Abstract
Condensin is a 5 subunit complex that plays an important role in the structure of chromosomes during mitosis. It is known that phosphorylation of condensin subunits by cdc2/cyclin B at the beginning of mitosis is important for condensin activity, but the sites of these phosphorylation events have not been identified nor has their role in regulating condensin function. Here we identify two threonine residues in the CAP-G subunit of condensin, threonines 308 and 332, that are targets of cdc2/cyclin B phosphorylation. Mutation of these threonines to alanines results in defects in CAP-G localization with chromosomes during mitosis. These results are the first to identify phosphorylation sites within the condensin complex that regulate condensin localization with chromosomal DNA.
Keywords: condensin, CAP-G, Cdc2 phosphorylation, mitosis
Introduction
Mitosis is characterized by a tightly regulated series of events characterized by condensation and subsequent segregation of chromosomes. Chromosome condensation represents a critical step in the process of mitosis leading to the formation of two daughter cells, each with a set of properly segregated and replicated chromosomes. Failure to correctly condense and segregate chromosomes can lead to aneuploidy which is characterized by abnormal chromosome number and is linked to birth defects and cancer [1,2].
Condensin I was originally identified in extracts of Xenopus laevis eggs [3,4]. Condensin I is composed of two SMC (structural maintenance of chromosomes) subunits, CAP-E and CAP-C as well as three non-SMC subunits (CAP-D2, CAP-G, and CAP-H) [5, 6]. CAP-C (SMC4) and CAP-E (SMC2) form a heterodimer and are members of an ATPase family known as SMC or structural maintenance of chromosomes which are involved in chromosome dynamics [5]. Similar to other SMC proteins, there are two ATP molecules located between the ATP binding domains at the distal ends of the heterodimer [7]. The three non-SMC subunits CAP-G, CAP-D2, and CAP-H form a subcomplex that binds to the distal head domains of CAP-C and CAP-E [8].
Studies have demonstrated that purified Xenopus or human condensin I can introduce positive supercoils into relaxed circular DNA when topoisomerase I and ATP are present [9-11]. Studies in S. cerevisiae have determined that the non-SMC subunits are required for condensin I binding to DNA and are also critical for the segregation and condensation of chromosomes [12]. Loss of human CAP-E, CAP-C, or CAP-G resulted in aberrant chromosome morphology [6]. A similar effect on chromosomes was seen with loss of CAP-D2 [13]. Loss of Barren, the Drosophila protein orthologous to CAP-H, leads to abnormal segregation of chromosomes and subsequently causes lagging chromatids [14].
The non-SMC subunits of Condensin I appear to be phosphorylated by Cdc2 in a mitotic specific manner which regulates its positive supercoiling activity as well as its localization to chromosomes [3,10,11,15,16]. Depletion of Cdc2 in Xenopus extracts reduced phosphorylation of condensin I subunits and abolished condensation activity [10]. Cdc2 and Aurora B are serine/threonine kinases which are both crucial in regulating numerous processes leading to cell division, albeit through different pathways [17]. A recent study indicated that both Cdc2 and Aurora B regulate chromosome binding of condensin during mitosis, with about 50% of the chromosomal localization dependent on Cdc2 whereas 12.5% was dependent on Aurora B kinase [16].
Preliminary studies in Xenopus laevis have determined putative Cdc2 phosphorylation sites for XCAP-D2 [10]. However, the specific phosphorylation sites for the remaining non-SMC subunits have not yet been elucidated and the role of these individual phospho-sites in regulating condensin function is poorly understood. The goal of this study was to determine the mitotic phosphorylation sites in human CAP-G (hCAP-G) and determine the significance of this phosphorylation with respect to its chromosomal localization during mitosis. Through peptide array analysis, we discovered three potential Cdc2 phosphorylation sites in hCAP-G. Mutation of threonine 308 led to significant changes in the localization of the CAP-G subunit in mitotic cells. However, mutation of both threonines 308 and 332 to alanine resulted in an even greater effect on localization of CAP-G with chromosomes during mitosis as detected by fluorescence microscopy analysis of cells expressing this mutant.
Materials and Methods
Peptide array analysis
To determine potential Cdc2 phosphorylation sites in CAP-G, peptide array analysis was performed (Jerini, Inc.) In this analysis an overlapping array of peptides representing the CAP-G sequence was spotted on a solid support and incubated with Cdc2/cyclin B (NEB) in the presence of γ-32P-ATP, washed, and then labeled peptides were visualized by autoradiography.
Plasmids/Antibodies
A human CAP-G cDNA (GenBank accession # AF331796) was directionally subcloned into the XhoI/KpnI multi-cloning site of the pEGFP C2 vector (Clontech) to create an N-terminal green fluorescent protein (GFP) fusion of hCAP-G. To generate threonine to alanine mutants, site-directed mutagenesis was performed according to the manufacturer's instructions (Stratagene). Constructs were verified by sequencing (Davis Sequencing).
Cell culture, transfection, and enrichment of mitotic cell populations
HeLa cells were grown in DMEM, 10% fetal bovine serum (FBS), 50 μg/ml gentamicin at 37°C with 5% CO2. These cells were transiently transfected with pEGFP-CAP-G, pEGFP-CAP-GT308A, pEGFP-CAP-GT332A, pEGFP-CAP-GT931A, or pEGFP-CAP-GT308A/T332A using jetPEI (Bridge Bioscience) according to the manufacturer's instructions. Enrichment of mitotic cells was achieved by treating the cells with 250ng/mL nocodazole (Sigma-Aldrich) for 18 hours for fluorescence analysis of mitotic cells or 500ng/mL for 6 hours for the experiments involving chromosome spreads [13].
Fluorescence microscopy analysis
HeLa cells were seeded onto coverslips that had been acid-washed, flamed, and then coated with laminin (5μg/mL) (Sigma). Cells were transfected and treated with nocodazole as described above. Cells were washed once in ice-cold 1×PBS, followed by fixation in 4% paraformaldehyde and permeabilization in 0.5% Triton X-100. After washes in 1×PBS, coverslips were mounted onto slides with Vectashield mounting medium plus 1.5μg/mL DAPI (4′, 6 diamidino-2-phenylindole) (Vector Laboratories). The GFP-CAP-G constructs were visualized using a Nikon fluorescence microscope with a 100× oil immersion objective and a Nikon Spotcam digital-imaging camera.
Results
Phosphorylation sites in CAP-G
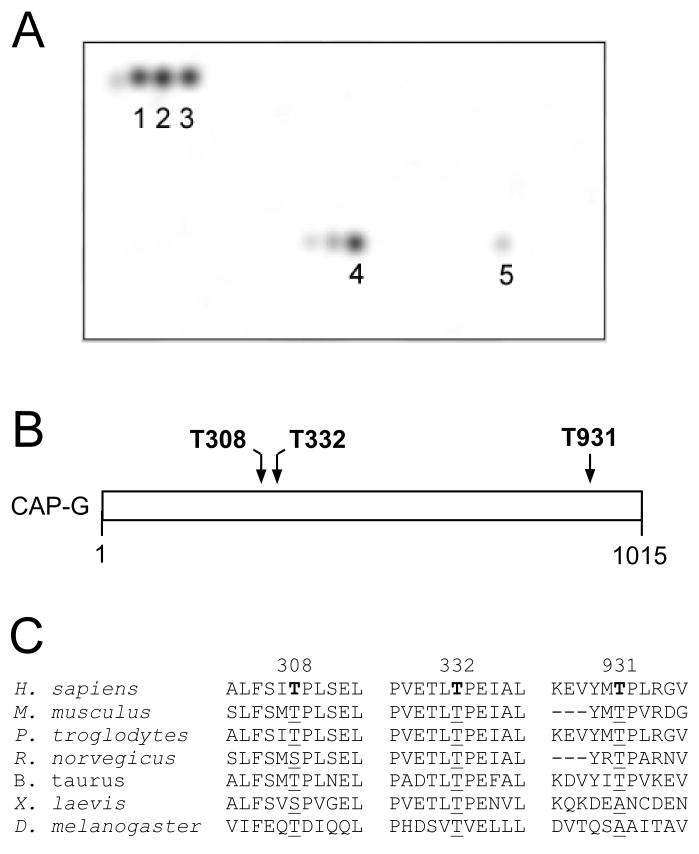
In order to obtain a better understanding of CAP-G phosphorylation during mitosis, a peptide array analysis was utilized to determine potential Cdc2 sites within the protein. In this analysis, an overlapping array of peptides representing the full length sequence of the CAP-G protein was spotted onto a solid support and incubated with Cdc2/cyclin B (NEB) in the presence of γ-32P-ATP. The results of this experiment, shown in Figure 1A, indicate that there are three potential Cdc2 phosphorylation sites. The overlapping phosphopeptide set marked 1, 2, 3 correspond to a candidate Cdc2 site at threonine 931, set 4 corresponds to another at threonine 332, and the phosphopeptide marked 5 represents a site at threonine 308. Figure 1B represents a schematic of the CAP-G protein showing the localization of these three potential Cdc2 phosphorylation sites. A comparison of the evolutionary conservation of the sequences at each of the three candidate phosphorylation sites is shown in Figure 1C, and indicates that the sequences at threonines 308 and 332 are slightly more evolutionarily conserved than the threonine 931 site.
Fig. 1.
Identification of Cdc2 phosphorylation sites in the CAP-G condensin subunit. An overlapping array of peptides representing the complete CAP-G sequence was spotted on a solid support and then incubated with Cdc2/cyclin B (NEB) in the presence of γ-32P-ATP. (A) The results of phosphopeptide analysis showing three potential Cdc2/cyclin B phosphorylation sites in the CAP-G protein are shown (assay by Jerini, Inc.). The overlapping phosphopeptide set marked 1, 2, 3 corresponds to a candidate Cdc2 site at threonine 931, set 4 corresponds to another at Threonine 332, and the phosphopeptide marked 5 represents a site at Threonine 308. (B) Schematic showing the location of these three candidate phosphorylation sites within the CAP-G protein. (C) Comparison of the evolutionary conservation of the sequences at the three candidate phosphorylation sites.
Chromosomal localization of wildtype and phospho-site mutant CAP-G proteins during mitosis
In fission yeast, Cdc2 phosphorylation is responsible for the nuclear localization of condensin in mitosis [18]. Therefore, we hypothesized that altering the putative phosphorylation sites in hCAP-G would affect the localization of this subunit with DNA during mitosis. To test this hypothesis, site-directed mutagenesis was used to mutate the threonine sites to alanine. HeLa cells were transiently transfected with pEGFP-CAP-G, pEGFP-CAP-GT308A, pEGFP-CAP-GT332A, pEGFP-CAP-GT931A, or pEGFP-CAP-GT308A/T332A and then treated with nocodazole to enrich for mitotic cells. The cells were then subjected to fluorescence microscopy analysis in the presence of DAPI staining to examine the localization of the wildtype vs. mutant CAP-G proteins with chromosomal DNA in the mitotic cells. The results of this experiment, shown in Figure 2, indicate that mutation of either threonine 308 or 332 to alanine is associated with a decreased localization of CAP-G with chromosomal DNA during mitosis. In contrast, mutation of threonine 931 to alanine does not appear to affect the localization of CAP-G with chromosomes DNA during mitosis. Simultaneous mutation of both threonine 308 and threonine 332 to alanines also results in decreased localization of CAP-G with chromosomal DNA in mitotic cells, and the effect appears to be larger than that observed for the single threonine mutants (threonine 308 or threonine 332). The results of this experiment suggest that phosphorylation of threonines 308 and 332 is important for correct CAP-G localization with chromosomes in mitotic cells. The results of Western blot analysis of the levels of the transfected wildtype and threonine-to-alanine mutant GFP-CAP-G proteins, normalized to amounts of β-actin, is shown in Figure 3.
Fig. 2.
Phosphorylation of CAP-G at threonines 308 and 332 is important for its localization with chromosomes during mitosis. HeLa cells were transiently transfected with GFP-hCAP-G, GFP-hCAP-GT308A, GFP-hCAP-GT332A, GFP-hCAP-GT931A, and GFP-hCAP-GT308A/T332A constructs and then nocodazole treated to enrich for mitotic cell populations. The cells were then subjected to fluorescence microscopy, with DAPI staining used to detect the DNA. Bar represents 10 μm.
Fig. 3.

Western blot assay of levels of wildtype and threonine-to-alanine mutant GFP-CAP-G proteins. Extracts of HeLa cells transiently transfected with the constructs encoding wildtype and the different threonine-to-alanine mutant GFP-CAP-G proteins were subjected to SDS-PAGE and Western blot assay using anti-GFP and anti-β-actin antibodies.
In order to obtain a more quantitative measure of the effects of the different phosphosite mutations on the ability of CAP-G to localize with chromosomal DNA, we examined the localizations of GFP-CAP-G proteins carrying the various mutations in a number of different cells. Figure 4 contains a graph showing the results of this analysis, presented as the percent of cells transfected with each construct that exhibited localization of the transfected GFP-CAP-G wildtype or mutant protein with chromosomal DNA in mitotic cells. The results of this experiment further support the importance of phosphorylation of CAP-G at threonines 308 and 332 for correct CAP-G localization with chromosomal DNA in mitotic cells.
Fig. 4.
Quantitative comparison of effects of phosphosite mutations on CAP-G localization with chromosomal DNA. This graph shows the results of quantitation of the percent of cells transfected with each construct that exhibited localization of the transfected GFP-CAP-G wildtype or mutant protein with chromosomal DNA. Data is presented as the means +/- s.d., and each is from 3 data sets.
Discussion
DNA condensation represents a critical step at the beginning of mitosis and allows proper segregation of chromosomes which is essential for the formation of two daughter cells in cytokinesis each with an identical set of replicated DNA. Failure to correctly condense and segregate chromosomes can lead to aneuploidy which is characterized by abnormal chromosome number and is linked to birth defects and cancer [1]. Thus, it is important to understand the mechanisms that regulate the activity of factors involved in mitotic chromosome structure, such as condensin, which was the goal of the current study.
Cdc2 phosphorylation of the non-SMC subunits during mitosis regulates the positive supercoiling activity of condensin I as well as condensin localization on chromosomes [3,10,11,15,16]. Despite the importance of the overall Cdc2 phosphorylation of the non-SMC subunits to condensin function, the specific phosphorylation sites of CAP-G and CAP-H have not been determined, although the putative Cdc2 sites have been mapped for Xenopus CAP-D2 [10]. The experiments described in our current study have examined three putative phosphorylation sites at threonines 308, 332, and 931 in the hCAP-G subunit of condensin. The results of these experiments showed that loss of the threonine 308 and 332 phosphorylation sites appears to correlate with altered localization of CAP-G with the DNA in mitosis, suggesting that phosphorylation of CAP-G at these sites is important for condensin function during mitosis.
Previous studies investigated an epigenetic phenomenon known as gene bookmarking, in which specific gene promoters remain relatively uncompacted in comparison to most genomic DNA [19-23]. A subsequent study defined the mechanism by which heat shock transcription factor 2 (HSF2) mediates gene bookmarking at the hsp70 promoter, one of the promoters known to be involved in this phenomenon. HSF2 binds to the heat shock elements (HSEs) in hsp70 and other heat shock gene promoters during mitosis and recruits the phosphatase PP2A, while simultaneously interacting with the CAP-G subunit of human condensin [24]. This interaction leads to the dephosphorylation/inactivation of CAP-G and the condensin complex and reduces compaction at this specific region of chromosomal DNA. The reduced compaction at the hsp70 promoter allows rapid reassembly to a transcriptionally competent state in early G1 phase of the cell cycle and ensures the ability of the cell to induce this protective heat shock protein if stress conditions occur [24]. Our results here showing the importance of CAP-G phosphorylation for CAP-G function activity further strengthens our previous results suggesting that dephosphorylation of this condensin subunit is a means for blocking condensin activity during gene bookmarking events in specific chromosomal regions.
Given the importance of Cdc2 phosphorylation in CAP-G localization and regulating condensin function in mitosis, future studies to determine the exact Cdc2 sites for the remaining non-SMC subunits will be necessary in determining the mechanism of this phosphorylation to the critical function of condensin I in DNA compaction during mitosis. In addition, cells contain another condensin complex called condensin II that also plays a role in mitotic chromosome structure [1,2]. Thus, it will also be important to investigate the role of cell cycle phosphorylation events in regulating the functional properties of condensin II.
Acknowledgments
This research was supported by NIH grant GM64606 to K.D.S. We thank other members of the laboratory for insightful comments during the course of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Legagneux V, Cubizolles F, Watrin E. Multiple roles of Condensins: a complex story. Biol Cell. 2004;96:201–13. doi: 10.1016/j.biolcel.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Hirano T. Chromosome shaping by two condensins. Cell Cycle. 2004;3:26–8. [PubMed] [Google Scholar]
- 3.Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–21. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- 4.Hirano T, Mitchison TJ. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–58. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 5.Hagstrom KA, Meyer BJ. Condensin and cohesin: more than chromosome compactor and glue. Nat Rev Genet. 2003;4:520–34. doi: 10.1038/nrg1110. [DOI] [PubMed] [Google Scholar]
- 6.Ono T, Losada A, Hirano M, Myers MP, Neuwald AF, Hirano T. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 2003;115:109–21. doi: 10.1016/s0092-8674(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 7.Melby TE, Ciampaglio CN, Briscoe G, Erickson HP. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J Cell Biol. 1998;142:1595–604. doi: 10.1083/jcb.142.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura SH, Hizume K, Murakami A, Sutani T, Takeyasu K, Yanagida M. Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr Biol. 2002;12:508–13. doi: 10.1016/s0960-9822(02)00719-4. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Cuvier O, Hirano T. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J Biol Chem. 2001;276:5417–20. doi: 10.1074/jbc.C000873200. [DOI] [PubMed] [Google Scholar]
- 10.Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282:487–90. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- 11.Kimura K, Hirano T. Dual roles of the 11S regulatory subcomplex in condensin functions. Proc Natl Acad Sci U S A. 2000;97:11972–7. doi: 10.1073/pnas.220326097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavoie BD, Hogan E, Koshland D. In vivo dissection of the chromosome condensation machinery: reversibility of condensation distinguishes contributions of condensin and cohesin. J Cell Biol. 2002;156:805–15. doi: 10.1083/jcb.200109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watrin E, Legagneux V. Contribution of hCAP-D2, a non-SMC subunit of condensin I, to chromosome and chromosomal protein dynamics during mitosis. Mol Cell Biol. 2005;25:740–50. doi: 10.1128/MCB.25.2.740-750.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhat MA, Philp AV, Glover DM, Bellen HJ. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with Topoisomerase II. Cell. 1996;87:1103–14. doi: 10.1016/s0092-8674(00)81804-8. [DOI] [PubMed] [Google Scholar]
- 15.Kimura K, Hirano T. ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell. 1997;90:625–34. doi: 10.1016/s0092-8674(00)80524-3. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto A, Murayama A, Katano M, Urano T, Furukawa K, Yokoyama S, Yanagisawa J, Hanaoka F, Kimura K. Analysis of the role of Aurora B on the chromosomal targeting of condensin I. Nucleic Acids Res. 2007;35:2403–12. doi: 10.1093/nar/gkm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari S. Protein kinases controlling the onset of mitosis. Cell Mol Life Sci. 2006;63:781–95. doi: 10.1007/s00018-005-5515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutani T, Yuasa T, Tomonaga T, Dohmae N, Takio K, Yanagida M. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 1999;13:2271–83. doi: 10.1101/gad.13.17.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christova R, Oelgeschlager T. Association of human TFIID-promoter complexes with silenced mitotic chromatin in vivo. Nat Cell Biol. 2002;4:79–82. doi: 10.1038/ncb733. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Balbas MA, Dey A, Rabindran SK, Ozato K, Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83:29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 21.Michelotti EF, Sanford S, Levens D. Marking of active genes on mitotic chromosomes. Nature. 1997;388:895–899. doi: 10.1038/42282. [DOI] [PubMed] [Google Scholar]
- 22.John S, Workman JL. Bookmarking genes for activation in condensed mitotic chromosomes. Bioessays. 1998;20:275–279. doi: 10.1002/(SICI)1521-1878(199804)20:4<275::AID-BIES1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Sarge KD, Park-Sarge OK. Gene bookmarking: keeping the pages open. Trends Biochem Sci. 2005;30:605–10. doi: 10.1016/j.tibs.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Xing H, Wilkerson DC, Mayhew CN, Lubert EJ, Skaggs HS, Goodson ML, Hong Y, Park-Sarge OK, Sarge KD. Mechanism of hsp70i gene bookmarking. Science. 2005;307:421–3. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]