2 poly(A) polymerase shown to polyadenylate other mRNAs at maturation (2, 33). The poly(A) tail is further increased at egg activation in a mechanism that requires active PNG kinase, and this may contribute to efficient translation of Cyclin A during early embryogenesis.
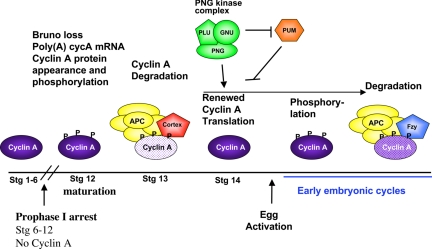
Fig. 5.
Model for the posttranscriptional regulation of Cyclin A in meiosis and early embryogenesis. The events affecting Cyclin A protein levels are schematized above the time line for meiosis and the restart of the cell cycle in embryogenesis. PNG begins to promote Cyclin A protein levels during oogenesis, but it is not known if Pumilio is inhibitory to Cyclin A translation at this time or only during embryogenesis. The Cort form of the APC/C targets Cyclin A for degradation during prometaphase I, but Cort is degraded after the completion of meiosis (10), so APC/CFzy controls Cyclin A destruction during the early embryonic divisions (40, 48, 49). PNG is needed during the early embryonic divisions for translation of Cyclin A, but this requirement is obviated if pum is mutated.
Two translational repressors, acting at distinct developmental times, appear to control Cyclin A translation (Fig. 5). Bruno has been shown to bind to the 3′UTR of the cyclin A mRNA and to be necessary to block protein accumulation during the prophase I arrest. Our observation that Bruno protein disappears at maturation raises the possibility that this may contribute to the abrupt appearance of Cyclin A protein at maturation. It will be necessary to identify the mechanism responsible for loss of Bruno protein to then determine whether its absence is required for Cyclin A translation at maturation. Given the importance of low Cyclin A protein levels for the prophase I arrest it appears that 2 mechanisms of a shortened poly(A) tail and presence of Bruno protein block its translation before maturation.
The PUM translational repressor is a likely candidate for regulating the translation of Cyclin A during embryogenesis. Mutation of the pum gene restores Cyclin A translation in png mutant embryos. PUM binds via its partner NANOS to a sequence motif, the Nanos Response Element (NRE) (34, 35). A degenerate NRE is present in the 3′UTR of the cyclin A mRNA with the sequence gc(u)ugu … 9 … auugua. An NRE mutant in the hb mRNA with a similar sequence has been shown to bind PUMILIO (36, 37). This raises the possibility that PUM binds to cyclin A mRNA, as it has been shown to do on the cyclin B mRNA (35). It remains to be determined whether PNG directly antagonizes PUM, but control of mitotic Cyclin translation by PUM during embryogenesis could provide an additional level of regulation of S–M oscillation during the early embryonic cycles.
PNG Kinase Affects Cyclin A Levels in Meiosis.
An unexpected result from these studies is that the levels of Cyclin A protein in png mutant stage 14 oocytes are even lower than in wild type. This indicates that during metaphase I new pools of Cyclin A protein are being translated in a PNG-dependent manner. It remains to be determined whether PUM inhibits Cyclin A translation during late oogenesis and, if so, if PNG functions to counteract this. This result also reveals that PNG is active before the completion of meiosis.
Control of Cyclin A Degradation in Meiosis.
We found that APC/CCort leads to the degradation of Cyclin A by metaphase I, and here we show that the most phosphorylated form of Cyclin A inappropriately persists in cort or mr (APC2) mutants. This form of Cyclin A is likely to arise by autophosphorylation, as supported by our observations that the C-terminal domain through which Cyclin A interacts with CDK1 is needed for phosphorylation, and that deletion of the region containing 2 of the 3 autophosphorylation sites results in a single phospho form of Cyclin A. We propose that during meiosis autophosphorylation of Cyclin A permits it to be targeted for degradation. During embryogenesis, autophosphorylation is not required for Cyclin A degradation, but in embryogenesis it is ubiquitinated by APC/CFzy (25). There could be a distinction between the requirements of Cort and Fzy, or phosphorylation could facilitate ubiquitination but not be absolutely required. Another possible explanation for the accumulation of the most phosphorylated form of Cyclin A in cort and mr mutants that is not excluded by these data are that this form accumulates because of persisting high levels of active Cyclin A/CDK1 in these mutants.
The observation that the most phosphorylated form of Cyclin A accumulates in the absence of APC/CCort function provides insights into how Cks30A may control Cyclin A levels. Swan and Schupbach found that in cks30A (rem) mutants the levels of Cyclin A protein are elevated in ovaries (17), and their immunoblot shows this to correspond to a form with slow electrophoretic mobility and therefore phosphorylated. This suggests that Cyclin A autophosphorylation is not compromised in cks30A mutants. Thus, as in human mitosis, Cks30A may directly affect Cyclin A ubiquitination by APC/CCort, as opposed to an indirect effect of increasing APC/C phosphorylation by increasing Cyclin A/CDK1 kinase activity (38).
Roles of Cyclin A in Meiosis.
Mutations in the cyclin A gene cause embryonic lethality, preventing direct tests of its function during meiosis (39). The abrupt appearance of the protein upon release of the prophase I arrest and its transient presence until metaphase I suggest that as in mitosis, Cyclin A/CDK1 activity is needed for progression through prophase and prometaphase. We speculate that the activation of Cyclin A/CDK1 kinase helps drive oocytes out of prophase I arrest and into the first division. If Cyclin A is needed for prophase and prometaphase II, low levels of the protein may be sufficient, but it is also possible that Cyclin B, which remains high until the completion of meiosis, suffices for meiosis II. It remains to be determined whether Cyclin A must be degraded by metaphase I for proper meiosis. In Drosophila embryonic mitoses, nondegradable Cyclin A prolongs prometaphase and can cause aberrant chromosome morphology (40, 41), but it does not block mitosis. If a parallel effect occurs in meiosis I detection would require precise live imaging of the timing of prometaphase I.
The parallel mechanisms to regulate Cyclin A levels both early in the meiotic cell cycle and during oocyte maturation and egg activation emphasize the importance of precise fine tuning of the amounts of Cyclin A protein during these meiotic stages. This paper shows that control pathways to meet this demand operate post transcriptionally.
Materials and Methods
Drosophila Stocks.
The cortRH65 and cortQW55 null alleles are described in refs. 42 and 43. The png1058 allele is a functional null (11, 18). plu6 and gnu305 are described in refs. 11 and 44. mr1 and mr2 are female-sterile alleles (27, 45). The UASp gnu-GFP transgenic line was obtained from Myles Axton (29). Embryos mutant for pum where obtained from females transheterozygous for In(3R)Msc and T(1;3)FC8, pum alleles obtained from Ruth Lehmann and Robin Wharton (46). The male sterile translocation strain T(Y;2)#11 cn bwD mr2/b cn mr1 bs2/SM6A was used for crosses to produce unfertilized eggs, because these males do not make sperm (45).
Egg Chamber Isolation and Immunoblots.
Egg chambers were dissected in Grace's media and staged using the morphological criteria in (47). Thirty micrograms of egg chamber protein extract was used for each lane for the immunoblots. Protein extracts were prepared as described in ref. 4. The phosphoforms of Cyclin A were separated on long 7.5% SDS/PAGE gels. Anti-Cyclin A antibody A19, a gift from P. O'Farrell (University of California, San Francisco), was used at 1:50. Anti-Cyclin B F2F3 (39) was used at 1:200, and rat anti-β-Tubulin (YL1/2 and YL1/34, Harlan Seralab) was used at 1:200. Alkaline phosphatase and HRP-conjugated secondary antibodies were used to detect the primary antibodies.
PAT Assays.
RNA isolation and PAT assays were as described in ref. 4. For the PAT assays on egg chamber RNA, the primer used was GCACCAGCAGCCGGATATTA. For embryo RNA, the primer was GAAATTGGAGGAAGCCACCG.
Phosphorylation Assays.
The 35S radiolabeled Cyclin A truncations [clones obtained from F. Sprenger (University of Regensburg, Germany) (26)] were in vitro-transcribed and -translated according to the manufacturers instructions (TNT T7 Coupled Reticulocyte Lysate System). Two microliters of IVT reaction was incubated in 15 μL of Drosophila embryo extracts (made in 1:4 volumes lysis buffer: 25 mM Hepes, 100 mM NaCl2, 1 mM EGTA, 10% glycerol) for 30 min at room temperature. These were then separated on 7.5% SDS/PAGE gels and exposed to film. To determine the phosphatase sensitivity of the Cyclin A isoforms, embryo and ovary extracts were made in lysis buffer and incubated with and without Lambda phosphatase (NEB) for 30 min at 37C. Extracts were then separated by 7.5% SDS/PAGE and detected by Western blot analysis as described above.
Embryo Translation Assay.
RNA production and injection into embryos and luciferase assays were done as described in ref. 4.
Acknowledgments.
We thank Mary Lilly (National Institute of Child Health and Human Development, National Institutes of Health) for helpful discussions and for providing the Bruno antibody, Frank Sprenger for the cyclin A deletion plasmids, and Pat O'Farrell for the Cyclin A monoclonal antibody. This work was supported by National Institutes of Health Grant GM39341. T.L.O.-W. is an American Cancer Society Research Professor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–229. doi: 10.1016/j.bbagrm.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit P, Papin C, Kwak JE, Wickens M, Simonelig M. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development. 2008;135:1969–1979. doi: 10.1242/dev.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit B, et al. An essential cytoplasmic function for the nuclear poly(A) binding protein, PABP2, in poly(A) tail length control and early development in Drosophila. Dev Cell. 2005;9:511–522. doi: 10.1016/j.devcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Vardy L, Orr-Weaver TL. The Drosophila PNG kinase complex regulates the translation of Cyclin B. Dev Cell. 2007;12:157–166. doi: 10.1016/j.devcel.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs H, Knoblich J, Lehner C. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998;12:3741–3751. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang Y, et al. The inhibition of Polo kinase by Matrimony maintains G2 arrest in the meiotic cell cycle. PLos Biol. 2007;5:e323. doi: 10.1371/journal.pbio.0050323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Stetina JR, et al. α-Endosulfine is a conserved protein required for oocyte meiotic maturation in Drosophila. Development. 2008;135:3697–3706. doi: 10.1242/dev.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horner VL, Wolfner MF. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev Dyn. 2008;237:527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- 9.Swan A, Schupbach T. The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development. 2007;134:891–899. doi: 10.1242/dev.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesin JA, Orr-Weaver TL. Developmental role and regulation of cortex, a meiosis-specific Anaphase-Promoting Complex/Cyclosome activator. PLoS Genet. 2007;3:e202. doi: 10.1371/journal.pgen.0030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamanski F, Orr-Weaver TL. The Drosophila plutonium and pan gu genes regulate entry into S phase at fertilization. Cell. 1991;66:1289–1300. doi: 10.1016/0092-8674(91)90050-9. [DOI] [PubMed] [Google Scholar]
- 12.Freeman M, Glover D. The gnu mutation of Drosophila causes inappropriate DNA synthesis in unfertilized and fertilized eggs. Genes Dev. 1987;1:924–930. doi: 10.1101/gad.1.9.924. [DOI] [PubMed] [Google Scholar]
- 13.Persson JL, et al. Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction. 2005;130:411–422. doi: 10.1530/rep.1.00719. [DOI] [PubMed] [Google Scholar]
- 14.Lilly MA, de Cuevas M, Spradling AC. Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev Biol. 2000;218:53–63. doi: 10.1006/dbio.1999.9570. [DOI] [PubMed] [Google Scholar]
- 15.Morris JZ, Hong A, Lilly MA, Lehmann R. twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development. 2005;132:1165–1174. doi: 10.1242/dev.01672. [DOI] [PubMed] [Google Scholar]
- 16.Sugimura I, Lilly MA. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev Cell. 2006;10:127–135. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Swan A, Barcelo G, Schupbach T. Drosophila Cks30A interacts with Cdk1 to target Cyclin A for destruction in the female germline. Development. 2005;132:3669–3678. doi: 10.1242/dev.01940. [DOI] [PubMed] [Google Scholar]
- 18.Fenger DD, et al. PAN GU: A protein kinase that inhibits S phase and promotes mitosis in early Drosophila development. Development. 2000;127:4763–4774. doi: 10.1242/dev.127.22.4763. [DOI] [PubMed] [Google Scholar]
- 19.Lee LA, Elfring LK, Bosco G, Orr-Weaver TL. A genetic screen for suppressors and enhancers of the Drosophila PAN GU cell cycle kinase identifies Cyclin B as a target. Genetics. 2001;158:1545–1556. doi: 10.1093/genetics/158.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilliland WD, Hughes SE, Vietti DR, Hawley RS. Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes. Dev Biol. 2008;325:122–128. doi: 10.1016/j.ydbio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Gilliland WD, et al. The multiple roles of Mps1 in Drosophila female meiosis. PLoS Genet. 2007;3:e113. doi: 10.1371/journal.pgen.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doane W. Completion of meiosis in uninseminated eggs of Drosophila melanogaster. Science. 1960;132:677–678. doi: 10.1126/science.132.3428.677. [DOI] [PubMed] [Google Scholar]
- 23.Heifetz Y, Yu J, Wolfner MF. Ovulation triggers activation of Drosophila oocytes. Dev Biol. 2001;234:416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- 24.Edgar BA, Sprenger F, Duronio RJ, Leopold P, O'Farrell PH. Distinct molecular mechanisms regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994;8:440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran V, Matzkies M, Dienemann A, Sprenger F. Cyclin A degradation employs preferentially used lysines and a cyclin box function other than Cdk1 binding. Cell Cycle. 2007;6:171–181. doi: 10.4161/cc.6.2.3716. [DOI] [PubMed] [Google Scholar]
- 26.Kaspar M, Dienemann A, Schulze C, Sprenger F. Mitotic degradation of cyclin A is mediated by multiple and novel destruction signals. Curr Biol. 2001;11:685–690. doi: 10.1016/s0960-9822(01)00205-6. [DOI] [PubMed] [Google Scholar]
- 27.Kashevsky H, et al. The anaphase promoting complex/cyclosome is required during development for modified cell cycles. Proc Natl Acad Sci USA. 2002;99:11217–11222. doi: 10.1073/pnas.172391099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee LA, Van Hoewyk D, Orr-Weaver TL. The Drosophila cell cycle kinase PAN GU forms an active complex with PLUTONIUM and GNU to regulate embryonic divisions. Genes Dev. 2003;17:2979–2991. doi: 10.1101/gad.1132603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renault AD, et al. giant nuclei is essential in the cell cycle transition from meiosis to mitosis. Development. 2003;130:2997–3005. doi: 10.1242/dev.00501. [DOI] [PubMed] [Google Scholar]
- 30.Page AW, Orr-Weaver TL. Activation of the meiotic divisions in Drosophila oocytes. Dev Biol. 1997;183:195–207. doi: 10.1006/dbio.1997.8506. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X-H, et al. Spatial and temporal control of mitotic cyclins by the Gnu regulator of embryonic mitosis in Drosophila. J Cell Sci. 2004;117:3571–3578. doi: 10.1242/jcs.01240. [DOI] [PubMed] [Google Scholar]
- 32.Tadros W, et al. SMAUG is the major regulator of maternal mRNA destabilization in Drosophila and is translationally activated by the PAN GU kinase. Dev Cell. 2007;12:143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Cui J, Sackton KL, Horner VL, Kumar KE, Wolfner MF. Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation. Genetics. 2008;178:2017–2029. doi: 10.1534/genetics.107.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickens M, Bernstein DS, Kimble J, Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 2002;18:150–157. doi: 10.1016/s0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- 35.Kadyrova LY, Habara Y, Lee TH, Wharton RP. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development. 2007;134:1519–1527. doi: 10.1242/dev.002212. [DOI] [PubMed] [Google Scholar]
- 36.Murata Y, Wharton RP. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell. 1995;80:747–756. doi: 10.1016/0092-8674(95)90353-4. [DOI] [PubMed] [Google Scholar]
- 37.Zamore PD, Williamson JR, Lehmann R. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA. 1997;3:1421–1433. [PMC free article] [PubMed] [Google Scholar]
- 38.Wolthuis RM, et al. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol Cell. 2008;30:290–302. doi: 10.1016/j.molcel.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Lehner CF, O'Farrell PH. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of Cyclins A, B, and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs HW, Keidel E, Lehner CF. A complex degradation signal in Cyclin A required for G1 arrest, and a C-terminal region for mitosis. EMBO J. 2001;20:2376–2386. doi: 10.1093/emboj/20.10.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page AW, Orr-Weaver TL. The Drosophila genes grauzone and cortex are necessary for proper female meiosis. J Cell Sci. 1996;109:1707–1715. doi: 10.1242/jcs.109.7.1707. [DOI] [PubMed] [Google Scholar]
- 44.Freeman M, Nusslein-Volhard C, Glover D. The dissociation of nuclear and centrosomal division in gnu, a mutation causing giant nuclei in Drosophila. Cell. 1986;46:457–468. doi: 10.1016/0092-8674(86)90666-5. [DOI] [PubMed] [Google Scholar]
- 45.Reed BH, Orr-Weaver TL. The Drosophila gene morula inhibits mitotic functions in the endo cell cycle and the mitotic cell cycle. Development. 1997;124:3543–3553. doi: 10.1242/dev.124.18.3543. [DOI] [PubMed] [Google Scholar]
- 46.Barker DD, Wang C, Moore J, Dickinson LK, Lehmann R. Pumilio is essential for function but not for distribution of the Drosophila abdominal determinant Nanos. Genes Dev. 1992;6:2312–2326. doi: 10.1101/gad.6.12a.2312. [DOI] [PubMed] [Google Scholar]
- 47.Spradling AC. In: The development of Drosophila melanogaster. Bate M, Martinez Arias A, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. pp. 1–70. [Google Scholar]
- 48.Dawson IA, Roth S, Akam M, Artavanis-Tsakonas S. Mutations at the fizzy locus cause metaphase arrest in Drosophila melanogaster embryos. Development. 1993;117:359–376. doi: 10.1242/dev.117.1.359. [DOI] [PubMed] [Google Scholar]
- 49.Dawson IA, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of Cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]