Abstract
Despite the increasing use of dendritic cell (DC) vaccination in clinical trials, optimal conditions for the generation of functionally mature DCs remain to be established. The current standard DC maturation protocol for clinical trials has been used as an inflammatory cytokine cocktail [tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and prostaglandin E2], but this cocktail induced insufficient maturation of DCs derived from elutriated monocytes when cultured in X-VIVO 15. The aim of this study was to define effective combinations of stimulators for generating functionally mature DCs from elutriated monocytes under current good manufacturing practice conditions. We compared the functional capacity of DCs in response to all possible pairwise combinations of four different classes of stimuli: TNF-α, peptidoglycan, polyinosinic : polycytidylic acid [poly(I:C)] and soluble CD40 ligand (CD40L). Maturation status of DCs stimulated with combination of four stimuli was similar to that of the cytokine cocktail as assessed by the cell surface phenotype. However, only the combination of poly(I:C) + CD40L induced complete functional activation of the whole DC population, assessing IL-12p70 production, allostimulatory activity, migratory response to CCL19 and T helper 1-polarizing capacity. Thus, the protocol based on the combination of poly(I:C) and CD40L is more effective for the induction of clinical-grade DCs from elutriated monocytes than the standard cytokine cocktail.
Keywords: dendritic cells, elutriation, functional activation, maturation stimuli, poly(I:C)
Introduction
Dendritic cells (DCs) play a pivotal role in cellular immunity by processing antigens in peripheral tissues and presenting them to T cells in secondary lymphoid tissues, thereby initiating primary immune responses [1,2]. These unique characteristics make DCs potentially suitable for immunotherapy against malignant and infectious diseases [3,4]. Since the first published clinical trial of DC vaccination in 1995, numerous studies have been performed and have demonstrated the safety of such strategies, but in most cases the clinical outcomes did not meet expectations because of suboptimal dosages, immunological potency of the injected DC and host immunodeficiency imposed by the tumours. To overcome these obstacles, new approaches to improve DC-mediated immunotherapeutic strategies are under investigation, including the use of different vaccine cell formats, cell numbers, vaccination schedules, sites of vaccination and maturation stages of DCs [5,6].
As the number of clinical studies using monocyte-derived DCs for cancer immunotherapy continues to increase, it is essential to provide advanced protocols for generating sufficient quantities of well-characterized, highly immunogenic DCs according to current good manufacturing practice (cGMP) guidelines. The elutriation method for monocyte enrichment is based on the physical separation of cells by counterflow centrifugation, in which cells are separated by size and density [7]. Elutriation with the Elutra™ cell separation system (Gambro BCT, Lakewood, CO, USA) gives a high recovery of monocytes directly from leukapheresis products that can be differentiated further into DCs suitable for vaccination protocols [8]. Because the whole process of elutriation can be conducted in a closed system, clinical-grade DCs can be obtained easily. DCs generated from elutriated monocytes show the same characteristics as DCs made from monocytes purified by plastic adherence [7–9].
Several known molecules or molecular cocktails have been reported to trigger DC maturation, including: (i) proinflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 [10]; (ii) molecules derived from pathogenic agents, such as lipopolysaccharide, lipoteichoic acid, cytidine–phosphate–guanosine oligonucleotides [10–12], double-stranded RNA or polyinosinic : polycytidylic acid [poly(I:C)][13]; and (iii) T cell-dependent signal such as CD40 ligand (CD40L) and interferon (IFN)-γ[14]. The effect of combinations of the aforementioned maturation factors on the maturation of elutriated monocyte-derived DCs remains relatively unexplored. Currently, most clinical trials utilize a cytokine cocktail that includes IL-1β, IL-6, TNF-α and prostaglandin E2 (PGE2) [15]. However, mature DCs (mDCs) with cytokine cocktail failed to produce IL-12p70. Therefore, we sought to determine whether combinations of various maturation factors may co-operate in DC maturation from elutriated monocytes and thus provoke the potent T helper 1 (Th1)-biased DC activity for clinical application. To this end, we selected maturation factors from three broad classes of stimuli: microbes and their products recognized by Toll-like receptors (TLRs) [peptidoglycan (PGN) and poly(I:C)], a proinflammatory mediator (TNF-α) and an activated T cell-derived factor (CD40L).
Materials and methods
Leukapheresis and elutriation
A total of seven leukapheresis products were obtained from seven healthy donors after obtaining written informed consent using the COBE Spectra Apheresis System (Gambro BCT), as described previously [16]. The study protocols were approved by the local ethics committee and met the guidelines for blood donation. Continuous counterflow elutriation was performed with the Elutra™ cell separator (Gambro BCT), and with Hanks's buffered salt solution (HBSS; Bio-Whittaker, Walkersville, MD, USA) used as elutriation buffer in automation mode. The leukapheresis product was loaded via the inlet pump into the constantly rotating (2400 rpm) elutriation chamber. In automation mode, the cell separator produced five elutriation fractions, each specified by a centrifuge speed, loading or elutriation buffer flow rate and process volume. The final monocyte-rich fraction was collected from the chamber into the final collection bag after the complete stop of the centrifuge. All procedures were conducted according to the manufacturer's recommendations.
The DC generation
After removing the HBSS elutriation buffer, elutriated monocytes were plated on T75 culture flasks (Greiner Bio-One GmbH, Frickenhausen, Germany) in complete medium, i.e. X-VIVO 15 (Bio-Whittaker), 100 U/ml penicillin, 100 mg/ml streptomycin and 2 mM l-glutamine, 2% human AB plasma. Adherent cells were cultured in 250 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (kindly provided by LG Life Sciences, Daejon, Korea) and 1000 U/ml recombinant human IL-4 (BD Biosciences, San Jose, CA, USA) for 6 days. Cultures were fed every other day by removing half the supernatant and adding fresh medium with a full dose of cytokines. On day 6, immature (iDCs) were harvested and replated at 1 × 106/ml on 24-well plates (Geiner). To induce maturation, iDCs were stimulated with one or a combination of the following reagents for 48 h: TNF-α (10 ng/ml; BD Biosciences), poly(I:C) (20 µg/ml; Sigma Chemical Co., St Louis, MO, USA), Staphylococcus aureus PGN (10 µg/ml; Fluka, Milwaukee, WI, USA), soluble human recombinant CD40L (1 µg/ml; Chemicon, Temecula, CA, USA), cytokine cocktail consisting of 10 ng/ml IL-1β, 10 ng/ml IL-6, 10 ng/ml TNF-α (all from BD Biosciences) and 10−7 M PGE2 (Sigma). After 48 h, the cells were harvested and analysed.
Flow cytometry analysis
To examine cell surface markers, samples for immunophenotyping were taken at the start of the culture and after DC maturation (day 8). The following commercial monoclonal antibodies (mAb) were used: anti-CD11c, -CD14, -CD1a, -CD83, -CD86, -human leucocyte antigen D-related (HLA-DR), CCR7, CD40 and CD80 (all from BD Biosciences). Contamination with natural killer cells, T cells and B cells was detected using CD16, CD56, CD3 and CD19 mAb (all from BD Biosciences). Cells were collected and analysed using the Cytomics™ Flow Cytometer (Beckman Coulter, Fullerton, CA, USA). Non-relevant antibodies of the recommended isotypes were included as controls. Data analysis was performed by WinMDI (Scripps Institute, La Jolla, CA, USA) or cxp and fcs 3·0 software for FC500 (Beckman Coulter). Cells were gated electronically according to light-scatter properties to exclude cell debris or dead cells stained with propidium iodide (Sigma). Results were expressed as median fluorescence intensity.
Allogeneic mixed leucocyte reaction assay
The DCs were harvested, washed and resuspended in RPMI-1640 + 10% fetal bovine serum (FBS). A total of 2 × 105 allogeneic T cells were incubated with irradiated DCs (30 Gy) at different responder : stimulator ratios ranging between 10:1 and 1280:1 in 96-well flat-bottomed plates. After 4 days of co-culture, the cells were pulsed with [3H]-thymidine (0·5 µCi/ml final concentration) and incorporation was measured after 16 h. Responses were reported as mean of triplicate counts per minute ± standard error of the mean (s.e.m.) minus the background counts.
Enzyme-linked immunosorbent assay
The culture supernatants were collected and frozen at −20°C for cytokine quantification. Production of IL-12p70 and IL-10 in cell culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA) using the OptEIA ELISA kit (BD Biosciences).
Flow cytometric detection of IFN-γ and IL-4 in DC-activated T cells
Intracellular cytokine expression was assessed in T cells using previously described methods, with minor modifications [17]. DCs, immature or mature, were co-cultured with allogeneic T cells for 7 days at a 1:10 ratio. Maturation stimuli were poly(I:C) (20 µg/ml) alone or a combination of poly(I:C) (20 µg/ml) with TNF-α (10 ng/ml), PGN (10 µg/ml) and CD40L (1 µg/ml). Controls were made without addition of DCs. CD4+ T cells were then washed extensively and restimulated with 20 ng/ml of phorbol 12-myristate 13-acetate and 1 µg/ml of ionomycin (all from Sigma) for 8 h. Brefeldin A (10 µg/ml; Sigma) was added during the last 6 h to accumulate most of the cytokine in the Golgi complex. Cells were then fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences) and incubated with fluorescein isothiocyanate (FITC)-labelled anti-IFN-γ and phycoerythrin-labelled anti-IL-4 mAbs (BD Biosciences). Cells were collected and analysed as described above.
Migration assay
The DC migration towards CCL19 [major inflammatory protein (MIP)-3β] or IL-8 (both from Peprotech, Rocky Hill, NJ, USA) was measured in 24-well Transwell plates with polycarbonate filters of 5 µm pore size (Corning Costar, New York, NY, USA); 600 µl of serum-free culture medium (RPMI-1640) with either 10 ng/ml of CCL19 or 50 ng/ml of IL-8 was added to the bottom of the chambers. DCs (1 × 105) were added to the upper chamber in a total volume of 100 µl medium. After 2 h of incubation, the migrated cells in the bottom chamber were collected and counted with a flow cytometer for 1 min at high flow speed.
Statistical analysis
All the experiments in this study were conducted at least three times. If not indicated otherwise, experimental values are given as means ± s.e.m. of triplicate assays. Statistical comparisons were performed by analysis of variance test using spss for Windows version 11·0 (SPSS Inc., Chicago, IL, USA). Any P-value below 0·05 was considered statistically significant.
Results
Generation of DCs from elutriated monocytes
With a total of seven leukapheresis products, elutriations were performed independently. In the leukapheresis products, the total mononuclear cell count was 7·3 ± 0·4 × 109 cells and the mean percentage of CD14+ monocytes was 15·8 ± 4·2%, which increased to 68·9 ± 5·8% after elutriation (data not shown). We found that the generation of DCs from elutriated monocytes was inhibited significantly in the absence of FBS or human AB plasma due to irreversible adherence and macrophage differentiation. As it is important to obtain a high yield of mDCs for vaccination purposes, we compared the yields of DC upon culture with conventional media (RPMI-1640 supplemented with 10% FBS) to X-VIVO 15 in the presence of various concentration of human AB plasma. GM-CSF and IL-4 were added to the elutriated monocytes in the presence of different concentrations of human AB plasma, and after 6 days of culture iDCs were matured with the cytokine cocktail (TNF-α, IL-1β, IL-6 and PGE2). After 48 h, cells were harvested and the yield and phenotype of DCs were analysed. DC yield was defined as percentage of cultured monocytes (Table 1). As dislodging DCs cultured with X-VIVO 15 (without plasma) required more handling than harvesting cells from other conditions, the yield of DCs was influenced significantly by the presence of human AB plasma. We found that 2% AB plasma was optimal for DC yield, which was comparable to that of DCs cultured with RPMI-1640 + 10% FBS. Furthermore, the addition of human plasma induced monocytes to become more mDCs, as evidenced by phenotype analysis (Fig. 1a).
Table 1.
Yield of dendritic cells (DCs) as percentage of the cultured monocytes (n = 3).
| Media | Yield (% ± s.d.) |
|---|---|
| RPMI-1640 + 10% FBS | 72·3 ± 10·3 |
| X-VIVO 15 | 37·7 ± 5·9 |
| X-VIVO 15 + 2% AB plasma | 52·3 ± 8·7 |
| X-VIVO 15 + 5% AB plasma | 55·9 ± 9·3 |
| X-VIVO 15 + 10% AB plasma | 60·7 ± 9·3 |
FBS, fetal bovine serum; s.d., standard deviation.
Fig. 1.
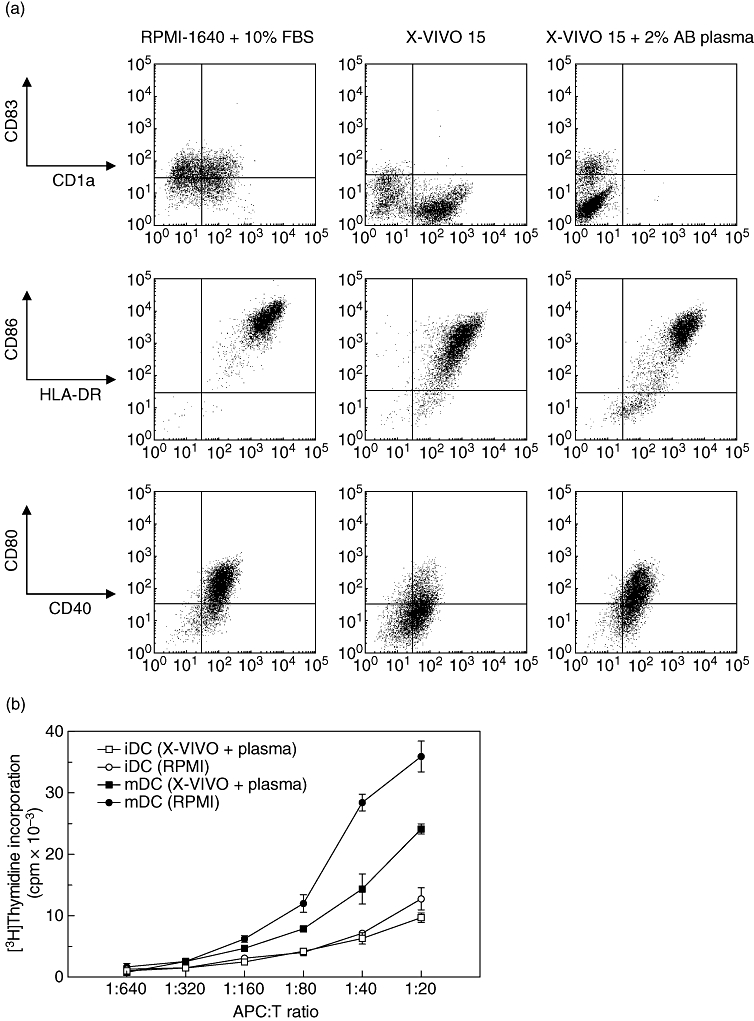
Phenotype and allostimulatory activity of mature dendritic cells (mDCs) generated from elutriated monocytes. (a) Elutriated monocyte-derived immature DCs (iDCs) were stimulated with tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and prostaglandin E2(PGE2) for 48 h in the presence of RPMI-1640 + 10% fetal bovine serum (FBS), serum-free X-VIVO 15 and X-VIVO 15 with 2% human AB plasma. Expression of CD83, CD1a, human leucocyte antigen D-related (HLA-DR), CD86, CD40 and CD80 was analysed by flow cytometry. Compared with the DCs cultured with RPMI-1640 + 10% FBS, DCs cultured with X-VIVO 15 expressed low level of CD83. The representative result of one1 of four experiments is shown. (b) Allogeneic mixed leucocyte reaction assay. The mDCs were generated in the presence of RPMI-1640 + 10% FBS or X-VIVO 15 + 2% human AB plasma stimulated with TNF-α, IL-1β, IL-6 and PGE2. Allogeneic peripheral blood mononuclear cells (105 cells/well) as responders were stimulated with graded numbers of mature and immature DCs (ratio ranging between 1:20 and 1:640). Proliferation was measured by [3H]-thymidine incorporation on day 5. Results from one representative of three independent experiments are shown.
The DCs generated in the presence of human plasma displayed a high expression of HLA-DR, CD86 with moderate CD40 and CD80 that are comparable to that cultured with RPMI-1640 and 10% FBS. It is of note that mDCs cultured with serum-free X-VIVO 15 expressed high levels of CD1a but little CD83, whereas the addition of plasma increased the fraction of CD83 expressing DCs. Meanwhile, the addition of human plasma completely abolished CD1a expression from DCs cultured with X-VIVO 15. As reported previously [8], CD14 expression by mature as well as iDCs was minimal in our setting. Although DCs cultured in RPMI-1640 + 10% FBS exhibited stronger allostimulatory capacity than those cultured in X-VIVO 15 + 2% human AB plasma (Fig. 1b), we selected X-VIVO + 2% human AB plasma as a GMP-grade medium for considering clinical applicability. The phagocytic capacity of resultant iDCs measured by the cellular uptake of FITC-labelled dextran was comparable to that of conventional iDCs generated from CD14+ purified monocytes cultured in RPMI-1640 (data not shown). As reported previously [18], the contamination of granulocytes (14·0 ± 3·0%) in the elutriated product had no significant effect on the viability, phenotype, cytokine production and stimulatory capacity of the DCs generated.
Phenotypical analysis of DCs matured with different stimuli
To test the responsiveness of iDCs to single or combinations of four different maturation stimuli, we evaluated the expression of CD80, CD86, CD40, CD83, HLA-DR and CCR7 after 48 h exposure of iDCs to TNF-α, CD40L, poly(I:C) and PGN. As a control, iDCs were matured with a cytokine cocktail containing IL-1β, IL-6, TNF-α and PGE2. DCs matured with all the stimuli displayed typical mDC morphology and were indistinguishable from DCs matured with cytokine cocktail. Although the yields and viabilities of mDCs were not significantly different from one another (data not shown), phenotypical analysis revealed that DC maturation was affected differently by the stimuli employed (Fig. 2). Exposure of DC to single or combinations of the maturation factors for 48 h induced expression of CD80, CD86, CD40 and HLA-DR with considerably weaker expression of CD83 and CCR7. CD83 and CD40 were up-regulated significantly only by cytokine cocktail expressed. Although poly(I:C) induced the expression of CD86, HLA-DR and CCR7 on DCs comparable to that of a cytokine cocktail, little or no additive effect of the combination of poly(I:C) with other stimuli was detected. PGN alone or in combination with CD40L or TNF-α had little impact on marker expression during DC maturation.
Fig. 2.
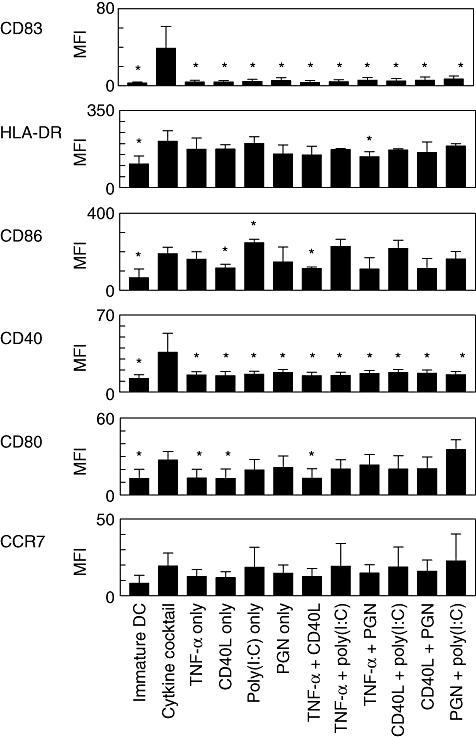
Phenotypic profiles of dendritic cells (DCs) stimulated with different combinations of maturation factors. Immature DCs were stimulated for 48 h with single or pairwise combinations of tumour necrosis factor (TNF)-α, CD40L, polyinosinic : polycytidylic acid [poly(I:C)] and peptidoglycan (PGN), and maturation status was assessed by expression of cell surface markers (CD83, HLA-DR, CD86, CD40, CD80 and CCR7). As a positive control, matured DCs matured with cytokine cocktail (TNF-α, interleukin-1β, IL-6 and prostaglandin E2) were included. Values represent mean fluorescence intensity (MFI) ± standard error of the mean from three independent experiments. *Increased or decreased MFI with respect to control (DCs matured with cytokine cocktail) is statistically significant (P < 0·05).
Immunostimulatory capacity of DCs in an allogeneic mixed lymphocyte reaction
To evaluate the allostimulatory capacities of DCs matured with different stimuli, we performed an allogeneic mixed leucocyte reaction assay. DCs matured with different stimuli induced proliferative responses more effectively than iDCs (Fig. 3a). Increases in allogeneic T cell proliferation were apparent in all DC : T cell ratios tested and were consistent for all donors tested (data not shown). DCs matured with poly(I:C) + CD40L presented much stronger allostimulatory activity than DCs matured with cytokine cocktail, as well as other stimuli. There was no significant difference in allostimulatory capacity between DCs matured with cytokine cocktail and DCs matured with poly(I:C) alone, TNF-α + poly(I:C) or PGN + poly(I:C). The other combinations of stimuli failed to provide either additive or synergistic effects on T cell proliferation.
Fig. 3.
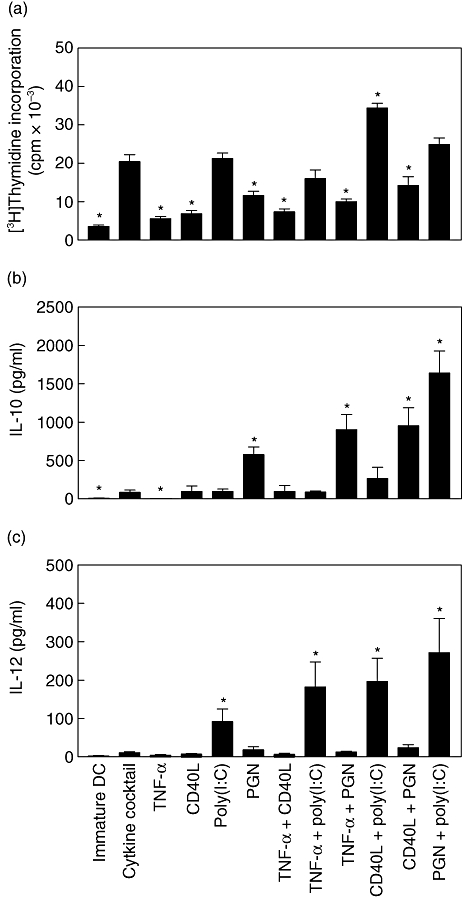
Allogeneic T cell proliferation and cytokine secretion by dendritic cells (DCs) matured with different combinations of maturation stimuli. (a) Capacity of mature DCs (mDCs) generated with different combinations of maturation stimuli to induce allogeneic T cell proliferation. Immature DCs were stimulated with the indicated maturation stimuli for 48 h, and the mDCs were used to stimulate proliferation of allogeneic peripheral blood mononuclear cells (2 × 105 cells/well) at different stimulator : responder ratios. Data from the stimulator : responder ratio at 1:10 are presented as mean counts of incorporated [3H]-thymidine. Results shown represent the mean ± standard error of the mean (s.e.m.) of four independent experiments. *Increased or decreased cell proliferation with respect to control (DCs matured with cytokine cocktail) is statistically significant (P < 0·05). (b) IL-10 and (c) IL-12p70 were measured in culture supernatants 48 h later. Results are expressed as mean ± s.e.m. of six independent experiments. *Increased cytokine production with respect to control (DCs matured with cytokine cocktail) is statistically significant (P < 0·05).
Cytokine production by DCs matured with different stimuli
The level of IL-12 production by DCs is a major factor driving the development of Th1 cells. IL-10, a pleiotropic cytokine known to have inhibitory effects on the accessory functions of DCs, appears to play a central role in preventing overly pathological Th1 or Th2 responses in a variety of settings. Thus, we studied the production of IL-10 and IL-12p70 by DCs differentiated under the influence of the above factors (Fig. 3b and c). The production of IL-12p70 and IL-10 by either iDC or mDCs cultured with cytokine cocktail was low or just detectable. Major enhancement of IL-10 production was caused by PGN. While PGN, CD40L and TNF-α alone did not induce IL-12p70, only poly(I:C) induced consistently high levels of IL-12p70 and low levels of IL-10. Among the combinations, PGN + poly(I:C), CD40L + poly(I:C) and TNF-α + poly(I:C) were the strongest stimulators of IL-12p70 secretion. In contrast, PGN + TNF-α and PGN + CD40L induced high levels of IL-10 and low levels of IL-12p70. Only PGN + poly(I:C) induced high levels of both IL-10 and IL-12p70. The differences observed in cytokine expression were not due to apoptosis, as fewer than 10% of cells stained positively for annexin V after maturation (data not shown). We could also not detect transforming growth factor-β1 production (data not shown). Thus, T cell stimulatory capacity of DCs correlated with the state of their phenotypic maturation as reflected by the levels of co-stimulatory and maturation-associated molecules as well as Th1-biased cytokine production.
Polarization of T cell by DCs matured with different stimuli
We evaluated T cell cytokine responses induced by DC maturation conditions. Purified CD4+ T cells were cultured with various mDCs, and Th1/Th2 polarization was monitored by measuring cytoplasmic IFN-γ or IL-4 production (Fig. 4). In line with strong IL-12 production, poly(I:C) alone induced allogeneic CD4+ T cells preferentially to differentiate towards a Th1 response, and this was enhanced further by a combination with TNF-α or CD40L as judged by elevated IFN-γ-producing cells with minimal expression of IL-4 by intracellular cytokine staining. However, TNF-α, CD40L or PGN alone or in combination induced IFN-γ-producing T cells only weakly (data not shown).
Fig. 4.
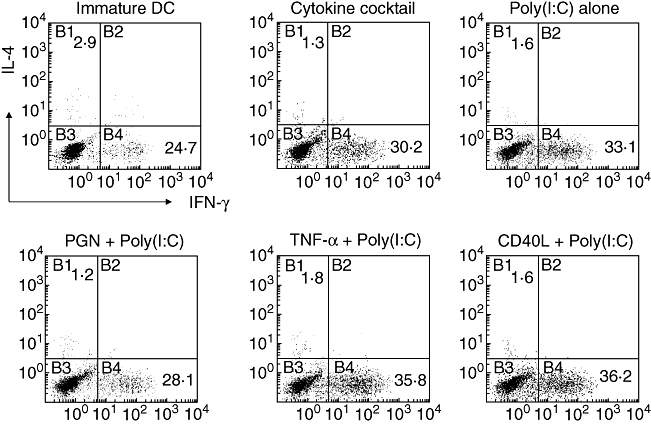
Intracellular cytokine expression of dendritic cell (DC)-activated T cells. Immature DCs, DCs matured with either cytokine cocktail, polyinosinic : polycytidylic acid [poly(I:C)] (20 µg/ml) alone or a combination of poly(I:C) (20 µg/ml) with tumour necrosis factor-α (10 ng/ml), peptidoglycan (10 µg/ml) and CD40L (1 µg/ml) were co-cultured with allogeneic CD4+ T cells. After 7 days of co-culture, T cells were restimulated with phorbol 12-myristate 13-acetate and ionomycin for 8 h. Brefeldin A was added to the culture during the last 6 h before staining to prevent cytokine release. Quadrants were set according to the fluorescence intensities of isotype-matched control antibodies with irrelevant specificity. Results from one representative of three independent experiments are shown.
The DCs matured with CD40L and poly(I:C) are capable to migrate
Maturation of DCs consistently up-regulated CCR7, driving homing to lymphoid organs. However, CCR7 expression does not always guarantee acquisition of CCL19/CCL21 responsiveness [19]. We assessed CCL19-driven chemotaxis of mDCs and found that they possessed the capacity to sense CCL19 gradients (Fig. 5). While iDCs were able to migrate in response to IL-8, none of the mDCs showed a significant migration over the level of DCs matured with cytokine cocktail. When MIP-3β was introduced, most of the mDCs showed a comparable migratory capacity, which was approximately 40% of the capacity of the DCs matured by cytokine cocktail. DCs matured with CD40L + poly(I:C), however, exerted a comparable level of migratory activity to that of cytokine cocktail.
Fig. 5.
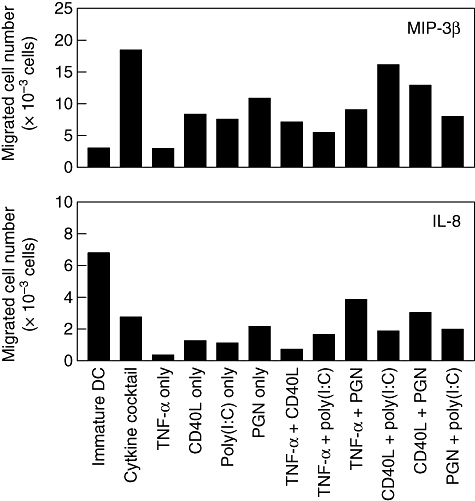
Chemotactic response of dendritic cells (DCs) in response to major inflammatory protein (MIP)-3β and interleukin (IL)-8. DCs (1 × 105) were seeded in the upper compartment of Transwell plates and incubated for 2 h at 37°C in the presence or absence of MIP-3β (10 ng/ml) or IL-8 (50 ng/ml) added to the lower compartment. DC counts in the lower chamber wells were measured by flow cytometry. Chemotaxis in the absence of any chemokines (medium) was the negative control. Results from one representative of three independent experiments are shown.
Discussion
The DCs have been used extensively in immunotherapy for their properties of initiator and modulator of immune responses, but there are some issues to be addressed for the clinical application of DCs. First, a large number of DCs must be generated under sterile cGMP conditions without using xenogeneic materials such as FBS. Secondly, a well-defined protocol should be prepared to generate DCs of stable phenotype and complete maturation.
The aim of this study was to define effective combinations of stimulators for generating functionally mDCs from elutriated monocytes. The maturation status of DCs is important for adequate T cell recruitment, activation, expansion and differentiation. Maturation of elutriated monocyte-derived DCs with a conventional cytokine cocktail resulted in phenotypically mature but functionally inefficient DCs when X-VIVO 15 was employed. Therefore, we compared mDCs across categories of maturation stimuli with regard to phenotype, cytokine production, allogeneic T cell stimulation of DCs, migratory capacity and Th1/Th2 polarization. These stimuli include PGN, poly(I:C), CD40L and TNF-α alone or in pairwise combinations.
The PGN has been shown to signal via TLR-2 and the intracellular pattern recognition receptor Nod2 [20,21]. While the ability of PGN to activate TLR-2 has been questioned recently, a recent study by Dziarski and Gupta [22] revealed that S. aureus PGN is indeed a TLR-2 activator. TLR-3 and melanoma differentiation-associated protein-5 has been implicated in the recognition of poly(I:C) and dsRNA recognition pattern receptors [23,24]. Some of the parameters of DC maturation, such as up-regulation of major histocompatibility complex (MHC) and co-stimulatory molecules, can be triggered by any of the stimuli mentioned. IL-12p70 production, however, is induced only by certain microbial stimuli, such as ligands of TLR-3 and TLR-4, but not by TNF-α or TLR-2 ligands [21]. The mechanism by which different maturation signals co-operate in the DC maturation and function is not yet defined. It has been reported that TLR synergy leads to sustained c-jun phosphorylation and enhances the expression of sets of cytokine genes including IL-12p70 [25], Th1 responses [26] and CTL responses in vivo[27].
While mDCs stimulated with the aforementioned stimulators showed typical DC morphology and expressed high levels of HLA class II, there were some differences in patterns of phenotypes and cytokine production, implying the existence of qualitative differences. Poly(I:C) was capable of inducing DC maturation as measured by the up-regulation of cell surface markers such as CD86, CD80, CCR7 and HLA-DR as well as allogeneic T cell stimulation. Combinations with other cytokines, however, failed to augment these maturation markers.
CD83 is a hallmark of mDCs which also express high levels of MHC, co-stimulatory, adhesion and activation molecules [28,29]. Although the function of the CD83 on DCs remains unclear, studies have indicated its role in the modulation of antigen presentation and T cell activation [30,31]. While previous studies have suggested that expression of CD83 [32] or CD1a [33] correlated with IL-12 expression and the functional outcome of resulting DCs, this was not reflected in our results. Only a fraction of cells expressed CD83 on mDCs cultured with various combinations of maturation factors in X-VIVO 15 supplemented with human AB plasma, suggesting that the maturation factors may provide insufficient signals for DC maturation. This, however, is not a new finding, as Napoletano et al.[34] showed that induction of CD83 was minimal in the serum-free condition. In our setting, the intensity of CD83 was enhanced when cells were cultured with human plasma-supplemented X-VIVO 15, implying that plasma components contribute to the expression of CD83. The cause of discrepancy between our data and the previous reports is not clear. It could be due to the type of cells (elutriated monocytes versus enriched or purified peripheral blood monocytes), cellular manipulation procedures (elutriation versus magnetic affinity cell sorting purification) or culture media and/or instrument setting of flow cytometry in the experiments. Another variation that can give rise to the difference may come from the source and/or concentration of serum employed.
In this study the cytokine cocktail induced the highest levels of CD83, but little IL-12p70 could be detected in culture supernatant. This is due most probably to suppression of IL-12 production by PGE2[35,36], and the extensive implementation of this maturation protocol in clinical trials might explain the limited success of DC vaccination therapy to date. The production of high levels of IL-12p70 was attained only in the presence of poly(I:C). IL-12 derived from DCs plays a central role in the development of IFN-γ-producing Th1 cells [37,38] and this was confirmed by cytoplasmic staining of IFN-γ in DC-activated T cells. PGN alone induced strong IL-10 production and this was enhanced further by combinations with most other stimuli. In general, signals that induce high IL-12 secretion such as poly(I:C) [39] promoted Th1 responses and stimuli that enhanced IL-10 production with or without inducing secretion of IL-12 (i.e. PGN) elicited less allostimulatory activity and Th1 responses in our system. Although DCs matured with either TNF-α + poly(I:C) or PGN + poly(I:C) displayed enhanced IL-12 secretion and some of the maturation markers of DCs, they were not comparable to that of DCs matured with CD40L + poly(I:C) in an allostimulatory capacity. While IL-12 production of DC matured with poly(I:C) or poly(I:C) + PGN was more efficient than DCs matured with cytokine cocktails, the T cell stimulatory capacity of DC was comparable only to conventional DCs, implying that this parameter is not sufficient for DC capacity. This is due most probably to weak induction of MHC and co-stimulatory molecules (such as CD80, CD86) by these stimuli, thus limiting the allostimulatory capacity of mDCs.
One of the major problems in the use of ex vivo-generated DCs for cancer immunotherapy is their poor ability to home into draining lymph nodes. This could be explained by poor expression of chemokine receptor CCR7, which guides DCs to secondary lymphoid organs, the sites of initiation of T cell responses in vivo[19,40,41]. We found that DCs matured with CD40L + poly(I:C) express CCR7 and had high migration in response to MIP-3β, but not to IL-8, suggesting that these DCs are likely to be mDCs that have the potential to migrate in vivo. As all combinations of maturation factors induced weakly the expression of CCR7 of DCs generated from elutriated monocytes, the differences observed in the migration assay stress the importance of assaying the functionality of the expressed CCR7 in a migration assay in order to assess the actual migratory capacity of DCs.
CD40L + poly(I:C)-mDCs showed good capacity to migrate towards CCL19 and in vitro characteristics that are important for optimized DCs for immunotherapy: both the capacity to migrate and to produce IL-12p70. Furthermore, the CD40L + poly(I:C) combination being a useful type 1 immunity-inducing maturation cocktail with better T cell stimulation capacity, as evidenced by allostimulation, may yield superior anti-tumour immune responses and a more effective clinical outcome than DCs generated by cytokine cocktail. Some of the data presented contradict previous reports, and these discrepancies (in immunophenotypes and cytokine production) may reflect the differences of DC progenitors (CD14+ cells versus elutriated monocytes) or culture media (conventional versus X-VIVO 15). Alternatively, the differences noted here may be due to different kinetics of expression. A deeper understanding of the cellular events triggered by the activation of each single maturation stimulus or combinations of different stimuli is a prerequisite for the rational design of successful immunotherapy and vaccination strategies.
In conclusion, we have demonstrated that the requirements for generating functionally mature mDCs from elutriated monocytes. DCs matured with a combination of poly(I:C) and CD40L are well suited for immunotherapy, particularly because it has been feasible to perform large-scale monocyte isolation as well as ex vivo DC generation in closed systems using only reagents of cGMP grade throughout the whole procedure. Comparative clinical trials need to be initiated to determine whether this approach can resultin more potent DC vaccines for the induction of specific T cell-mediated anti-tumour responses to overcome the limited success of DC vaccination to date.
Acknowledgments
This research was supported by a grant SC-2130 from the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, Republic of Korea.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377–413. doi: 10.1080/08830180600992456. [DOI] [PubMed] [Google Scholar]
- 3.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Ann Rev Immunol. 2000;18:245–73. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240–8. doi: 10.1007/s00262-003-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–46. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 6.Song SY, Kim HS. Strategies to improve dendritic cell-based immunotherapy against cancer. Yonsei Med J. 2004;45:48–52. doi: 10.3349/ymj.2004.45.Suppl.48. [DOI] [PubMed] [Google Scholar]
- 7.Berger TG, Strasser E, Smith R, et al. Efficient elutriation of monocytes within a closed system (Elutra) for clinical-scale generation of dendritic cells. J Immunol Methods. 2005;298:61–72. doi: 10.1016/j.jim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Kim HO, Baek EJ, Choi Y, Kim HS, Lee MG. Monocyte enrichment from leukapheresis products by using the Elutra cell separator. Transfusion. 2007;47:2290–6. doi: 10.1111/j.1537-2995.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 9.Adamson L, Palmborg A, Svensson A, et al. Development of a technology platform for large-scale clinical grade production of DC. Cytotherapy. 2004;6:363–71. doi: 10.1080/14653240410004934. [DOI] [PubMed] [Google Scholar]
- 10.De Becker G, Moulin V, Pajak B, et al. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol. 2000;12:807–15. doi: 10.1093/intimm/12.6.807. [DOI] [PubMed] [Google Scholar]
- 11.De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann G, Wiener GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96:9305–10. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdijk RM, Mutis T, Esendam B, et al. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 14.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–8. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 15.Lee AW, Truong T, Bickham K, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl.)(4):A8–22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 16.Strasser EF, Berger TG, Weisbach V, et al. Comparison of two apheresis systems for the collection of CD14+ cells intended to be used in dendritic cell culture. Transfusion. 2003;43:1309–16. doi: 10.1046/j.1537-2995.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 17.Della Bella S, Nicola S, Riva A, Biasin M, Clerici M, Villa ML. Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha. J Leukoc Biol. 2004;75:106–16. doi: 10.1189/jlb.0403154. [DOI] [PubMed] [Google Scholar]
- 18.ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, Vrielink H, Marieke van Ham S. Generation of dendritic cells for immunotherapy is minimally impaired by granulocytes in the monocyte preparation. Immunobiolgy. 2006;211:633–40. doi: 10.1016/j.imbio.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Sanchez N, Riol-Blanco L, Rodriguez-Fernandez JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol. 2006;176:5153–9. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Kitani A, Murray PJ, Strober W. Nod2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–8. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 21.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–33. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005;73:5212–16. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 24.Sasai M, Shingai M, Funami K, et al. K-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J Immunol. 2006;177:8676–83. doi: 10.4049/jimmunol.177.12.8676. [DOI] [PubMed] [Google Scholar]
- 25.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with Toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73:7967–76. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warger T, Osterloh P, Rechtsteiner G, et al. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 28.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 29.Prazma CM, Tedder TF. Dendritic cell CD83: a therapeutic target or innocent bystander? Immunol Lett. 2008;115:1–8. doi: 10.1016/j.imlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse M, Rosorius O, Kratzer F, et al. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J Exp Med. 2000;191:1581–90. doi: 10.1084/jem.191.9.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirano N, Butler MO, Xia Z, et al. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528–36. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosca PJ, Hobeika AC, Clay TM, et al. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–504. [PubMed] [Google Scholar]
- 33.Chang CC, Wright A, Punnonen J. Monocyte-derived CD1a+ and CD1a− dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J Immunol. 2000;165:3584–91. doi: 10.4049/jimmunol.165.7.3584. [DOI] [PubMed] [Google Scholar]
- 34.Napoletano C, Pinto D, Bellati F, et al. A comparative analysis of serum and serum-free media for generation of clinical grade DCs. J Immunother. 2007;30:567–76. doi: 10.1097/CJI.0b013e318046f396. [DOI] [PubMed] [Google Scholar]
- 35.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–9. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 36.Jongmans W, Tiemessen DM, van Vlodrop IJ, Mulders PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J Immunother. 2005;28:480–7. doi: 10.1097/01.cji.0000171290.78495.66. [DOI] [PubMed] [Google Scholar]
- 37.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 38.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 39.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 41.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]


