Abstract
Introduction
Neuroblastoma is the most common pediatric extracranial solid cancer. This tumor is characterized by metaiodobenzylguanidine (MIBG) avidity in 90% of cases, prompting the use of radiolabeled MIBG for targeted radiotherapy in these tumors.
Methods
The available English language literature was reviewed for original research investigating in vitro, in vivo, and clinical applications of radiolabeled MIBG for neuroblastoma.
Results
MIBG is actively transported into neuroblastoma cells by the norepinephrine transporter. Preclinical studies demonstrate substantial activity of radiolabeled MIBG in neuroblastoma models, with 131I-MIBG showing enhanced activity in larger tumors compared to 125I-MIBG. Clinical studies of 131I-MIBG in patients with relapsed or refractory neuroblastoma have identified myelosuppression as the main dose-limiting toxicity, necessitating stem cell reinfusion at higher doses. Most studies report a response rate of 30–40% with 131I-MIBG in this population. More recent studies have focused on the use of 131I-MIBG in combination with chemotherapy or myeloablative regimens.
Conclusions
131I-MIBG is an active agent for the treatment of patients with neuroblastoma. Future studies will need to define the optimal role of this targeted radiopharmaceutical in the therapy of this disease.
Keywords: Metaiodobenzylguanidine, neuroblastoma, pediatric, radionuclide
Introduction
Neuroblastoma is the most common extracranial malignant solid tumor of childhood. The peak incidence occurs during early childhood and approximately 650 new cases are diagnosed in the United States each year [1]. The tumor is derived from the sympathetic nervous system. The most common site of origin is within the adrenal medulla, although the tumor also commonly arises elsewhere along the sympathetic chain.
Neuroblastoma is notable for its heterogeneous clinical behavior. Several key clinical and biological prognostic features have been identified that appear to influence the behavior of these tumors. The two most important clinical features are age and stage. Younger patients have a lower risk of developing recurrent disease and therefore improved overall survival. The threshold at which a patient is considered “young” has shifted in recent years from less than 1 year to less than 18 months of age at initial diagnosis [2,3]. All patients with newly diagnosed neuroblastoma undergo a series of staging studies to evaluate the extent of disease. Approximately 50% of patients have distant hematogenous metastases at the time of diagnosis, most commonly to the bone or bone marrow [4]. These patients will undergo dedicated imaging of the primary tumor and bone marrow biopsies. As a tumor derived from the sympathetic nervous system, these tumors typically express the norepinephrine transporter which mediates active intracellular uptake of radiolabeled metaiodobenzylguanidine (MIBG) in approximately 90% of patients [5,6]. Patients with newly-diagnosed neuroblastoma therefore typically also have a diagnostic MIBG scan performed to identify sites of bone metastasis or diffuse bone marrow involvement [7]. The results of these staging studies are used to assign a patient a stage based upon the International Neuroblastoma Staging System (INSS) [8]. In addition to these clinical features, several biological prognostic factors have also been identified. The most important biological prognostic factor is amplification of the MYCN oncogene, which has consistently been reported as an independent adverse prognostic factor [9]. Tumor histopathologic grading according to the Shimada classification system, tumor ploidy, gain of chromosome 17q, and deletions of 1p and 11q are other biological prognostic factors evaluated in these tumors [10–13].
The treatment of neuroblastoma depends upon a patient’s estimated risk of relapse, based upon these identified clinical and biological prognostic features. For those patients with low-stage localized tumors (INSS 1 and 2) and favorable biological features, surgical resection alone is almost always curative. For patients with locally aggressive tumors (INSS 3) but favorable biology, the combination of chemotherapy and surgical resection is the standard approach with excellent outcomes [14]. Patients with metastatic disease at initial diagnosis who are greater than 18 months of age and patients with MYCN amplified locoregional tumors are treated with intensive multimodal therapy with chemotherapy, surgical resection, local radiation, and consolidation with high-dose therapy with autologous hematopoietic stem cell rescue [15]. These patients also benefit from 13-cis-retinoic acid given post-consolidation as a differentiating agent for minimal residual disease [15,16]. While this intensive approach has been shown to improve outcome, patients with high-risk disease frequently relapse and fewer than 50% of these patients will be long-term survivors [15,16].
The poor outcome in patients with high-risk disease and the observation that 90% of tumors are MIBG-avid provide the rationale for utilizing MIBG as a targeted radionuclide in these patients. This review will summarize the available preclinical and clinical experience of using MIBG as a therapeutic agent for neuroblastoma. The review will include a discussion of approaches that combine MIBG with other active agents for neuroblastoma. The review will conclude with an overview of the reported late effects of MIBG therapy in this patient population as well as the practical considerations involved in administering MIBG therapy to young patients.
Preclinical Studies of Radiolabeled MIBG in Neuroblastoma
A relatively small number of studies have evaluated radiolabeled MIBG in preclinical models of neuroblastoma. Most of these studies have focused on MIBG uptake into neuroblastoma cells, though other groups have also investigated mechanisms of the cytotoxicity of this agent in neuroblastoma. Early investigators observed that only a subset of neuroblastoma cell lines demonstrated specific uptake of radiolabeled MIBG, while other neuroblastoma cell lines showed only passive diffusion of the drug into cells [17]. The specific uptake is reduced by inhibition of the Na-K-ATPase and also competitively inhibited by norepinephrine [17–19]. These results suggested the possibility that MIBG is actively taken up into neuroblastoma cells by the norepinephrine transporter. This possibility was supported by studies demonstrating attenuation of MIBG uptake into neuroblastoma cells in the presence of imipramine, an inhibitor of norepinephrine transport [19–21]. In addition, MIBG uptake into neuroblastoma cells shows a strong correlation with norepinephrine transporter expression levels [5,22,23]. Neuroblastoma cells that do not actively take up MIBG become MIBG avid when transfected with the norepinephrine transporter gene [24]. Similarly, non-neural cells can be engineered to take up MIBG by transfection with the norepinephrine transporter gene [25,26]. These data indicate that neuroblastoma cells take up MIBG via specific active uptake by the norepinephrine transporter gene. Once taken up into neuroblastoma cells, most MIBG appears to be stored in the cytoplasm and mitochondria, rather than in the neurosecretory granules that store norepinephrine [19,21,27,28].
Several factors may modulate MIBG uptake by neuroblastoma cells (Table 1). Some of these findings may have implications for the clinical application of radiolabeled MIBG to patients with neuroblastoma. MIBG uptake appears to be reduced in the presence of hypoxia and by moderate hyperthermia [17,29]. Pretreatment with cisplatin or doxorubicin, two active chemotherapy agents used in the clinical treatment of neuroblastoma, has been shown to significantly increase MIBG uptake by neuroblastoma in vitro and in vivo [30,31]. This effect may be due to an increase in norepinephrine transporter gene expression after chemotherapy exposure [30]. Pretreatment with γ-interferon augments MIBG uptake [32,33]. The evaluation of retinoic acid pretreatment on MIBG uptake has produced mixed results depending on the cell line evaluated. In one early study, retinoic acid had no effect on MIBG uptake [32]. A separate study using a different cell line indicated that retinoic acid pretreatment improved both MIBG uptake and retention [20]. A third study showed that retinoic acid together with γ-interferon increases MIBG uptake into neuroblastoma cells [33]. The effect of retinoic acid on neuroblastoma MIBG uptake will require further study to clarify these conflicting results.
Table 1.
Modulators of MIBG uptake by neuroblastoma cells.
One group has evaluated the efficacy of 131I-MIBG combined with topotecan in preclinical models of neuroblastoma [34]. These studies demonstrated that this combination produced synergistic inhibition of tumor cell growth, particularly when topotecan was given simultaneously with 131I-MIBG. Cells treated with topotecan either simultaneously with or 24 hours after 131I-MIBG treatment appeared to decrease DNA repair mechanisms [34]. These results provide the rationale for some of the combination approaches applied clinically and discussed below.
The mechanism of cytotoxicity of MIBG has been evaluated in preclinical models of neuroblastoma. Experiments in which neuroblastoma cells were exposed in vitro to radiolabeled or unlabeled MIBG demonstrated that unlabeled MIBG at doses < 10 µM does not contribute to the cytotoxicity of radiolabeled MIBG [35]. Instead, targeted radiation exposure accounts for all of the cytotoxicity of radiolabeled MIBG. These results were confirmed in another report indicating that unlabeled MIBG at 1–2 µM was not cytotoxic [36]. In contrast, higher dose unlabeled MIBG may be directly cytotoxic [37–39]. Higher dose unlabeled MIBG produced moderate cytotoxicity in a range of cell types, including fibroblasts, leukemia cells, and neuroblastoma cells [39]. Unlabeled MIBG at doses ≥ 10 µM results in dose-dependent inhibition of neuroblastoma cell growth and an increase in markers of oxidative stress in neuroblastoma cells [37,38]. The small contribution to cytotoxicity of unlabeled MIBG is unlikely to be clinically relevant in human therapies and even less so with a new no-carrier-added formulation (see below).
Several studies have evaluated radiolabeled MIBG in other neuroblastoma models. 125I-MIBG concentrates uniformly throughout 300–400 micron neuroblastoma spheroids, while an antibody directed against neuroectodermal tissues concentrates mainly on the periphery of the spheroids [40]. 131I-MIBG administered to mice with neuroblastoma xenografts concentrates in the neuroblastoma xenografts compared to normal tissues, with peak tumor uptake 6 hours after infusion [41]. Xenograft growth was attenuated compared to control-treated mice for up to 12 days following 131I-MIBG treatment. 131I-MIBG administered to produce a tumor absorbed dose of 5 Gy was as effective as 5 Gy external beam radiation in reducing tumor growth [42]. This finding is significant since the dose rate following 131I-MIBG therapy is much lower than the dose rate from external beam radiation. Moreover, fractionated administration of 131I-MIBG does not appear to improve the efficacy of this therapy compared to the same total dose given as a single treatment [42]. In fact, at least one experiment has suggested that multiple treatments with radiolabeled MIBG may be less effective compared with a single treatment. This experiment demonstrated that a second treatment with radiolabeled MIBG to neuroblastoma cells in vitro resulted in reduced cytotoxicity compared to the first treatment using the same dose [35]. The mechanism for this diminished efficacy is unclear, particularly since in vivo experiments have demonstrated that radiolabeled MIBG storage by neuroblastoma xenografts does not differ between initial and subsequent treatments [42].
The choice of iodine isotope for use with MIBG has been evaluated in preclinical models of neuroblastoma. Given the longer effective range of 131I beta particles compared to the much shorter effective range of 125I electrons, several groups have hypothesized that 125I-MIBG might be better agents for the treatment of microscopic disease. In mathematical models of this issue, 125I-MIBG was anticipated to produce higher dose rates in tumors ≤ 100 microns in diameter compared to 131I-MIBG [43]. In contrast, 131I-MIBG was anticipated to out-perform 125I-MIBG for larger tumors. When this comparison was made using neuroblastoma spheroids of varying sizes, 125I-MIBG and 131I-MIBG were equally efficacious in the treatment of 100 micron spheroids. The effect of 125I-MIBG on tumor growth remained relatively constant as spheroid size increased. In contrast, 131I-MIBG became increasingly efficacious as spheroid size increased [43]. A second group replicated these findings, noting that 125I-MIBG was more effective in treating neuroblastoma monolayers or 240 micron spheroids [44]. 125I-MIBG was ineffective in treating 400 micron spheroids, while 131I-MIBG performed well in treating these larger spheroids [36,44]. 125I-MIBG has also been compared to 131I-MIBG in neuroblastoma xenograft models of both macroscopic and microscopic tumors [42]. In the macroscopic tumor model, 125I-MIBG was considerably less effective at slowing tumor growth compared to 131I-MIBG. In the microscopic tumor model, 125I-MIBG had no antitumor effect. While 131I-MIBG showed some decrease in tumor growth in this microscopic disease model, the effect was much less pronounced compared with external beam total body irradiation [42]. These results support the current use of 131I as the radioisotope of choice for MIBG therapy in neuroblastoma, although combination approaches may also be of future interest. These results also support the clinical observation that 131I-MIBG therapy may be less efficacious for treating bone marrow micrometastatic disease (see below) [45].
An 131I-MIBG formulation with no-carrier-added and therefore higher specific activity than conventional 131I-MIBG has also been evaluated in preclinical models of neuroblastoma. This formulation appears to enter neuroblastoma cells using the same norepinephrine transporter as conventional 131I-MIBG [46]. The cytotoxicity of the no-carrier-added formulation decreased as increasing amounts of unlabeled MIBG were added to neuroblastoma spheroids [46,47]. High specific activity 125I-MIBG appeared to be more cytotoxic than conventional 131I-MIBG in 500 micron neuroblastoma spheroids [48]. In neuroblastoma xenografts, tumor uptake was enhanced in mice receiving the no-carrier-added formulation compared to conventional 131I-MIBG [47], although this result was not replicated by a different group [49]. Given the theoretical advantages of this formulation, no-carrier-added 131I-MIBG has now entered clinical trials in children with neuroblastoma.
Clinical Determinants of MIBG Uptake in Patients with Neuroblastoma
Only 90% of patients with neuroblastoma have MIBG-avid tumors [5,6]. The clinical determinants of MIBG-avidity remain largely unclear, though two studies have begun to address this issue. In the first study, researchers attempted to correlate the intensity of 123I-MIBG tumor uptake on diagnostic scans with clinical and biologic features in 26 patients with neuroblastoma [50]. Aside from a suggestion of increased 123I-MIBG uptake in larger tumors, none of the other variables correlated with intensity of 123I-MIBG uptake, including degree of differentiation and MYCN amplification. In a second study of 54 patients with neuroblastoma, 11 tumors did not express the norepinephrine transporter by RT-PCR [5]. All six tumors that did not take up MIBG on diagnostic scans were included in this group, demonstrating a strong correlation between norepinephrine transporter expression and MIBG uptake in the clinical setting. The mechanism of MIBG uptake in the five MIBG-avid tumors that did not express the norepinephrine transporter is not clear.
Clinical Studies of MIBG Monotherapy in Neuroblastoma
Pharmacokinetics
Relatively few data describe the clearance of radiolabeled MIBG in children with neuroblastoma. In one study, six children with neuroblastoma received 100–200 mCi of 131I-MIBG and had urinary MIBG levels measured following the infusion [51]. A median of 57% and 70% of the administered dose was excreted in the urine by 24 and 48 hours post-infusion, respectively. The elimination half-life during the first 44 hours post-infusion was 10.6 hours, which was slightly faster than in adult patients with neuroendocrine tumors also included in the study. A second study in seven children with neuroblastoma confirmed that 70% of the administered dose is excreted in the urine by 48 hours post-infusion [52,53]. This study included pharmacokinetic plasma sampling for one week following 131I-MIBG infusion and demonstrated a mean terminal half-life of 37 hours. A third study obtained pharmacokinetic data on 17 children with neuroblastoma who received tracer doses of 123I-MIBG or 131I-MIBG [54]. This study demonstrated rapid clearance of MIBG from the blood, with 10% or less remaining in the blood one hour after injection. Not surprisingly, blood radioactivity did not significantly contribute to the whole body dose [55].
Early Experience
The earliest studies of MIBG therapy for patients with neuroblastoma focused mainly on the toxicity and feasibility of this approach. Some groups reported their objective response rates (complete or partial response) in these studies, though the value of this information is somewhat limited by the small sample sizes in many of these studies. The results of those pilot studies with 10 or more patients are presented here and summarized in Table 2.
Table 2.
131I-MIBG monotherapy studies for patients with relapsed or refractory neuroblastoma. Response rate refers to percent of evaluable patients with at least a partial response to therapy as their best overall response.
| Reference(s) | Number of Patients | 131I-MIBG Activity per Cycle | Response Rate (Complete or Partial Response) | Number of Complete Responses |
|---|---|---|---|---|
| Pilot or Single Institution Studies | ||||
| [56] | 11 | 90–450 mCi (100–400 mCi/m2) |
18% | 0 |
| [57] | 11 | 70–256 mCi | 18% | 1 |
| [58] | 12 | Mean 10.3 mCi/kg | 66% | 2 |
| [59] | 15 | To Yield 1 Gy Whole Body Dose |
NE | NE |
| [60] | 17 | 3.8–14.1 mCi/kg | 31% | 0 |
| [62] | 42 | 67–148 mCi | 17% | 2 |
| [63] | 43 | 75–162 mCi | 30% | 1 |
| Formal Phase I Studies | ||||
| [67,68] | 14 | 50–220 mCi | 0 | 0 |
| [69] | 25 | To Yield 1 Gy, 2 Gy, or 2.5 Gy Whole Body Dose | 33% | 0 |
| [70] | 30 | 90–819 mCi (2.6–18.2 mCi/kg) | 37% | 1 |
| Formal Phase II Studies | ||||
| [71] | 26 | Median 70 mCi | 0 | 0 |
| [72] | 53 | 100–200 mCi | 56% | 7 |
| [45] | 164 | 18 mCi/kg (12 mCi/kg if no stem cells) | 36% | 13 |
NE = Not evaluable
Our institution treated 11 patients with refractory neuroblastoma with 100–400 mCi/m2 of 131I-MIBG [56]. The objective response rate was 18%, with two partial responses. Thrombocytopenia was the most prominent toxicity.
The Universita Cattolica in Rome treated 11 patients with refractory neuroblastoma with 70–256 mCi per cycle of therapy [57]. Seven patients received multiple cycles of therapy. The objective response rate was 18%, including one partial response and one complete response. Palliation of pain was reported in all evaluable patients.
A German study included 12 evaluable patients with relapsed or refractory neuroblastoma treated with 131I-MIBG at a mean dose of 10.3 mCi/kg per cycle (range 3.6–20 mCi/kg) [58]. The objective response rate was 66%, with 2 complete responses and 6 partial responses. The median survival was 369 days after 131I-MIBG therapy. Therapy was tolerable, with predominantly hematologic toxicity.
Another German series reported on 15 patients with relapsed or refractory neuroblastoma treated with 131I-MIBG at a dose calculated to achieve 1 Gy whole body radiation dose [59]. Some patients received concomitant chemotherapy, surgery, or stem cell transplant, making treatment response difficult to assess. Nevertheless, ten patients had some degree of disease improvement with this therapy as assessed by imaging, though formal partial and complete responses were not graded.
The Children’s Hospital of Philadelphia treated 17 patients with refractory neuroblastoma with 131I-MIBG ranging in dose from 3.8–14.1 mCi/kg [60]. The objective response rate in evaluable patients was 31%, with all four partial responses seen in the ten patients treated with 7 mCi/kg or greater 131I-MIBG. Of those patients with tumor pain at the time of MIBG therapy, 82% had a subjective decrease in pain following treatment.
An Italian center treated 21 evaluable patients with relapsed or refractory neuroblastoma [61]. Patients received 73–148 mCi of 131I-MIBG per cycle of therapy. The overall response rate was 35.7%, with no complete responses reported.
The group from Genoa reported on 42 patients with relapsed or refractory neuroblastoma treated with 67–148 mCi of 131I-MIBG per cycle of therapy, with 27 patients receiving more than one cycle [62]. Seven patients had a complete or partial response, for an objective response rate of 16.7%. None of the 8 patients with bone marrow involvement cleared their bone marrow with this therapy. Five patients survived for at least two years following 131I-MIBG therapy.
A follow-up study from this same group included 43 patients with relapsed or refractory disease with a range of 75–162 mCi of 131I-MIBG repeated up to every 4 weeks in patients benefiting from therapy [63,64]. Thirty-seven patients received multiple courses of therapy. Myelosuppression was the main toxicity. Thirteen patients (30.2%) had a complete or partial response to therapy, including one patient with a complete response.
Investigators at the University of Michigan have evaluated 125I-MIBG in the treatment of relapsed or refractory neuroblastoma [65,66]. Ten patients received 224–814 mCi of 125I-MIBG. Five of these patients survived for at least 18 months. At least two of these patients had bulk disease at the time of 125I-MIBG therapy. This result is somewhat surprising in light of preclinical data suggesting that 125I-MIBG should be most effective in treating neuroblastoma clusters less than 400 microns in size.
Formal Phase I and II Studies
Three formal phase I dose escalation studies of 131I-MIBG monotherapy in patients with neuroblastoma have been performed. In the first study, 14 patients with relapsed or refractory neuroblastoma at the University of Michigan received 131I-MIBG at doses escalating from 50–220 mCi [67,68]. Myelosuppression was the most notable toxicity and appeared to be more severe in patients who had previously undergone myeloablative therapy. Three patients had minor responses and one patient had a mixed response. Patients survived a median of 5.6 months following the therapy.
The United Kingdom Children’s Cancer Study Group initiated another phase I study of 131I-MIBG for patients with refractory stage 3 or 4 neuroblastoma [69]. Patients received a test dose of 131I-MIBG followed by dosimetry studies to calculate a treatment dose that would deliver escalating whole body radiation doses. Two patients received a whole body dose of 1 Gy, 13 patients received 2 Gy, and 10 patients received 2.5 Gy. The main acute toxicities observed were transient mild blood pressure alterations as well as nausea and vomiting. The most prominent toxicity was myelosuppression, with the severity increasing as the whole body radiation dose increased. Thrombocytopenia was more severe than neutropenia. No patients developed febrile neutropenia. The objective response rate was 33%, with no complete responses noted. A dose-response effect was not observed. The median survival was 12 months.
In the third phase I study, 30 patients at our institution with relapsed or refractory neuroblastoma received 131I-MIBG at escalating doses from 2.6 to 18.2 mCi/kg (90–819 mCi) [70]. Nonhematologic toxicity again was mild across all dose levels and consisted mainly of nausea, vomiting, transient blood pressure changes, and transient xerostomia. Myelosuppression was the most significant toxicity with this therapy and was again dose-dependent. None of the patients treated with 12 mCi/kg or less experienced prolonged neutropenia and therefore did not require autologous stem cell rescue. In contrast, 2 of 5 patients treated with 15 mCi/kg and 4 of 9 patients treated with 18 mCi/kg required stem cell rescue. The maximum tolerated dose for patients without stem cell support was therefore 12 mCi/kg. The objective response rate was 37%, with most of the responses observed in patients receiving 12 mCi/kg or higher 131I-MIBG. The median survival time following treatment was 6 months.
Twenty-six patients with refractory or relapsed neuroblastoma on a French phase II study received a median of 70 mCi (range 30–108 mCi) 131I-MIBG [71]. Twelve patients received multiple cycles of therapy given at a median of one month intervals between treatments. No patients had an objective response, though ten patients had stable disease for at least 8 weeks. Several patients had pain reduction. Myelosuppression was the main toxicity.
A Dutch phase II study of 53 patients with relapsed or refractory neuroblastoma prescribed 100–200 mCi of 131I-MIBG [72]. The objective response rate in this study was 56%, including 7 complete responses. Only 9 patients had progressive disease as their best response to this therapy.
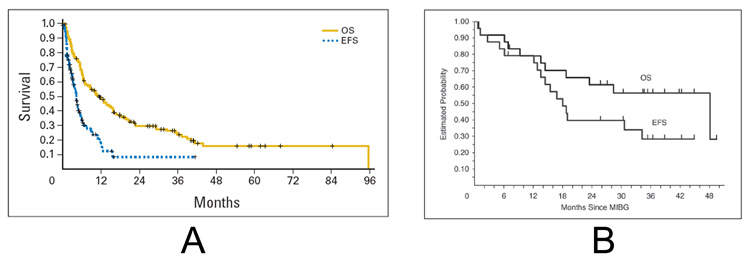
A phase II from UCSF, CHOP, and the University of Michigan treated 164 patients with 131I-MIBG at a dose of 18 mCi/kg or 12 mCi/kg for patients with or without available autologous stem cells [45]. The overall response rate was 36%, though an additional 34% of patients had stable disease following therapy. The response rate in the 16 patients treated at the 12 mCi/kg dose level was 25% compared to 37% for patients treated at the 18 mCi/kg dose level, though this difference was not statistically significant. Predictors of response included older age, disease isolated to soft tissue alone or bone and bone marrow alone, fewer previous treatment regimens, and longer time from diagnosis to 131I-MIBG treatment. The 1-year event-free survival was 18% and the 2-year overall survival was 29% (Figure 1A).
Figure 1.
A. Overall (OS) and event-free survival (EFS) of patients with relapsed or refractory neuroblastoma treated on a phase II study of high-dose 131I-MIBG [45]. B. Overall and event-free survival of patients with refractory neuroblastoma treated on a phase I study of high-dose 131I-MIBG followed by myeloablative chemotherapy with carboplatin, etoposide, and melphalan [87]. (Figures reprinted with permission from the American Society of Clinical Oncology.)
Use of Multiple MIBG Treatments in Patients with Neuroblastoma
Most of the studies described above included patients who received multiple courses of MIBG therapy. While these studies have demonstrated the feasibility of this strategy, only two reports have specifically studied multiple treatments with 131I-MIBG. The first study retrospectively reported on the experience with multiple 131I-MIBG treatments at our institution in 28 patients with relapsed or refractory neuroblastoma [73]. Patients in this series typically received approximately 18 mCi/kg 131I-MIBG separated by a median of 98 days. Fourteen patients had a complete or partial response after the first cycle of therapy. Of the 13 patients with stable disease after the first cycle of therapy, two patients had a partial response and one patient had a mixed response after the second cycle of therapy. These results suggest that the majority of the clinical benefit with 131I-MIBG therapy occurs after the first cycle of therapy, although additional responses may be observed with subsequent cycles.
A New Approaches to Neuroblastoma Therapy (NANT) consortium study has prospectively evaluated the use of two 131I-MIBG infusions given sequentially 14 days apart followed 14 days later by stem cell rescue [74]. In this phase I dose escalation study, 20 evaluable patients received a cumulative 131I-MIBG dose ranging from 22 to 50 mCi/kg over the course of the two infusions. No dose limiting toxicities were noted, though 6 patients at the highest dose level developed grade 3 nonhematologic toxicities. All patients engrafted appropriately after stem cell reinfusion. The overall objective response rate was 10%, though five of 11 (45%) patients with measurable disease had a partial response based on CT scan criteria alone. Ten of 21 patients had a partial response by MIBG scan. The overall objective response rate, though, was only 10%, due mainly to persistence or progression of bone marrow disease in 13 of 15 patients. These results indicate that treatment with two sequential high doses of 131I-MIBG is tolerable, but that clearing bone marrow metastatic remains a challenge with 131I-MIBG monotherapy.
MIBG in Combination with Other Therapies in Neuroblastoma
Given the success of radiolabeled MIBG monotherapy in treating patients with relapsed or refractory neuroblastoma, several groups have evaluated this agent in combination with other active agents for neuroblastoma. An Italian group has reported on their experience using 131I-MIBG in combination with cisplatin, an active agent against neuroblastoma and a radiation sensitizer [75,76]. Five patients with relapsed or refractory disease were treated with cisplatin on the first day of therapy and then 100 mCi 131I-MIBG on the second day of therapy. This treatment was repeated one week later. Of the five patients treated with one course of this therapy, two patients had a complete response, two patients had a partial response, and one patient had a mixed response. The main reported toxicity of this regimen was myelosuppression.
This experience was extended to a larger group of patients treated with cisplatin, 131I-MIBG, and other active agents in neuroblastoma [77]. Sixteen patients with relapsed or refractory neuroblastoma received cisplatin and cyclophosphamide with or without etoposide and vincristine. All patients received 200 mCi 131I-MIBG on day 10. Myelosuppression was again the main toxicity. Twelve of 16 patients (75%) had a partial response with this therapy. The remaining four patients included three with stable disease and one with a mixed response. No long-term outcome data were provided in this report. The response rate with this combination therapy compares favorably with response rates of < 40% observed with 131I-MIBG monotherapy, particularly given the relatively low dose of 131I-MIBG used in this study.
A group in the United Kingdom has evaluated 131I-MIBG together with the camptothecin topotecan [78]. Like cisplatin, topotecan is also an active drug against neuroblastoma with radiation sensitizing properties. Eight patients with relapsed neuroblastoma were treated with topotecan on Days 1–5 and 15–19 along with 12 mCi/kg of 131I-MIBG on days 1 and 15. All patients received hematopoietic stem cell support on Day 27. This combination was well-tolerated and without unanticipated toxicities. Response data were not provided from this pilot study. An ongoing NANT study is evaluating the combination of 131I-MIBG and another camptothecin, irinotecan, in this patient population.
Given that oxygen enhances radiation toxicity and also appears to increase MIBG uptake by neuroblastoma cells, a Dutch group evaluated 131I-MIBG in combination with hyperbaric oxygen therapy [79]. All patients received 200 mCi 131I-MIBG during the first cycle of therapy followed by 100 mCi in any subsequent treatments. A historical control group of 36 patients with relapsed or refractory neuroblastoma received 131I-MIBG alone. This group had a mean survival of 15.4 months and an overall survival of 12% at 28 months. A second group of 27 patients received 4–5 days of hyperbaric oxygen therapy starting 2–4 days after 131I-MIBG treatment. This group had an overall survival of 32% at 28 months. This result suggests a possible improvement in outcome with the addition of hyperbaric oxygen therapy, recognizing the limitations of drawing definitive conclusions using historic controls. Toxicity was comparable between groups. This strategy will require further study to validate these results.
MIBG as a Component of Myeloablative Therapy in Neuroblastoma
As myeloablative therapy has been demonstrated to improve the outcomes for newly diagnosed patients with advanced neuroblastoma [15,80,81], several groups have evaluated 131I-MIBG in combination with myeloablative regimens. One of the first reports of this strategy treated five patients with relapsed or refractory neuroblastoma with a median of 300 mCi 131I-MIBG on day 0 [82]. Patients then received high-dose chemotherapy on days 7–12 using carboplatin and melphalan with our without vincristine and etoposide. Stem cells were given on day 14. Toxicity was as expected for this type of high-dose chemotherapy regimen. All patients engrafted neutrophils. One patient with extensive bone marrow tumor involvement did not engraft platelets. Two patients survived at least 17 months following this therapy.
Another early pilot series included five patients with advanced neuroblastoma treated with 131I-MIBG at a dose estimated to produce 2 Gy of whole body radiation on day 0 [83]. Patients then received melphalan on day 10 and then 12.6 Gy of total body irradiation over days 12–15 followed by stem cell infusion. Toxicity consisted mainly of expected myelosuppression and mucositis. All patients had bone marrow recovery demonstrating the feasibility of incorporating 131I-MIBG into a myeloablative regimen.
Follow-up studies have focused on combining 131I-MIBG with high-dose combination chemotherapy. Groups in Germany and at the University of Michigan performed pilot studies in a total of 23 patients with advanced neuroblastoma using 131I-MIBG therapy followed by carboplatin, etoposide, and melphalan [84,85]. In one study, 11 patients received 131I-MIBG at a median dose of 15.7 mCi/kg [84]. Once patients were released from radiation isolation, they began high-dose chemotherapy with carboplatin, etoposide, and melphalan on days -8 to -2. In the other study, 12 patients were treated with 12 mCi/kg 131I-MIBG on day -21 and carboplatin, etoposide, and melphalan on days -7 to -4 [85]. Stem cells were re-infused on day 0 in both studies. Engraftment was prompt in all patients and toxicity was generally as expected for patients receiving this type of high-dose chemotherapy. Nine patients not already in complete remission achieved a complete remission with this type of therapy.
An Italian study evaluated 131I-MIBG in combination with busulfan and melphalan in 17 patients with refractory neuroblastoma [86]. Patients received a median 131I-MIBG dose of 7 mCi/kg followed 7–10 days later by busulfan and melphalan. Engraftment following stem cell infusion occurred as expected. Compared to a historical control group treated with similar high-dose chemotherapy but without 131I-MIBG, patients treated with 131I-MIBG seemed to have more gastrointestinal toxicity and required greater nutritional support. Two patients achieved a complete remission with this combination approach.
With these studies demonstrating the feasibility of incorporating 131I-MIBG into autologous transplant regimens, the NANT consortium performed a phase I dose escalation study of 131I-MIBG on day -21 in combination with carboplatin, etoposide, and melphalan on days -7 to -3 for patients with refractory neuroblastoma [87]. In this trial, the dose of chemotherapy was held constant and the dose of 131I-MIBG was escalated starting at 12 mCi/kg. Two separate cohorts were evaluated based upon renal function at time of study enrollment, with chemotherapy dose reduction in the cohort of patients with a glomerular filtration rate of 60–99 mL/min/1.73 m2. An 131I-MIBG dose of 12 mCi/kg was identified as the maximum tolerated dose in the normal renal function cohort based on two of six and two of four patients with dose limiting toxicities at both the 15 and 18 mCi/kg dose levels, respectively. The six patients treated with 12 mCi/kg in the low renal function group had a higher than expected incidence of hepatic toxicity and dose escalation was not attempted in this cohort. The overall response rate was 22% with a median overall survival of 48 months (Figure 1B). Based on this promising experience in patients with refractory disease, the NANT consortium is currently conducting a phase II study of this combination in patients with refractory neuroblastoma. In addition, the Children’s Oncology Group will soon open a pilot study of 131I-MIBG together with carboplatin, etoposide, and melphalan followed by autologous hematopoietic stem cell transplant for consolidation in patients with newly diagnosed neuroblastoma.
MIBG Therapy for Newly Diagnosed Patients with Neuroblastoma
With the success of 131I-MIBG in treating patients with relapsed or refractory neuroblastoma, several studies have incorporated this agent into the treatment of patients with newly diagnosed neuroblastoma. At one center in Amsterdam, patients with stage 4 neuroblastoma were eligible to enroll in a study of 131I-MIBG given at a fixed dose of 200 mCi followed 4–6 weeks later by a second infusion of 100 mCi [88,89]. If the primary tumor was resectable after these two courses, patients proceeded to surgery. Otherwise, they received additional courses of 131I-MIBG until surgery. After surgery, patients went on to receive conventional chemotherapy followed by high-dose chemotherapy with autologous stem cell rescue. Forty-one patients with stage 4 disease were treated on this protocol. The primary outcome for this study was response after two cycles of 131I-MIBG. The objective response rate was 66%, including one patient with a complete response [89]. In addition, 58% of patients had a bone marrow complete response after two cycles of 131I-MIBG. Of the group of 24 patients who received only 131I-MIBG and surgery before initiating chemotherapy, 14 patients (58%) had a complete response after 131I-MIBG and surgery. Four patients (9.8%) developed progressive disease after two courses of 131I-MIBG. An additional seven patients developed progressive disease following surgery. Only 17 patients went on to receive high-dose chemotherapy with stem cell rescue. The 5-year overall survival for the 41 patients was 14.6% [89]. These results demonstrate a higher response rate of 131I-MIBG in the up-front setting compared with the relapse setting. However, this agent must be incorporated into an overall treatment strategy that emphasizes the importance of both conventional chemotherapy and high-dose chemotherapy in the treatment of these patients.
Three consecutive German national trials have evaluated the role of 131I-MIBG in patients with stage 4 neuroblastoma and residual MIBG-positive disease after induction therapy. In the NB85 trial, 47 patients without a complete response to induction therapy received 131I-MIBG therapy with a mean dose of 8.9 mCi/kg per course [90]. The objective response rate was 46.8%. The outcome of a small subset of these patients treated with 131I-MIBG and a comparison group of similar patients non-randomly chosen to not receive 131I-MIBG showed no survival advantage for 131I-MIBG-treated patients. In the NB97 trial, 111 patients had residual disease after induction therapy [91]. Of these patients, 36 patients non-randomly received 131I-MIBG therapy prior to autologous transplant and 30 patients did not receive 131I-MIBG therapy prior to transplant. The median dose of 131I-MIBG on this trial was approximately 12 mCi/kg. The 3-year event-free survival for patients receiving 131I-MIBG therapy was 49% compared to 33% for patients not receiving 131I-MIBG. This difference was not statistically significant. The 3-year overall survival was identical between these two groups (59%). In a multivariate analysis, the use of 131I-MIBG therapy did not improve outcomes. Outcomes did not differ between patients who received an 131I-MIBG dose above or below the median dose of 12 mCi/kg. The ongoing NB2004 trial incorporates 131I-MIBG therapy at a standard dose of 12 mCi/kg for patients with residual MIBG-positive disease after induction therapy. A prospective randomized trial of 131I-MIBG combined with consolidation high-dose therapy compared to consolidation high-dose therapy alone will be required to establish the impact of 131I-MIBG in consolidation.
Use of Dosimetry and Post-treatment Scans in MIBG Therapy for Neuroblastoma
Dosimetry has been evaluated in therapeutic MIBG studies for several indications. Several groups have utilized dosimetry either from a tracer MIBG dose or from an initial therapeutic MIBG dose in order to prescribe an 131I-MIBG activity calculated to produce a given whole body radiation dose [69,74,78,92]. This strategy has been effective, with calculated 131I-MIBG doses typically yielding the desired whole body radiation dose. For example, one study treated 8 patients with two sequential doses of 131I-MIBG with a goal total whole body radiation dose of 4 Gy [78]. The second dose of 131I-MIBG was calculated based upon first dose dosimetry. The measured total whole body radiation doses ranged from 3.73 –4.65 Gy. One group determined that patients with neuroblastoma received a median of 1 cGy of whole body radiation per millicurie of 131I-MIBG administered [93]. The whole body radiation dose increases approximately linearly with administered 131I-MIBG dose [55]. In addition, excreted urinary activity correlates well with whole body dosimetry readings [55].
Dosimetry has also been used to estimate tumor-specific radiation dose following 131I-MIBG therapy. Due to differences in intensity of tumor uptake, tumor-specific radiation dose did not correlate with prescribed 131I-MIBG dose in one study [55]. In contrast, tumor-specific radiation dose correlated with treatment response, with an increased probability of treatment response in patients with tumor-specific radiation doses > 10 Gy [55]. In addition, tumor dosimetry was also used by one group to establish the effective tumor half-life of 131I-MIBG in 18 patients as ranging from 35 to 95 hour [93].
A third application of dosimetry in 131I-MIBG therapy has been to determine organ-specific doses. Since 131I-MIBG is cleared through the urine, the bladder could receive a potentially limiting radiation dose. In five patients treated without bladder catheters, the mean bladder dose was 27 Gy, or approximately 11 cGy per mCi of 131I-MIBG administered [93]. The liver and lung absorbed doses are approximately 2 and 1.3 times the whole body absorbed dose, respectively [94]. Red marrow dose appears similar to whole body dose [55].
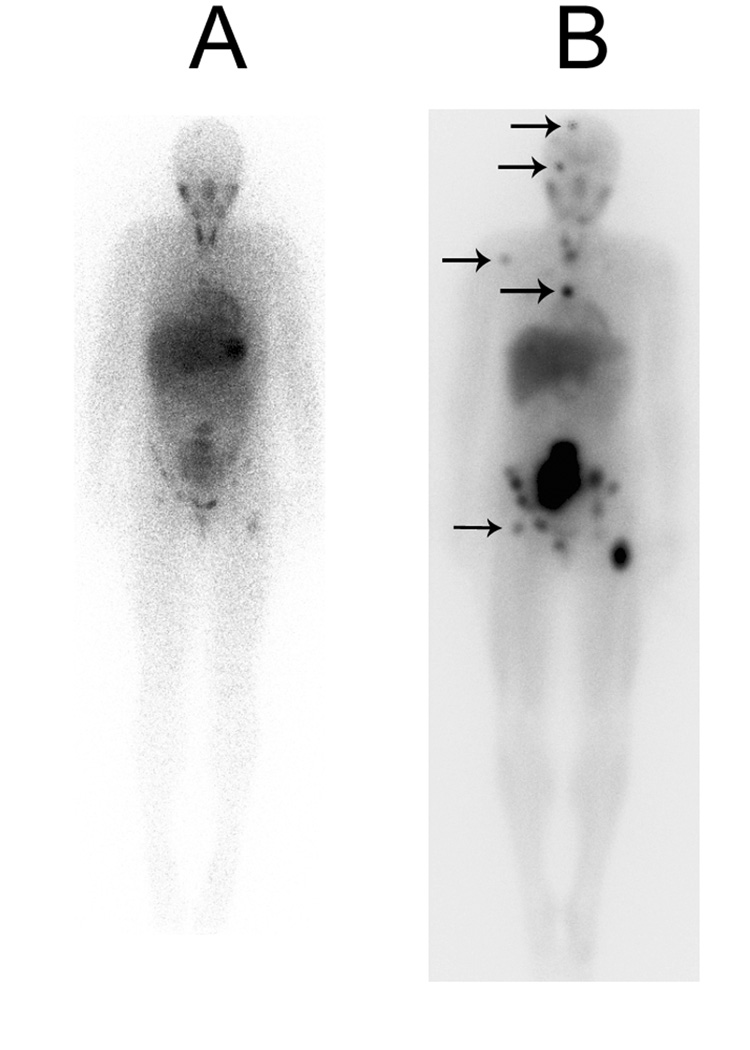
Three reports have compared diagnostic MIBG (131I-or 123I-MIBG) scans with scans obtained shortly after administration of treatment doses of 131I-MIBG [95–97]. In all three studies, scans performed after treatment doses of 131I-MIBG revealed considerably more metastatic lesions than diagnostic scans (Figure 2). Additional sites of disease were detected in more than two-thirds of post-treatment scans. The impact of the improved sensitivity of the post-treatment scans on patient management was minimal in two of these studies as tumor stage was altered in only 2 of 15 patients and 1 of 18 patients, respectively [95,96]. Given the increased sensitivity of the post-treatment scans, it is important to compare the follow-up scan (1–2 months after treatment) to the pre-therapy scan rather than to the immediate post-treatment scan. Further study will be necessary to determine how best to utilize the additional information obtained on immediate post-treatment scans.
Figure 2.
Increased detection of neuroblastoma metastases on an MIBG scan obtained 5 days following 15 mCi/kg 131I-MIBG (B) compared to a routine diagnostic scan obtained 24 hours after the administration of 5 mCi 123I-MIBG (A). Arrows indicate tumor uptake seen on the post-treatment scan and not definitely seen on the diagnostic scan.
Acute Toxicity and Late Effects of MIBG Therapy in Patients with Neuroblastoma
Hematologic toxicity, most notably thrombocytopenia, has been reported as the main toxicity in nearly all studies of 131I-MIBG therapy. The hematologic toxicity of 53 patients with relapsed or refractory neuroblastoma treated with 18 mCi/kg of 131I-MIBG has been described in detail by our group [98]. In this series, 36% of patients required stem cell support for prolonged myelosuppression. Those patients who did not meet criteria for stem cell reinfusion nevertheless required platelet transfusion support for a median of 3 weeks before recovery. In contrast, patients were typically neutropenic for approximately 1 week before recovering their neutrophils. Hematologic toxicity was more pronounced in patients with bone marrow tumor involvement and in patients who received higher whole body radiation doses [55,98]. These results are consistent with an earlier report indicating that whole body absorbed dose was one of the best predictors of hematologic toxicity following 131I-MIBG therapy [99].
In one series of nine patients with neuroblastoma treated with 12–18 mCi/kg of 131I-MIBG, five patients developed bilateral parotid gland swelling shortly after 131I-MIBG infusion [100]. These symptoms were associated with transient serum amylase elevations in the absence of serum lipase elevations, indicating a salivary gland process. None of the patients developed long-term xerostomia. This toxicity is not unexpected due to the known physiologic uptake of MIBG in salivary glands.
To date, MIBG therapy has mainly been used for the treatment of refractory or relapsed neuroblastoma. Since this patient population has a dismal prognosis, little is known about the late effects of MIBG therapy on these young patients. Moreover, since these patients have been heavily pre-treated with other toxic therapies, the contribution of radiolabeled MIBG to a specific late effect can be difficult to ascertain. Despite these limitations, several groups have reported their experience with thyroid dysfunction and second malignancies in patients treated with 131I-MIBG for neuroblastoma.
Primary hypothyroidism appears to develop in a significant number of patients with neuroblastoma treated with 131I-MIBG due to uptake of free 131I by the thyroid gland. Estimates of the incidence of hypothyroidism vary widely between studies and depend upon the definition of hypothyroidism. In a series of 14 patients with neuroblastoma who survived at least two years from the time of 131I-MIBG therapy, 8 patients developed symptomatic hypothyroidism requiring thyroid replacement therapy [101]. Five of these patients had not received total body external beam radiation as part of their neuroblastoma therapy. An additional 4 patients developed asymptomatic elevations in thyroid stimulating hormone (TSH) levels such that 12/14 patients (85%) in this study exhibited some degree of thyroid dysfunction despite prophylaxis with Lugol’s solution for 7 days before and after 131I-MIBG therapy. A larger analysis included 42 patients treated with 131I-MIBG [102]. Despite 14 days of prophylaxis with Lugol’s solution, thyroid uptake of 131I was noted in 21% of MIBG diagnostic or post-treatment scans. Twenty-two patients (52%) developed elevations in TSH levels. In all but four patients, the TSH elevation was permanent. All patients were asymptomatic and no patients showed abnormal T4 levels. The average time to onset of TSH elevation was 1.4 years. One small series reported that a quarter of patients treated with 131I-MIBG developed thyroid nodules [103].
Two reports have indicated that thyroid uptake on MIBG scans does not correlate with development of thyroid dysfunction [102,103]. In a series of 5 patients with neuroblastoma treated with multiple 131I-MIBG courses ranging in dose from 50 to 150 mCi per dose, two of five patients developed hypothyroidism [104]. Thyroid dosimetry was performed on these patients and demonstrated a wide range of absorbed dose by the thyroid despite a uniform thyroid prophylaxis regimen. The results suggested that patients who developed hypothyroidism had higher thyroid absorbed doses.
The use of a more aggressive thyroid-blocking regimen may reduce the risk of thyroid toxicity. Thyroxine, methimazole, and Lugol’s solution was tested in one series of patients and resulted in TSH elevations in 35% of patients [105]. At our institution and in the NANT consortium, the standard thyroid-blocking regimen consists of both potassium iodide for 45 days as well as potassium perchlorate for 5 days following 131I-MIBG infusion. Following 18 mCi/kg 131I-MIBG therapy, this strategy resulted in a reported incidence of 7% of hypothyroidism requiring thyroid replacement therapy [98].
Three reports describe patients with second malignancies following 131I-MIBG therapy for neuroblastoma. In one case series from our institution, three out of 95 children treated with 131I-MIBG with refractory neuroblastoma developed secondary myelodysplastic syndrome (MDS) or acute myeloid leukemia [106]. These cases all developed within 1 year of 131I-MIBG therapy and were characterized by variable losses of chromosome 5, 7, or 11 or by gain of chromosome 12. The cumulative incidence of developing a secondary leukemia or MDS was less than 4% at 5 years. The second report of five patients included two with leukemia, two with sarcoma, and one with malignant schwannoma diagnosed from 1.5 to 14 years following 131I-MIBG therapy [107]. Curiously, the malignant schwannoma and one of the sarcomas arose within a residual differentiating neuroblastoma tumor mass, suggesting a role of the targeted radiation in secondary tumorigenesis. One patient included on the large phase II study of 131I-MIBG developed peritoneal mesothelioma following therapy [45]. These cases indicate a possible increased risk of second cancers in patients treated with 131I-MIBG. Given this possibility, long-term survivors after 131I-MIBG therapy require ongoing surveillance.
Practical Aspects of Administering 131I-MIBG Therapy to Children
Neuroblastoma is a tumor that peaks in incidence in toddlers. As such, most of the patients with neuroblastoma who receive 131I-MIBG therapy are children. Use of high-dose 131I-MIBG in this patient population poses challenges resulting from the radiation safety requirements associated with the administration of this therapy. These treatments require close collaboration between pediatric oncologists, nuclear medicine physicians and technologists, nursing staff, radiation safety officers, social workers, and child life specialists [108]. Potential pediatric candidates for 131I-MIBG therapy should be screened carefully to ensure that they can comply with the radiation safety requirements. We routinely utilize Foley catheters during 131I-MIBG treatments, particularly in young patients in order to increase their safety and the safety of their caregivers. Since it would be unacceptable for a caregiver to remain in prolonged close contact with the patient immediately following 131I-MIBG administration, children must be of an appropriate developmental level to reliably remain in radiation isolation for several days. They must also be of an age at which they are not expected to remove urinary or central venous catheters if left unattended. At our institution, child life specialists provide patients with age-appropriate activities to occupy them during their time in radiation isolation. In addition, a mirror allows children in radiation isolation to see their caregivers waiting just outside of the radiation isolation room. A video camera allows the medical staff to monitor these patients without having to enter the radiation isolation room. While these strategies are very useful, in extreme circumstances, younger patients may require sedative medications in order to safely receive 131I-MIBG therapy. Young children require greater assistance with meals, toileting, and personal hygiene than older patients. In order to minimize radiation exposure to nursing staff at the 131I-MIBG treatment center, caregivers (typically parents) usually provide this type of care after receiving radiation safety instructions. Female caregivers should be screened for possible pregnancy prior to assuming this role. For diapered patients, soiled diapers require special handling since 131I-MIBG is cleared in the urine and stool. Young children may also be reluctant to take the required oral thyroid blocking medications. Placement of a nasogastric tube for medication administration may be helpful in these instances.
Future Directions
Both preclinical and clinical data demonstrate the substantial activity of radiolabeled MIBG in neuroblastoma. Recent studies have evaluated more novel approaches. These approaches include combinations of 131I-MIBG with myeloablative regimens, with chemotherapy agents with radiation sensitizing properties, or with biologic agents. As additional experience with these combination approaches is obtained, the use of these combination strategies will need to be rationally incorporated into the upfront management of newly diagnosed patients with high-risk disease. Combination approaches that address the decreased activity of 131I-MIBG for treating small tumor deposits, such as bone marrow metastases, are required. As 131I-MIBG therapy becomes more prevalent, continued study of the psychosocial implications and late effects of this treatment in a pediatric population will be necessary.
Table 3.
Combination studies of 131I-MIBG in patients with relapsed or refractory neuroblastoma. Response rate refers to percent of evaluable patients with at least a partial response to therapy as their best overall response.
| Reference(s) | Number of Patients | 131I-MIBG Activity per Cycle | Combination Regimen | Response Rate (Complete Response |
|---|---|---|---|---|
| Nonmyeloablative Approaches | ||||
| [75,76] | 5 | 100 mCi × 2 doses, 1 week apart | Cisplatin 1 day prior to 131IMIBG | 80% |
| [77] | 16 | 200 mCi on Day 10 | Cisplatin and Cyclophosphamide on Days 1–4 with or without Vincristine and Etoposide | 75% |
| [78] | 8 | 12 mCi/kg on Days 1 and 15 | Topotecan on Days 1–5 and 15–19 | NR |
| [79] | 27 | 200 mCi | Hyperbaric Oxygen for 4–5 Days after 131I-MIBG | NR |
| Myeloablative Approaches | ||||
| [82] | 5 | 300 mCi on Day 0 | Carboplatin and Melphalan on Days 7–12 with or without Vincristine and Etoposide | NR |
| [83] | 5 | To Yield 2 Gy Whole Body Dose | Melphalan and 12.6 Gy Total Body Irradiation on Days 10–15 | NR |
| [84] | 11 | Median of 15.7 mCi/kg ~1 Week Prior to Chemotherapy | Carboplatin, Etoposide, and Melphalan on Days -8 to -2 | 36% |
| [85] | 12 | 12 mCi/kg on Day - 21 | Carboplatin, Etoposide, Melphalan on Days -7 to -4 | 67% |
| [86] | 17 | Median of 7 mCi/kg | Busulfan and Melphalan 7–10 Days after 131I-MIBG | 47% |
| [87] | 22 | 12–18 mCi/kg on Day -21 | Carboplatin, Etoposide, Melphalan on Days -7 to -4 | 27% |
NR = Not reported
Acknowledgments
Support: Supported in part by the National Institute of Health grants PO1 CA81403, CCSG CA82103, as well by donations from the Campini Foundation, the Conner Research Fund, the Katie Dougherty Foundation, and Alex's Lemonade Stand Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodman M, Gurney J, Smith M, Olshan A, et al. Sympathetic Nervous System Tumors. In: Ries L, Smith M, Gurney J, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda: National Institutes of Health: National Cancer Institute, SEER Program; 1999. pp. 65–72. [Google Scholar]
- 2.London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. Journal of Clinical Oncology. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt ML, Lal A, Seeger RC, Maris JM, Shimada H, O'Leary M, et al. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: a Children's Cancer Group Study. Journal of Clinical Oncology. 2005;23:6474–6480. doi: 10.1200/JCO.2005.05.183. [DOI] [PubMed] [Google Scholar]
- 4.DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Carlin S, Mairs RJ, McCluskey AG, Tweddle DA, Sprigg A, Estlin C, et al. Development of a real-time polymerase chain reaction assay for prediction of the uptake of meta-[(131)I]iodobenzylguanidine by neuroblastoma tumors. Clin Cancer Res. 2003;9:3338–3344. [PubMed] [Google Scholar]
- 6.Treuner J, Feine U, Niethammer D, Muller-Schaumburg W, Meinke J, Eibach E, et al. Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet. 1984;1:333–334. doi: 10.1016/s0140-6736(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 7.Howman-Giles R, Shaw PJ, Uren RF, Chung DK. Neuroblastoma and other neuroendocrine tumors. Semin Nucl Med. 2007;37:286–302. doi: 10.1053/j.semnuclmed.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment [see comments] Journal of Clinical Oncology. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 9.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 10.Attiyeh EF, London WB, Mosse YP, Wang Q, Winter C, Khazi D, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 11.Bown N, Cotterill S, Lastowska M, O'Neill S, Pearson AD, Plantaz D, et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med. 1999;340:1954–1961. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- 12.Look AT, Hayes FA, Shuster JJ, Douglass EC, Castleberry RP, Bowman LC, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. Journal of Clinical Oncology. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Shimada H, Chatten J, Newton WA, Jr, Sachs N, Hamoudi AB, Chiba T, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 14.Matthay KK, Perez C, Seeger RC, Brodeur GM, Shimada H, Atkinson JB, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. Journal of Clinical Oncology. 1998;16:1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 15.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 16.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children’s Oncology Group study. Journal of Clinical Oncology. 2008 doi: 10.1200/JCO.2007.13.8925. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck J, Bruchelt G, Girgert R, Treuner J, Niethammer D. Specific uptake of m-[125I]iodobenzylguanidine in the human neuroblastoma cell line SK-N-SH. Cancer Res. 1985;45:6366–6370. [PubMed] [Google Scholar]
- 18.Iavarone A, Lasorella A, Servidei T, Riccardi R, Troncone L, Mastrangelo R. Biology of metaiodobenzylguanidine interactions with human neuroblastoma cells. J Nucl Biol Med. 1991;35:186–190. [PubMed] [Google Scholar]
- 19.Montaldo PG, Lanciotti M, Casalaro A, Cornaglia-Ferraris P, Ponzoni M. Accumulation of m-iodobenzylguanidine by neuroblastoma cells results from independent uptake and storage mechanisms. Cancer Res. 1991;51:4342–4346. [PubMed] [Google Scholar]
- 20.Iavarone A, Lasorella A, Servidei T, Riccardi R, Mastrangelo R. Uptake and storage of m-iodobenzylguanidine are frequent neuronal functions of human neuroblastoma cell lines. Cancer Res. 1993;53:304–309. [PubMed] [Google Scholar]
- 21.Smets LA, Loesberg C, Janssen M, Metwally EA, Huiskamp R. Active uptake and extravesicular storage of m-iodobenzylguanidine in human neuroblastoma SK-N-SH cells. Cancer Res. 1989;49:2941–2944. [PubMed] [Google Scholar]
- 22.Lode HN, Bruchelt G, Seitz G, Gebhardt S, Gekeler V, Niethammer D, et al. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of monoamine transporters in neuroblastoma cell lines: correlations to meta-iodobenzylguanidine (MIBG) uptake and tyrosine hydroxylase gene expression. Eur J Cancer. 1995;31A:586–590. doi: 10.1016/0959-8049(95)00039-l. [DOI] [PubMed] [Google Scholar]
- 23.Mairs RJ, Livingstone A, Gaze MN, Wheldon TE, Barrett A. Prediction of accumulation of 131I-labelled meta-iodobenzylguanidine in neuroblastoma cell lines by means of reverse transcription and polymerase chain reaction. Br J Cancer. 1994;70:97–101. doi: 10.1038/bjc.1994.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham S, Boyd M, Brown MM, Carlin S, McCluskey A, Livingstone A, et al. A gene therapy approach to enhance the targeted radiotherapy of neuroblastoma. Med Pediatr Oncol. 2000;35:708–711. doi: 10.1002/1096-911x(20001201)35:6<708::aid-mpo49>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Altmann A, Kissel M, Zitzmann S, Kubler W, Mahmut M, Peschke P, et al. Increased MIBG uptake after transfer of the human norepinephrine transporter gene in rat hepatoma. J Nucl Med. 2003;44:973–980. [PubMed] [Google Scholar]
- 26.Boyd M, Cunningham SH, Brown MM, Mairs RJ, Wheldon TE. Noradrenaline transporter gene transfer for radiation cell kill by 131I meta-iodobenzylguanidine. Gene Ther. 1999;6:1147–1152. doi: 10.1038/sj.gt.3300905. [DOI] [PubMed] [Google Scholar]
- 27.Gaze MN, Huxham IM, Mairs RJ, Barrett A. Intracellular localization of metaiodobenzyl guanidine in human neuroblastoma cells by electron spectroscopic imaging. Int J Cancer. 1991;47:875–880. doi: 10.1002/ijc.2910470615. [DOI] [PubMed] [Google Scholar]
- 28.Lashford LS, Hancock JP, Kemshead JT. Meta-iodobenzylguanidine (mIBG) uptake and storage in the human neuroblastoma cell line SK-N-BE(2C) Int J Cancer. 1991;47:105–109. doi: 10.1002/ijc.2910470119. [DOI] [PubMed] [Google Scholar]
- 29.Armour A, Mairs RJ, Gaze MN, Wheldon TE. Modification of meta-iodobenzylguanidine uptake in neuroblastoma cells by elevated temperature. Br J Cancer. 1994;70:445–448. doi: 10.1038/bjc.1994.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armour A, Cunningham SH, Gaze MN, Wheldon TE, Mairs RJ. The effect of cisplatin pretreatment on the accumulation of MIBG by neuroblastoma cells in vitro. Br J Cancer. 1997;75:470–476. doi: 10.1038/bjc.1997.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meco D, Lasorella A, Riccardi A, Servidei T, Mastrangelo R, Riccardi R. Influence of cisplatin and doxorubicin on 125I-meta-iodobenzylguanidine uptake in human neuroblastoma cell lines [see comments] Eur J Cancer. 1999;35:1227–1234. doi: 10.1016/s0959-8049(99)00078-7. [DOI] [PubMed] [Google Scholar]
- 32.Montaldo PG, Carbone R, Ponzoni M, Cornaglia-Ferraris P. gamma-Interferon increases metaiodobenzylguanidine incorporation and retention in human neuroblastoma cells. Cancer Res. 1992;52:4960–4964. [PubMed] [Google Scholar]
- 33.Montaldo PG, Raffaghello L, Guarnaccia F, Pistoia V, Garaventa A, Ponzoni M. Increase of metaiodobenzylguanidine uptake and intracellular half-life during differentiation of human neuroblastoma cells. Int J Cancer. 1996;67:95–100. doi: 10.1002/(SICI)1097-0215(19960703)67:1<95::AID-IJC16>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.McCluskey AG, Boyd M, Ross SC, Cosimo E, Clark AM, Angerson WJ, et al. [131I]meta-iodobenzylguanidine and topotecan combination treatment of tumors expressing the noradrenaline transporter. Clin Cancer Res. 2005;11:7929–7937. doi: 10.1158/1078-0432.CCR-05-0982. [DOI] [PubMed] [Google Scholar]
- 35.Bruchelt G, Girgert R, Buck J, Wolburg H, Niethammer D, Treuner J. Cytotoxic effects of m-[131I]- and m-[125I]iodobenzylguanidine on the human neuroblastoma cell lines SK-N-SH and SK-N-LO. Cancer Res. 1988;48:2993–2997. [PubMed] [Google Scholar]
- 36.Gaze MN, Mairs RJ, Boyack SM, Wheldon TE, Barrett A. 131I-meta-iodobenzylguanidine therapy in neuroblastoma spheroids of different sizes. Br J Cancer. 1992;66:1048–1052. doi: 10.1038/bjc.1992.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornelissen J, Van Kuilenburg AB, Voute PA, Van Gennip AH. MIBG causes oxidative stress and up-regulation of anti-oxidant enzymes in the human neuroblastoma cell line SK-N-BE(2c) Int J Cancer. 1997;72:486–490. doi: 10.1002/(sici)1097-0215(19970729)72:3<486::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Cornelissen J, Wanders RJ, Van den Bogert C, Van Kuilenburg AB, Elzinga L, Voute PA, et al. Meta-iodobenzylguanidine (MIBG) inhibits malate and succinate driven mitochondrial ATP synthesis in the human neuroblastoma cell line SK-N-BE(2c) Eur J Cancer. 1995;31A:582–586. doi: 10.1016/0959-8049(95)00045-k. [DOI] [PubMed] [Google Scholar]
- 39.Smets LA, Bout B, Wisse J. Cytotoxic and antitumor effects of the norepinephrine analogue meta-iodo-benzylguanidine (MIBG) Cancer Chemother Pharmacol. 1988;21:9–13. doi: 10.1007/BF00262730. [DOI] [PubMed] [Google Scholar]
- 40.Mairs RJ, Angerson W, Gaze MN, Murray T, Babich JW, Reid R, et al. The distribution of alternative agents for targeted radiotherapy within human neuroblastoma spheroids. Br J Cancer. 1991;63:404–409. doi: 10.1038/bjc.1991.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sisson JC, Brown R, Zasadny K, Normolle D, Fisher S, Moon S, et al. Biodistribution and therapeutic effects of a radiolabeled metaiodobenzylguanidine in a human neuroblastoma xenograft. Antibody Immunoconjugates and Radiopharmaceuticals. 1992;5:23–36. [Google Scholar]
- 42.Rutgers M, Buitenhuis CK, van der Valk MA, Hoefnagel CA, Voute PA, Smets LA. [(131)I] and [(125)I] metaiodobenzylguanidine therapy in macroscopic and microscopic tumors: a comparative study in SK-N-SH human neuroblastoma and PC12 rat pheochromocytoma xenografts. Int J Cancer. 2000;90:312–325. [PubMed] [Google Scholar]
- 43.Weber W, Weber J, Senekowitsch-Schmidtke R. Therapeutic effect of m-[131I]-and m-[125I]iodobenzylguanidine on neuroblastoma multicellular tumor spheroids of different sizes. Cancer Res. 1996;56:5428–5434. [PubMed] [Google Scholar]
- 44.Cunningham SH, Mairs RJ, Wheldon TE, Welsh PC, Vaidyanathan G, Zalutsky MR. Toxicity to neuroblastoma cells and spheroids of benzylguanidine conjugated to radionuclides with short-range emissions. Br J Cancer. 1998;77:2061–2068. doi: 10.1038/bjc.1998.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. Journal of Clinical Oncology. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 46.Mairs RJ, Cunningham SH, Russell J, Armour A, Owens J, McKellar K, et al. No-carrier-added iodine-131-MIBG: evaluation of a therapeutic preparation. J Nucl Med. 1995;36:1088–1095. [PubMed] [Google Scholar]
- 47.Mairs RJ, Russell J, Cunningham S, O'Donoghue JA, Gaze MN, Owens J, et al. Enhanced tumour uptake and in vitro radiotoxicity of no-carrier-added [131I]meta-iodobenzylguanidine: implications for the targeted radiotherapy of neuroblastoma. Eur J Cancer. 1995;31A:576–581. doi: 10.1016/0959-8049(95)00052-k. [DOI] [PubMed] [Google Scholar]
- 48.Roa WH, Miller GG, McEwan AJ, McQuarrie SA, Tse J, Wu J, et al. Targeted radiotherapy of multicell neuroblastoma spheroids with high specific activity [125I]meta-iodobenzylguanidine. International Journal of Radiation Oncology, Biology, Physics. 1998:425–432. doi: 10.1016/s0360-3016(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 49.Vaidyanathan G, Friedman HS, Keir ST, Zalutsky MR. Localisation of [131I]MIBG in nude mice bearing SK-N-SH human neuroblastoma xenografts: effect of specific activity. Br J Cancer. 1996;73:1171–1177. doi: 10.1038/bjc.1996.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brans B, Laureys G, Schelfhout V, Wiele CVD, Potter CRD, Dhooge C, et al. Activity of iodine-123 metaiodobenzylguanidine in childhood neuroblastoma: lack of relation to tumour differentiation in vivo. European Journal of Nuclear Medicine. 1998;25:144–149. doi: 10.1007/s002590050207. [DOI] [PubMed] [Google Scholar]
- 51.Wafelman AR, Nortier YL, Rosing H, Maessen HJ, Taal BG, Hoefnagel CA, et al. Renal excretion of meta-iodobenzylguanidine after therapeutic doses in cancer patients and its relation to dose and creatinine clearance. Nucl Med Commun. 1995;16:767–772. doi: 10.1097/00006231-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Ehninger G, Klingebiel T, Kumbier I, Schuler U, Feine U, Treuner J, et al. Stability and pharmacokinetics of m-[131I]iodobenzylguanidine in patients. Cancer Res. 1987;47:6147–6149. [PubMed] [Google Scholar]
- 53.Klingebiel T, Treuner J, Ehninger G, Keller KD, Dopfer R, Feine U, et al. [131I]-metaiodobenzylguanidine in the treatment of metastatic neuroblastoma. Clinical, pharmacological and dosimetric aspects. Cancer Chemother Pharmacol. 1989;25:143–148. doi: 10.1007/BF00692356. [DOI] [PubMed] [Google Scholar]
- 54.Lashford LS, Moyes J, Ott R, Fielding S, Babich J, Mellors S, et al. The biodistribution and pharmacokinetics of meta-iodobenzylguanidine in childhood neuroblastoma. European Journal of Nuclear Medicine. 1988;13:574–577. doi: 10.1007/BF02574771. [DOI] [PubMed] [Google Scholar]
- 55.Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, et al. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med. 2001;42:1713–1721. [PubMed] [Google Scholar]
- 56.Matthay KK, Huberty JP, Hattner RS, Ablin AR, Engelstad BL, Zoger S, et al. Efficacy and safety of [131I]metaiodobenzylguanidine therapy for patients with refractory neuroblastoma. J Nucl Biol Med. 1991;35:244–247. [PubMed] [Google Scholar]
- 57.Troncone L, Rufini V, Riccardi R, Lasorella A, Mastrangelo R. The use of [131I]metaiodobenzylguanidine in the treatment of neuroblastoma after conventional therapy. J Nucl Biol Med. 1991;35:232–236. [PubMed] [Google Scholar]
- 58.Klingebiel T, Feine U, Treuner J, Reuland P, Handgretinger R, Niethammer D. Treatment of neuroblastoma with [131I]metaiodobenzylguanidine: long-term results in 25 patients. J Nucl Biol Med. 1991;35:216–219. [PubMed] [Google Scholar]
- 59.Hor G, Maul FD, Kornhuber B, Schwabe D, Hesse J, Manegold KH, et al. Outcome of [131I]metaiodobenzylguanidine therapy of neuroblastoma: seven years after. J Nucl Biol Med. 1991;35:207–215. [PubMed] [Google Scholar]
- 60.Kang TI, Brophy P, Hickeson M, Heyman S, Evans AE, Charron M, et al. Targeted radiotherapy with submyeloablative doses of 131I-MIBG is effective for disease palliation in highly refractory neuroblastoma. J Pediatr Hematol Oncol. 2003;25:769–773. doi: 10.1097/00043426-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Castellani MR, Chiti A, Seregni E, Bombardieri E. Role of 131I-metaiodobenzylguanidine (MIBG) in the treatment of neuroendocrine tumours. Experience of the National Cancer Institute of Milan. Q J Nucl Med. 2000;44:77–87. [PubMed] [Google Scholar]
- 62.Claudiani F, Garaventa A, Bertolazzi L, Villavecchia GP, Cabria M, Scopinaro G, et al. [131I]metaiodobenzylguanidine therapy in advanced neuroblastoma. J Nucl Biol Med. 1991;35:224–227. [PubMed] [Google Scholar]
- 63.Garaventa A, Bellagamba O, Lo Piccolo MS, Milanaccio C, Lanino E, Bertolazzi L, et al. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: a mono-institutional experience with 43 patients. Br J Cancer. 1999;81:1378–1384. doi: 10.1038/sj.bjc.6694223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garaventa A, Guerra P, Arrighini A, Bertolazzi L, Bestagno M, De Bernardi B, et al. Treatment of advanced neuroblastoma with I-131 meta-iodobenzylguanidine. Cancer. 1991;67:922–928. doi: 10.1002/1097-0142(19910215)67:4<922::aid-cncr2820670411>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 65.Sisson JC, Hutchinson RJ, Shapiro B, Zasadny KR, Normolle D, Wieland DM, et al. Iodine-125-MIBG to treat neuroblastoma: preliminary report [see comments] J Nucl Med. 1990;31:1479–1485. [PubMed] [Google Scholar]
- 66.Sisson JC, Shapiro B, Hutchinson RJ, Shulkin BL, Zempel S. Survival of patients with neuroblastoma treated with 125-I MIBG. Am J Clin Oncol. 1996;19:144–148. doi: 10.1097/00000421-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 67.Hutchinson RJ, Sisson JC, Miser JS, Zasadny KR, Normolle DP, Shulkin BL, et al. Long-term results of [131I]metaiodobenzylguanidine treatment of refractory advanced neuroblastoma. J Nucl Biol Med. 1991;35:237–240. [PubMed] [Google Scholar]
- 68.Hutchinson RJ, Sisson JC, Shapiro B, Miser JS, Normole D, Shulkin BL, et al. 131-I-metaiodobenzylguanidine treatment in patients with refractory advanced neuroblastoma. Am J Clin Oncol. 1992;15:226–232. doi: 10.1097/00000421-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Lashford LS, Lewis IJ, Fielding SL, Flower MA, Meller S, Kemshead JT, et al. Phase I/F;II study of iodine 131 metaiodobenzylguanidine in chemoresistant neuroblastoma: a United Kingdom Children's Cancer Study Group investigation. Journal of Clinical Oncology. 1992;10:1889–1896. doi: 10.1200/JCO.1992.10.12.1889. [DOI] [PubMed] [Google Scholar]
- 70.Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. Journal of Clinical Oncology. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 71.Lumbroso J, Hartmann O, Schlumberger M. Therapeutic use of [131I]metaiodobenzylguanidine in neuroblastoma: a phase II study in 26 patients. "Societe Francaise d'Oncologie Pediatrique" and Nuclear Medicine Co-investigators. J Nucl Biol Med. 1991;35:220–223. [PubMed] [Google Scholar]
- 72.Hoefnagel CA, Voute PA, De Kraker J, Valdes Olmos RA. [131I]metaiodobenzylguanidine therapy after conventional therapy for neuroblastoma. J Nucl Biol Med. 1991;35:202–206. [PubMed] [Google Scholar]
- 73.Howard JP, Maris JM, Kersun LS, Huberty JP, Cheng SC, Hawkins RA, et al. Tumor response and toxicity with multiple infusions of high dose (131)I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer. 2005;44:232–239. doi: 10.1002/pbc.20240. [DOI] [PubMed] [Google Scholar]
- 74.Matthay KK, Quach A, Huberty J, Franc B, Hawkins R, Jackson H, et al. 131I-Metaiodobenzylguanidine (131I-MIBG) Double Infusion with Autologous Stem Cell Rescue for Neuroblastoma: A New Approaches to Neuroblastoma Therapy (NANT) Phase I study. Journal of Clinical Oncology. 2008 doi: 10.1200/JCO.2007.15.7628. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mastrangelo R, Tornesello A, Lasorella A, Iavarone A, Mastrangelo S, Riccardi R, et al. Optimal use of the 131-I-metaiodobenzylguanidine and cisplatin combination in advanced neuroblastoma. J Neurooncol. 1997;31:153–158. doi: 10.1023/a:1005770405844. [DOI] [PubMed] [Google Scholar]
- 76.Mastrangelo R, Tornesello A, Riccardi R, Lasorella A, Mastrangelo S, Mancini A, et al. A new approach in the treatment of stage IV neuroblastoma using a combination of [131I]meta-iodobenzylguanidine (MIBG) and cisplatin. Eur J Cancer. 1995;31A:606–611. doi: 10.1016/0959-8049(95)00048-n. [DOI] [PubMed] [Google Scholar]
- 77.Mastrangelo S, Tornesello A, Diociaiuti L, Pession A, Prete A, Rufini V, et al. Treatment of advanced neuroblastoma: feasibility and therapeutic potential of a novel approach combining 131-I-MIBG and multiple drug chemotherapy. Br J Cancer. 2001;84:460–464. doi: 10.1054/bjoc.2000.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaze MN, Chang YC, Flux GD, Mairs RJ, Saran FH, Meller ST. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm. 2005;20:195–199. doi: 10.1089/cbr.2005.20.195. [DOI] [PubMed] [Google Scholar]
- 79.Voute PA, van der Kleij AJ, De Kraker J, Hoefnagel CA, Tiel-van Buul MM, Van Gennip H. Clinical experience with radiation enhancement by hyperbaric oxygen in children with recurrent neuroblastoma stage IV. Eur J Cancer. 1995;31A:596–600. doi: 10.1016/0959-8049(95)00073-r. [DOI] [PubMed] [Google Scholar]
- 80.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 81.Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer. 2005;44:348–357. doi: 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 82.Corbett R, Pinkerton R, Tait D, Meller S. [131I]metaiodobenzylguanidine and high-dose chemotherapy with bone marrow rescue in advanced neuroblastoma. J Nucl Biol Med. 1991;35:228–231. [PubMed] [Google Scholar]
- 83.Gaze MN, Wheldon TE, O'Donoghue JA, Hilditch TE, McNee SG, Simpson E, et al. Multi-modality megatherapy with [131I]meta-iodobenzylguanidine, high dose melphalan and total body irradiation with bone marrow rescue: feasibility study of a new strategy for advanced neuroblastoma [see comments] Eur J Cancer. 1995;31A:252–256. doi: 10.1016/0959-8049(94)e0036-4. [DOI] [PubMed] [Google Scholar]
- 84.Klingebiel T, Bader P, Bares R, Beck J, Hero B, Jurgens H, et al. Treatment of neuroblastoma stage 4 with 131I-meta-iodo-benzylguanidine, high-dose chemotherapy and immunotherapy. A pilot study. Eur J Cancer. 1998;34:1398–1402. doi: 10.1016/s0959-8049(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 85.Yanik GA, Levine JE, Matthay KK, Sisson JC, Shulkin BL, Shapiro B, et al. Pilot study of iodine-131-metaiodobenzylguanidine in combination with myeloablative chemotherapy and autologous stem-cell support for the treatment of neuroblastoma. Journal of Clinical Oncology. 2002;20:2142–2149. doi: 10.1200/JCO.2002.08.124. [DOI] [PubMed] [Google Scholar]
- 86.Miano M, Garaventa A, Pizzitola MR, Piccolo MS, Dallorso S, Villavecchia GP, et al. Megatherapy combining I(131) metaiodobenzylguanidine and high-dose chemotherapy with haematopoietic progenitor cell rescue for neuroblastoma. Bone Marrow Transplant. 2001;27:571–574. doi: 10.1038/sj.bmt.1702846. [DOI] [PubMed] [Google Scholar]
- 87.Matthay KK, Tan JC, Villablanca JG, Yanik GA, Veatch J, Franc B, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to Neuroblastoma Therapy Consortium Study. Journal of Clinical Oncology. 2006;24:500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 88.De Kraker J, Hoefnagel CA, Caron H, Valdes Olmos RA, Zsiros J, Heij HA, et al. First line targeted radiotherapy, a new concept in the treatment of advanced stage neuroblastoma. Eur J Cancer. 1995;31A:600–602. doi: 10.1016/0959-8049(95)00063-o. [DOI] [PubMed] [Google Scholar]
- 89.de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer. 2008;44:551–556. doi: 10.1016/j.ejca.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Klingebiel T, Berthold F, Treuner J, Schwabe D, Fischer M, Feine U, et al. Metaiodobenzylguanidine (mIBG) in treatment of 47 patients with neuroblastoma: results of the German Neuroblastoma Trial. Med Pediatr Oncol. 1991;19:84–88. doi: 10.1002/mpo.2950190203. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt M, Simon T, Hero B, Eschner W, Dietlein M, Sudbrock F, et al. Is there a benefit of 131 I-MIBG therapy in the treatment of children with stage 4 neuroblastoma? A retrospective evaluation of The German Neuroblastoma Trial NB97 and implications for The German Neuroblastoma Trial NB2004. Nuklearmedizin. 2006;45:145–151. quiz N139–140. [PubMed] [Google Scholar]
- 92.Buckley SE, Saran FH, Gaze MN, Chittenden S, Partridge M, Lancaster D, et al. Dosimetry for fractionated (131)I-mIBG therapies in patients with primary resistant high-risk neuroblastoma: preliminary results. Cancer Biother Radiopharm. 2007;22:105–112. doi: 10.1089/cbr.2007.301. [DOI] [PubMed] [Google Scholar]
- 93.Fielding SL, Flower MA, Ackery D, Kemshead JT, Lashford LS, Lewis I. Dosimetry of iodine 131 metaiodobenzylguanidine for treatment of resistant neuroblastoma: results of a UK study. European Journal of Nuclear Medicine. 1991;18:308–316. doi: 10.1007/BF02285457. [DOI] [PubMed] [Google Scholar]
- 94.Bolster AA, Hilditch TE, Wheldon TE, Gaze MN, Barrett A. Dosimetric considerations in 131I-MIBG therapy for neuroblastoma in children. Br J Radiol. 1995;68:481–490. doi: 10.1259/0007-1285-68-809-481. [DOI] [PubMed] [Google Scholar]
- 95.Giammarile F, Lumbroso J, Ricard M, Aubert B, Hartmann O, Schlumberger M, et al. Radioiodinated metaiodobenzylguanidine in neuroblastoma: influence of high dose on tumour site detection. European Journal of Nuclear Medicine. 1995;22:1180–1183. doi: 10.1007/BF00800601. [DOI] [PubMed] [Google Scholar]
- 96.Hickeson MP, Charron M, Maris JM, Brophy P, Kang TI, Zhuang H, et al. Biodistribution of post-therapeutic versus diagnostic (131)I-MIBG scans in children with neuroblastoma. Pediatr Blood Cancer. 2004;42:268–274. doi: 10.1002/pbc.10454. [DOI] [PubMed] [Google Scholar]
- 97.Parisi MT, Matthay KK, Huberty JP, Hattner RS. Neuroblastoma: dose-related sensitivity of MIBG scanning in detection. Radiology. 1992;184:463–467. doi: 10.1148/radiology.184.2.1620849. [DOI] [PubMed] [Google Scholar]
- 98.Dubois SG, Messina J, Maris JM, Huberty J, Glidden DV, Veatch J, et al. Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. Journal of Clinical Oncology. 2004;22:2452–2460. doi: 10.1200/JCO.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 99.Sisson JC, Shapiro B, Hutchinson RJ, Carey JE, Zasadny KR, Zempel SA, et al. Predictors of toxicity in treating patients with neuroblastoma by radiolabeled metaiodobenzylguanidine. European Journal of Nuclear Medicine. 1994;21:46–52. doi: 10.1007/BF00182305. [DOI] [PubMed] [Google Scholar]
- 100.Modak S, Pandit-Taskar N, Kushner BH, Kramer K, Smith-Jones P, Larson S, et al. Transient sialoadenitis: A complication of (131)I-metaiodobenzylguanidine therapy. Pediatr Blood Cancer. 2007 doi: 10.1002/pbc.21391. [DOI] [PubMed] [Google Scholar]
- 101.Picco P, Garaventa A, Claudiani F, Gattorno M, De Bernardi B, Borrone C. Primary hypothyroidism as a consequence of 131-I-metaiodobenzylguanidine treatment for children with neuroblastoma. Cancer. 1995;76:1662–1664. doi: 10.1002/1097-0142(19951101)76:9<1662::aid-cncr2820760924>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 102.van Santen HM, de Kraker J, van Eck BL, de Vijlder JJ, Vulsma T. High incidence of thyroid dysfunction despite prophylaxis with potassium iodide during (131)I-meta-iodobenzylguanidine treatment in children with neuroblastoma. Cancer. 2002;94:2081–2089. doi: 10.1002/cncr.10447. [DOI] [PubMed] [Google Scholar]
- 103.van Santen HM, de Kraker J, Vulsma T. Endocrine late effects from multi-modality treatment of neuroblastoma. Eur J Cancer. 2005;41:1767–1774. doi: 10.1016/j.ejca.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 104.Brans B, Monsieurs M, Laureys G, Kaufman JM, Thierens H, Dierckx RA. Thyroidal uptake and radiation dose after repetitive I-131-MIBG treatments: influence of potassium iodide for thyroid blocking. Med Pediatr Oncol. 2002;38:41–46. doi: 10.1002/mpo.1261. [DOI] [PubMed] [Google Scholar]
- 105.van Santen HM, de Kraker J, van Eck BL, de Vijlder JJ, Vulsma T. Improved radiation protection of the thyroid gland with thyroxine, methimazole, and potassium iodide during diagnostic and therapeutic use of radiolabeled metaiodobenzylguanidine in children with neuroblastoma. Cancer. 2003;98:389–396. doi: 10.1002/cncr.11523. [DOI] [PubMed] [Google Scholar]
- 106.Weiss B, Vora A, Huberty J, Hawkins RA, Matthay KK. Secondary myelodysplastic syndrome and leukemia following 131I-metaiodobenzylguanidine therapy for relapsed neuroblastoma. J Pediatr Hematol Oncol. 2003;25:543–547. doi: 10.1097/00043426-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 107.Garaventa A, Gambini C, Villavecchia G, Di Cataldo A, Bertolazzi L, Pizzitola MR, et al. Second malignancies in children with neuroblastoma after combined treatment with 131I-metaiodobenzylguanidine. Cancer. 2003;97:1332–1338. doi: 10.1002/cncr.11167. [DOI] [PubMed] [Google Scholar]
- 108.Brophy P, Schmus C, Balistreri L. Meeting the nursing challenges in treating children with 131I-MIBG. J Pediatr Oncol Nurs. 2004;21:9–15. doi: 10.1177/1043454203258606. [DOI] [PubMed] [Google Scholar]