Abstract
The purpose of this study is to evaluate the effects of neonatal thymectomy in the functional capacity of the immune system. We selected a group of 23 subjects, who had undergone thymectomy in their first 30 days of life, during an intervention for congenital heart disease. Several parameters of the immune system were evaluated during their first 3 years of life. Lymphocyte populations and subpopulations (including naive, memory and effector subpopulations), T cell receptor (TCR) Vβ repertoire, response of T cells following in vitro stimulation by mitogen, quantification of immunoglobulins, TCR excision circles (TRECS) and interleukin (IL)-7 were measured. We found that neonatal thymectomy produces long-term diminution in total lymphocyte counts, especially in naive CD4+ and CD8+ T cells. Additionally, TRECS were decreased, and plasma IL-7 levels increased. A statistically significant negative correlation was found between absolute CD4+ T cells and IL-7 (r = −0·470, P = 0·02). The patients did not suffer more infectious events than healthy control children, but thymectomy in neonates resulted in a significant decrease in T lymphocyte levels and TRECS, consistent with cessation of thymopoiesis. This could produce a compromise in immune function later in life, especially if the patients suffer T cell depletion and need a reconstitution of immune function.
Keywords: cardiac surgery, IL-7, thymectomy, TRECS
Introduction
T cells derive from fetal liver or bone marrow stem cells and migrate at a very early stage to the thymic primordium or thymus respectively. This central lymphoid organ provides the specialized microenvironment where receptor gene rearrangement and maturation of T cells occurs.
In humans, thymic export of T cells begins during early gestation, and a diverse T cell repertoire is established by the end of the first trimester [1]. Molecular analysis of the human thymus T cell receptor (TCR) Vβ repertoire at 15–17 weeks of gestation shows no difference from the newborn thymus Vβ repertoire [2]. Thymic involution starts at the age of 1 year and decreases at a rate of approximately 3% per year until young adulthood, and at a rate of 1% per year thereafter [3–4]. It is known that there is a progressive reduction in the production of new T cells by the thymus throughout time. However, data from patients with different clinical conditions associated to T cell depletion [human immunodeficiency virus (HIV) infection, treatment with chemotherapy and bone marrow transplantation] have confirmed that the thymus in these individuals is still capable of producing new T cells that could be functionally important for reconstitution of immune function [5–9].
Additionally, the importance of the prenatal thymus is observed clearly in patients with DiGeorge syndrome. This syndrome is a congenital disorder caused by developmental defects in the third pharyngeal pouch and fourth pharyngeal arch [10]. As a result, defects are found in the thymus gland that produce a mild to severe immunodeficiency by impaired production and function of T cells [11]. In the neonatal period, the effects of thymectomy are different in several species, depending on the thymus maturity at birth. In mice it results in partial immunodeficiency, principally affecting cell-mediated immune response [12]. In contrast, human neonatal thymectomy is not believed to have clinical consequences [13], and it is common to carry out thymectomy during open heart surgery for the correction of congenital cardiac malformations in order to increase exposure of the surgical field. Nevertheless, the scarce, short-term studies on the impact of human infantile thymectomy on the immune system conclude that these patients have a lower proportion and total number of T cells [13–17].
This study aims to address the relative contribution of the thymus on the immune function in a group of children with congenital heart disease who have undergone incidental thymectomy in the neonatal period. First, we monitored phenotypic and functional changes in the peripheral lymphocyte pool before thymectomy and in following studies during their first years of life (post-thymectomy). Additionally, we conducted an extensive phenotype of CD8+ T cells (naive, central memory, effector memory, and effector) [18] and CD4+ T cells [naive and memory subsets (CD45RA−CCR7+CD27+, CD45RA−CCR7−CD27+, CD45RA−CCR7−CD27−)][19]. Peripheral venous blood was also analysed for TCR Vβ repertoire.
Next, we defined the thymic function by sequential monitoring of TCR recombination excision circles (TRECS). In the last years, the concentration of TRECS has been suggested as a measure for quantifying thymic output in humans [6]. TRECS are episomal DNA circles that are generated during excisional rearrangement of TCR genes. These products are stable, are not duplicated during mitosis and are diluted with each cellular division. Then, the number of TRECS can serve as a parameter of thymopoiesis [20]. Finally, we measured interleukin (IL)-7 levels as this factor may play a critical role in lymphocyte development and homeostasis. IL-7 is required for thymopoiesis, contributes to post-thymic lymphocyte development and provides important survival signals for the naive T cell pool [21]. High plasma levels of IL-7 have been correlated with lymphopenia and low CD4+ T cells because of a variety of clinical conditions reflecting a homeostatic response to T cell development [22–24].
Patients and methods
Patients
Twenty-three children who had undergone thymectomy during an operation for congenital heart disease during the neonatal period (< 30 days of life) were enrolled into the study. The anatomic diagnosis of heart disease in each patient is reflected in Table 1. Information about the complete or partial removal of thymus was not always included in the surgical report, but cardiac surgeons tried to dissect out the whole thymus in an attempt to avoid bleeding. DiGeorge syndrome patients were excluded from this study by investigating the 22q11·2 chromosomal deletion in all children. One hundred and five healthy subjects (age range 0–42 months) were used as the control group (each control was used as single point in the longitudinal analyses).
Table 1.
Cardiac diagnosis.
| Cardiac diagnosis | Patients |
|---|---|
| Transposition of great vessels | 8 |
| Tetralogy of Fallot | 5 |
| Interventricular communication | 1 |
| Interventricular communication and aortic stenosis | 1 |
| Interventricular communication and hypoplasic aortic arch | 1 |
| Interventricular communication and transposition of great vessels | 1 |
| Interventricular communication and coarctation of the aorta | 2 |
| Pulmonary stenosis | 1 |
| Pulmonary atresia | 1 |
| Ventricular septal defect and pulmonary atresia | 1 |
| Tricuspid atresia | 1 |
The medical history of each patient including the original diagnosis, surgical intervention and hospital stay was retrieved from hospital records. All subjects came to paediatrics out-patient clinic every 6 months and their parents were inquired about the history of infections (otitis, bronchitis, pneumonia, meningitis, candidiasis and others), skin diseases, autoimmune diseases, neurological diseases, allergy and cancer.
A peripheral venous blood sample from the patients was collected the day before heart surgery (prethymectomy), and subsequent samples from the same patients were collected at intervals of approximately 6, 12, 18, 24 and 36 months after thymectomy (post-thymectomy). Informed consent was obtained according to the World Medical Association Declaration of Helsinki.
Flow cytometry
Whole blood cells were stained with the corresponding monoclonal antibodies to determine the percentage and the absolute number of the following lymphocyte populations: CD3+ (all T cells), CD3+CD4+ (helper T cells) and subsets CD45RA+ and CD45RO+, CD3+CD8+ (cytotoxic T cells) and subsets CD45RA+ and CD45RO+, CD19+ (all B cells), CD16+CD56+CD3−[all natural killer (NK) cells] and CD3+TCR γδ++ (T cells with γδ receptor).
Also, we performed a phenotypic analysis of CD8+ T cells [naive (CD45RA+CCR7+CD27+), central memory (CD45RA−CCR7+CD27+), effector memory (CD45RA−CCR7−CD27+) and effector (CD45RA+CCR7−CD27+ and CD45RA+/−CCR7−CD27−)][18] and CD4+ T cells [naive (CD45RA+CCR7+CD27+) and memory subsets (CD45RA−CCR7+CD27+, CD45RA−CCR7−CD27+, CD45RA−CCR7−CD27−)][19]. The four-colour analysis was performed in a Coulter EPICS XL-MCL flow cytometer (Fullerton, CA, USA) [25].
The TCR Vβ repertoire was analysed in CD4+ and CD8+ T cells using 24 monoclonal antibodies anti-TCR Vβ (Beckman-Coulter Immunotech, Marseille, France). The immunophenotypic panel of TCR Vβ used in this study detects approximately 70% of all TCR αβ+ T cells.
Proliferation assay
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by Ficoll-Paque densitycentrifugation; 8 × 105 PBMCs per well were cultured with one of the following mitogens: anti-CD3 antibody, phytohaemagglutinin (PHA) and phorbol myristate acetate (PMA) plus ionomycin. The cells were cultured in triplicate wells for 3 days at 37°C in a humidified incubator containing 5% CO2, then the wells were pulsed individually with 1 uCi of [3H]-thymidine and incubated during the last 18 h. The amount of radioactivity was measured in a scintillation counter with results expressed as counts per minute [26].
Quantification of immunoglobulins
Serum immunoglobulin levels (IgG, IgA and IgM) were measured by nephelometry (Immage system; Beckman, Brea, CA, USA).
Quantification of signal joint TRECS
Thymic generation of new T cells was studied by quantifying the production of TRECS in PBMCs by real-time quantitative polymerase chain reaction (PCR) in a LightCycler system (Roche Diagnostic GmbH, Mannheim, Germany). A standard curve was constructed using a plasmid that includes a fragment of 375 base pairs of the signal joint TRECS sequence (kindly supplied by M. A. Muñoz Fernandez) [6]. All samples were analysed in duplicate using a single lot of serially diluted plasmid as standard for all assays. The mean of the duplicate TRECS values was used for data analysis. To normalize for PBMCs equivalents in the input DNA we used a separate real-time PCR assay to quantify the β-globin sequence, as it is known that this gene is present at only two copies per cell, i.e. there are no pseudogenes.
Quantification of IL-7 in plasma samples
Plasma IL-7 levels were measured according to the manufacturer's instructions using a high-sensitivity immunoassay (Quantikine HS human IL-7 immunoassay; R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Student's t-test was used to compare differences between the mean values of the study and control groups using spss 12·0 software. Pearson's r correlation was used to investigate any possible correlation between variables. A P-value < 0·05 (*) was considered statistically significant and P < 0·01 (**) very significant.
Results
Patients and follow-up
The patients enrolled in this study were clinically healthy and none had required hospital admission because of infection. One patient was diagnosed with coeliac disease and another with alimentary allergy. Sequential blood samples were collected prethymectomy and post-thymectomy every 6 months until patients reached the age of 3 years. The results obtained in the immune studies from these subjects were compared with data obtained from a control group in the same age intervals.
Immunophenotype
The total number and percentage of T, B and NK lymphocytes in neonates with congenital heart disease were examined before the surgical intervention (prethymectomy sample) and were comparable to the control group.
After thymectomy and over the 3-year follow-up the patients showed progressive lymphopenia and a very significant reduction in both total number and percentage of CD3+ T lymphocytes compared with the control group. Analysis of the changes in each T cell subset showed that the decay slope was greater in CD4+ T lymphocytes than in CD8+ T lymphocytes. The CD4+ and CD8+ T cells were diminished mainly at the expense of CD45RA+ subsets (Fig. 1).
Fig. 1.
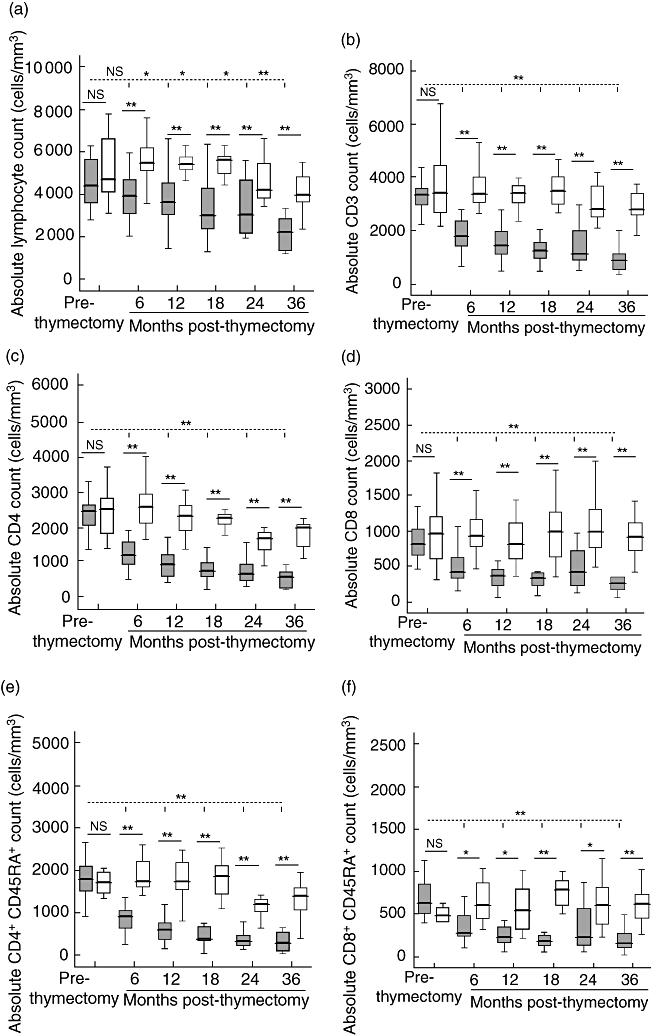
Peripheral lymphocyte count and T cell subsets. Absolute lymphocyte counts (a), CD3+ T cells (b), CD3+CD4+ T cells (c), CD3+CD8+ T cells (d), CD4+CD45RA+ T cells (e) and CD8+CD45RA+ T cells (f) for age-related controls (white box: < 30 days, 6 months, 12 months, 18 months, 24 months and 36 months), patients (grey box) before thymectomy (prethymectomy) and patients after thymectomy (6 months, 12 months, 18 months, 24 months and 36 months post-thymectomy). Boxes are interquartile ranges, box lines are median values and external lines are 5th and 95th percentiles. Significance was calculated using Student's t-test. P > 0·05 (not significant), *P < 0·05 and **P < 0·01. Discontinuous lines are statistical comparisons between pre- and post-thymectomy samples. Continuous lines are statistical comparisons between control groups and patient groups of the same age.
The decay is also observed when prethymectomy patients' samples were compared with post-thymectomy samples and is especially important in the first months post-thymectomy (6 and 12 months). After 36 months the T lymphocyte subsets were maintained in low numbers (Fig. 1).
Because of this reduction in CD45RA+ subsets, we conducted an extensive phenotype of CD8+ T cells and CD4+ T cells in a group of thymectomized children (n = 12, 5–7 years post-thymectomy). The data revealed that the percentage and absolute numbers of naive subset (CD45RA+CCR7+CD27+) in CD8+ and CD4+ T cells was decreased profoundly [percentage of naive CD8+ T cells: patients = 21·2 ± 9·7 (mean ± standard deviation), controls = 57·9 ± 16·4, P = 1·5 × 10−6; naive CD8+ T cells/µl: patients = 55·4 ± 26·8, controls = 391·5 ± 236·5, P = 7·6·10−5; percentage of naive CD4+ T cells: patients = 17·4 ± 8·3, controls = 51·8 ± 11·4, P = 3·10−7; naive CD4+ T cells/µl: patients = 79·7 ± 51·2, controls = 597·9 ± 345·4, P = 4·10−5] (Fig. 2). The CD8+ T cells showed predominantly an effector phenotype (CD45RA+CCR7−CD27+ and CD45RA+/−CCR7−CD27−) and an effector memory phenotype (CD45RA−CCR7−CD27+); the percentage of these subsets are significantly elevated (P = 0·0004 and 0·002 respectively), but absolute numbers were not statistically significant (Fig. 2). The central memory CD8+ T cells were normal. The CD4+ T cells showed a predominantly memory phenotype with elevated percentage of all memory subsets (statistically significant) (CD45RA−CCR7+CD27+, CD45RA−CCR7−CD27+ and CD45RA−CCR7−CD27−) and normal absolute numbers (Fig. 2).
Fig. 2.
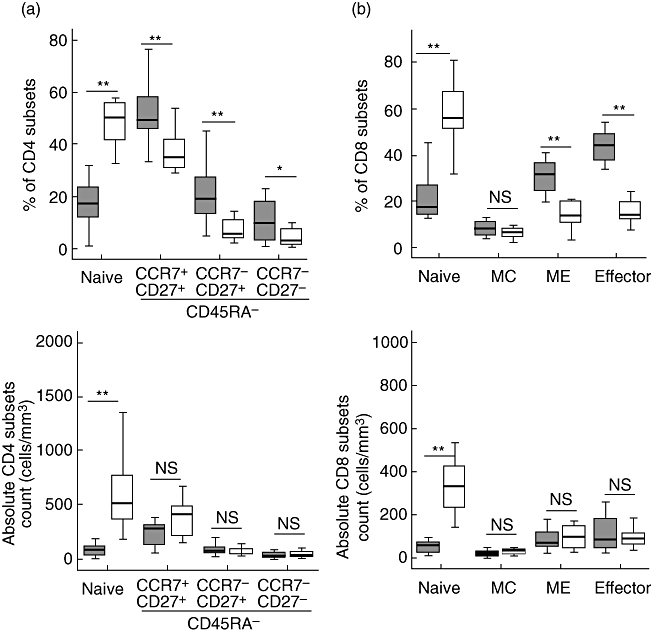
Peripheral percentage and absolute numbers of naive, memory and effector CD4 (a) and CD8 (b) T cell subsets. CD4 T cell subpopulations were defined as: naive (CD45RA+CCR7+CD27+) and memory (CD45RA−CCR7+CD27+, CD45RA−CCR7−CD27+, CD45RA−CCR7−CD27−). CD8+ T cell subpopulations were defined as: naive (CD45RA+CCR7+CD27+), central memory (CD45RA−CCR7+CD27+), effector memory (CD45RA−CCR7−CD27+) and effector (CD45RA+CCR7−CD27+ and CD45RA+/−CCR7−CD27−). Age-related controls (white box, n = 11, median age 9 years) and patients (grey box) after thymectomy (n = 12, 5–7 years post-thymectomy). Boxes are interquartile ranges, box lines are median values and external lines are 5th and 95th percentiles. Significance was calculated using Student's t-test. P > 0·05 (not significant), *P < 0·05 and **P < 0·01.
There were no differences in absolute numbers of lymphocytes expressing CD19 (B cells) and CD16/CD56 (NK cells) between patients and controls. TCR γδ+ absolute numbers showed only a slight diminution (statistically significant) after 18 months post-thymectomy.
Additionally, it would be interesting to know the repertoire of the TCR owing to the findings in the lymphocytic phenotype. Analysis of the TCR Vβ repertoire was performed across the CD8+ and CD4+ T cells in a group of thymectomized children (n = 11, 5–7 years post-thymectomy). The results show different expansions and/or contractions of the TCR Vβ repertoire in both CD4+ and CD8+ T cells from all the thymectomized patients tested (Table 2). The contractions in TCR Vβ usage in CD4+ T cells were less frequent compared with those observed in CD8+ T cells (15% of total contractions in CD4+versus 20% in CD8+ T cells). However, expansions were more frequent (12·5% of total expansions in CD4+versus 7·5% in CD8+ T cells). Contractions of CD8+ T cells expressing TCR Vβ 13·1, 13·6 and 14 were seen in at least five of 11 patients, and they were the most frequently detected shared contractions (Table 2). These observations are consistent with a skewed repertoire of TCR Vβ families in the thymectomized patients.
Table 2.
Flow cytometry analysis of the T cell receptor (TCR) Vβ usage in CD4 and CD8 T cells in 11 thymectomized patients.
| TCR Vb family | TCR Vβ usage in CD4+ T cells | TCR Vβ usage in CD8+ T cells | ||||
|---|---|---|---|---|---|---|
| Control range mean ± 1·5 SD (n = 11)† | Patients (n = 11) | Control range mean ± 1·5 SD (n = 11)† | Patients (n = 11) | |||
| Contraction‡ | Expansion§ | Contraction‡ | Expansion§ | |||
| Vβ 1 | 3·08 ± 0·71 | 0% | 36% | 4·25 ± 1·84 | 27% | 0% |
| Vβ 2 | 10·06 ± 1·88 | 18% | 36% | 5·68 ± 2·21 | 36% | 0% |
| Vβ 3 | 4·98 ± 3·71 | 9% | 0% | 4·29 ± 3·14 | 18% | 0% |
| Vβ 4 | 1·70 ± 0·83 | 18% | 0% | 1·77 ± 1·73 | 9% | 0% |
| Vβ 5·1 | 6·17 ± 1·89 | 0% | 18% | 2·67 ± 1·12 | 36% | 18% |
| Vβ 5·2 | 2·10 ± 0·69 | 0% | 18% | 1·57 ± 0·78 | 27% | 9% |
| Vβ 5·3 | 1·07 ± 0·52 | 0% | 0% | 1·40 ± 1·60 | 0% | 0% |
| Vβ 7·1 | 1·90 ± 0·74 | 36% | 0% | 2·91 ± 1·27 | 36% | 0% |
| Vβ 7·2 | 2·86 ± 1·64 | 27% | 0% | 3·91 ± 3·65 | 0% | 9% |
| Vβ 8 | 5·05 ± 1·16 | 27% | 9% | 4·32 ± 1·92 | 18% | 0% |
| Vβ 9 | 3·26 ± 0·96 | 9% | 0% | 2·15 ± 1·41 | 9% | 9% |
| Vβ 11 | 2·96 ± 0·80 | 27% | 18% | 5·94 ± 2·04 | 27% | 9% |
| Vβ 12 | 1·27 ± 0·42 | 18% | 0% | 1·20 ± 0·89 | 9% | 18% |
| Vβ 13·1 | 4·47 ± 0·75 | 27% | 18% | 3·58 ± 0·87 | 82% | 0% |
| Vβ 13·2 | 1·61 ± 1·09 | 9% | 27% | 2·04 ± 1·40 | 27% | 9% |
| Vβ 13·6 | 1·99 ± 0·62 | 0% | 0% | 1·35 ± 0·69 | 45% | 0% |
| Vβ 14 | 0·88 ± 0·27 | 0% | 27% | 0·76 ± 0·38 | 45% | 36% |
| Vβ 16 | 1·14 ± 0·42 | 18% | 18% | 1·34 ± 1·08 | 0% | 9% |
| Vβ 17 | 5·19 ± 1·58 | 18% | 9% | 3·99 ± 1·91 | 0% | 18% |
| Vβ 18 | 1·26 ± 0·39 | 36% | 9% | 0·55 ± 1·15 | 9% | 18% |
| Vβ 20 | 2·71 ± 1·61 | 0% | 9% | 3·19 ± 2·53 | 9% | 0% |
| Vβ 21·3 | 0·52 ± 0·31 | 36% | 9% | 1·29 ± 1·10 | 0% | 9% |
| Vβ 22 | 3·98 ± 1·45 | 0% | 9% | 3·13 ± 2·49 | 0% | 0% |
| Vβ 23 | 2·68 ± 0·47 | 36% | 36% | 2·61 ± 1·31 | 9% | 9% |
TCR Vβ expression is defined as percentage [mean ± standard deviation (s.d.)] of CD4+ and CD8+ T cells in age-matched controls (n = 11, median age 9 years);
contraction: percentage of patients with TCR Vβ expression lower than the control range (mean −1·5 s.d.);
expansion: percentage of patients with TCR Vβ expression higher than the control range (mean + 1·5 s.d.).
T cell function
All patients had a normal lymphoproliferative response to the assayed mitogens (anti-CD3, PHA and PMA + ionomycin) in both pre- and post-thymectomy samples. All proliferation assays showed results comparable with the controls and above the lower range established in our laboratory.
Quantification of immunoglobulins
The IgG, IgA and IgM levels in prethymectomy samples and in at least one post-thymectomy sample were normal. The levels in post-thymectomy samples were: IgG: 823 ± 212 mg/dl; IgA: 88 ± 46 mg/dl; IgM: 87 ± 31 mg/dl (n = 23, age range 2–6 years); all were within the normal range established in our laboratory for this age (IgG: 400–1100; IgA: 10–160; IgM: 50–180). Specific antigen response following vaccination was measured in only one patient (4 years after-thymectomy), showing normal response to pneumococcus polysaccharide IgG and IgG2.
Quantification of TRECS
To investigate the impact of thymectomy during immune development in young children it is important to examine the production of de novo T cells [15].
The TREC levels in neonates with congenital heart disease before surgical intervention (prethymectomy samples) were comparable to the control group (Fig. 3). In contrast, TREC levels after thymectomy were much lower than in age-matched healthy controls (P < 0·01). The decline of TRECS is also observed when comparing prethymectomy with post-thymectomy patient samples, and it is especially important in the first year after surgery (Fig. 3).
Fig. 3.
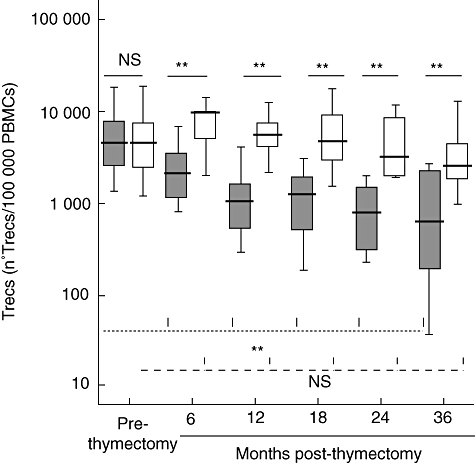
TRECS in age-related controls (white box: < 30 days, 6 months, 12 months, 18 months, 24 months and 36 months), patients (grey box) before thymectomy (prethymectomy) and patients after thymectomy (6 months, 12 months, 18 months, 24 months and 36 months post-thymectomy). Boxes are interquartile ranges, boxes lines are median values and external lines represent 5th and 95th percentiles. Significance was calculated using Student's t-test. P > 0·05 (not significant), *P < 0·05 and **P < 0·01. The higher discontinuous lines are statistical comparisons between prethymectomy and post-thymectomy patient samples and the lower discontinuous lines are statistical comparisons between < 30 days and the remaining control samples. Continuous lines are statistical comparisons between control groups and patient groups of the same age.
Quantification of IL-7
In the study group, IL-7 levels before thymectomy (2·5 ± 2·5 pg/ml) were comparable to the control group (3·8 ± 3·1 pg/ml) (P = 0·11). After thymectomy, the IL-7 concentration increased to 11·1 ± 9·9 pg/ml (1 year after thymectomy) and 13·9 ± 11·4 pg/ml (2 years after thymectomy). These results differ significantly from the controls and from prethymectomy samples (P < 0·01) (Fig. 4).
Fig. 4.
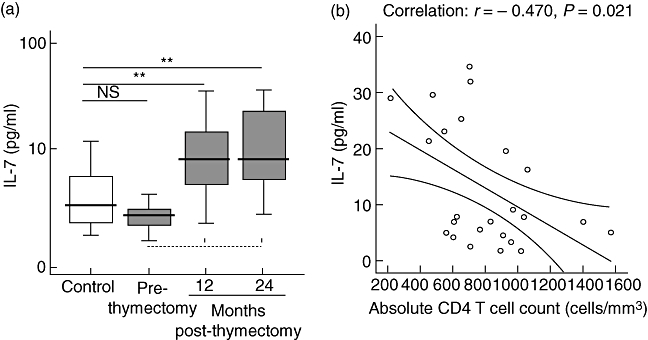
(a) Interleukin (IL)-7 in healthy controls (white box; n = 51, median age 19·3 months, range 1–42 months), patients (grey box) before thymectomy (prethymectomy) and patients after thymectomy (12 months and 24 months after thymectomy). Boxes are interquartile ranges, box lines are median values and external lines represent 5th and 95th percentiles. Significance was calculated using Student's t-test. P > 0·05 (not significant), *P < 0·05 and **P < 0·01. Discontinuous lines are statistical comparisons between prethymectomy and post-thymectomy samples. Continuous lines are statistical comparisons between control groups and patient groups of the same ages. (b) Correlation between IL-7 concentration and absolute CD4+ T cell count 2 years post-thymectomy. r = Pearson's correlation; P = bilateral signification.
Moreover, we analysed the correlation between IL-7 and CD4+ T cell percentage and absolute numbers. The plasma IL-7 level did not show correlation with the CD4+ T cell percentage. One year post-thymectomy there was no correlation between IL-7 and absolute CD4+ T cell counts (r = −0·07, P = 0·67); IL-7 levels correlated negatively with absolute CD4+ T cell counts only 2 years post-thymectomy (r = −0·47, P = 0·02) (Fig. 4). No correlation existed between IL-7 and TRECS quantification.
Discussion
The purpose of this study was to achieve a 3-year follow-up in a homogeneous cohort of children, all of them thymectomized in the neonatal period (< 1 month of age) to define the long-term outcomes of thymectomy in humans. With this longitudinal study we have defined more clearly the natural course of the immune functions in these children comparing data before and after thymectomy.
There are several human and animal models of thymic deficiency that differ from our thymectomized patients. The complete DiGeorge syndrome is a primary immunodeficiency classified in the group of ‘other well defined immunodeficiency’[26]. These patients present absence of the thymus because of fetal developmental anomalies. Thymus transplantation has shown a stable immunoreconstitution in these infants [27,28]. Other studies have described the effects of thymectomy in adults with myasthenia gravis [29]; extrapolation of these data to a paediatric population presents many challenges because of the different degrees of immunological maturity. In neonatal mice models, thymectomy leads to severe immunodeficiency affecting mainly a cell-mediated immune response [12]. On the contrary, in humans neonatal thymectomy is not believed to have clinical consequences [13].
Our results show clearly that thymectomy in neonates produces significant and enduring changes in peripheral T lymphocytes that are consistent with loss of ongoing thymopoiesis. We showed that this reduction in the CD4+ and CD8+ T cells was due mainly to impaired production or proliferation of naive T cells (Fig. 2). It is of interest to mention that the mean CD4+ T cell count in all post-thymectomy samples was diminished to immunodeficiency levels (Fig. 1c), as defined by guidelines for HIV infection (0–1 year of age, less than 1500 CD4 cells/mm3; 1–5 years of age, less than 750 CD4 cells/mm3) [30]. Eighteen months after thymectomy the mean CD4+ T cell percentage was also decreased to levels of evidence of moderate suppression, as defined by guidelines for HIV infection (15–24%) [30]. Four thymectomized patients showed a CD3 percentage (between 19% and 25%) approaching the critically low level of 20% that has been associated with a significant rise for clinical infections [13]. The diminution of CD8+ T cells is less important in our thymectomized patients. It is known that in situations of suboptimal thymic regenerative capacity, the ability to generate CD8 T cells is maintained, while CD4 T cell regeneration is impaired [31]. Several lines of evidence suggest that thymic-independent pathways of CD8+ T cell regeneration are responsible for the differences observed. These could, potentially, involve extrathymic generation of CD8+ T cells from haematopoietic precursors and/or peripheral expansion of mature CD8+ T cells. This peripheral expansion is reflected in the predominant effector and memory effector phenotype of CD8+ T cells after thymectomy. On the other hand, both CD4 and CD8 T cells showed a markedly skewed TCR repertoire, reflecting the lack of production of new lymphocytes with naive phenotype and pre-existing lymphocytes peripheral expansion. Hence, peripheral expansion of existing T cell pools may lead to T cell repertoires limited to those of existing memory T cells, with limited capacity to respond to new antigens [31].
In this study, we also quantified TREC levels in pre- and post-thymectomy samples (Fig. 3). The number of TRECS can serve as a parameter of thymopoiesis. We observed significantly decreased TREC levels in all patients with respect to the control group. This diminution was very profound in some patients, who showed undetectable TREC levels in the first 3 years after thymectomy, which could be consistent with cessation of thymopoiesis. Halnon et al. have published that patients without residual thymus had significantly lower TRECS than those with partial thymectomy [15].
Interleukin-7 has an important role at several stages of T cell development. Several data indicate that IL-7 results in an increased TCR rearrangement in human thymus, suggesting that immune reconstitution in human could be enhanced through stimulation of thymus-dependent T cell generation with exogenous IL-7 [32]. In this manner, IL-7 plays an important role in the development, homeostasis and activity of lymphoid cells. Additionally, follow-up studies in multiple clinical cohorts with T cell depletion have shown profound inverse relationships between circulating IL-7 and peripheral CD4 T cell numbers in children and adults with T cell depletion [21]. Furthermore, the role of IL-7 in thymic and extrathymic development of TCR γδ cells has been investigated. Thymic IL-7 is required for development of thymic TCR γδ cells, while peripheral IL-7 is sufficient for the development of extrathymic TCR γδ intestinal intra-epithelial lymphocytes [33]. One patient with a diagnosis of coeliac disease showed increased TCR γδ intestinal intra-epithelial lymphocytes, but more studies are necessary to conclude if the increased IL-7 observed in thymectomized patients will affect TCR γδ development.
There are very few reports available on plasma IL-7 levels in healthy children. In this study the IL-7 concentration in controls (n = 51 mean age 19·3 months, range 1–42 months) was 3·8 ± 3·1 pg/ml. Additionally, we report for the first time the quantification of IL-7 levels in thymectomized children. Our cohort of patients has high plasma levels of IL-7 associated with low counts of CD4 T cells. However, these high levels of IL-7 are ineffective, as no increase in thymic production or recovery of the CD4 T cell repertoire has been observed during the period of analysis.
Furthermore, in some autoimmune diseases such as juvenile rheumatoid arthritis and bullous pemphigoid, elevated circulating levels of IL-7 are observed. Thus, although the biological effect of high levels of IL-7 in response to T cell depletion is to enhance immune competence, in some situations it is predicted that elevated levels of IL-7 might also predispose to autoimmunity [34]. The patients enrolled in this study were clinically healthy and during the period of analysis only one showed evidence of an autoimmune event.
Because of thymic involution, it has been suggested that the adult thymus does not contribute to new T cell regeneration. However, other data have confirmed that in normal individuals there is an ongoing contribution of the thymus to the maintenance of the T cell compartment throughout life into late adulthood [5,6,8,35]. Some clinical conditions (HIV infection, chemotherapy and bone marrow transplantation) are associated with T cell depletion, and subsequently there is a need for T cell regeneration despite limited thymopoietic capability. The thymus in these patients is functional and it is required for reconstitution of immune function [6–9]. Moreover, the decreased production of recent thymic emigrants with age could explain the increased susceptibility of elderly individuals to new pathogens [36]. Given these possibilities, thymectomy could have an impact on immunity later in life, especially if the patients need a reconstitution of immune function.
Acknowledgments
This work was supported by grants from Fondo de Investigación Sanitaria PI06/0170 and PI06/0614. We would like to thank Maite Fernandez for her excellent assistance to the patients.
References
- 1.Haynes BF, Heinly CS. Early human T cell development analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal microenviroment. J Exp Med. 1995;181:1445–58. doi: 10.1084/jem.181.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonati A, Zanelli P, Ferrari S, et al. T cell receptor β-chain gene rearrangement and expression during human thymic ontogenesis. Blood. 1992;79:1472–83. [PubMed] [Google Scholar]
- 3.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epitelium is independent of puberty: a morphometric study. Scand J Immunol. 1985;22:563–75. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson BD, Douek DC, Killian S, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–75. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 6.Douek DC, McFarland RD, Keisser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:6990–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 7.Haynes BF, Hale LP, Weinhold KJ, et al. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J Clin Invest. 1999;103:453–60. doi: 10.1172/JCI5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow, transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 9.Heitger A, Neu N, Kern H, et al. Essential role of the thymus to reconstitute naïve (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90:850–7. [PubMed] [Google Scholar]
- 10.Thomas RA, Landing BH, Wells TR. Embryologic and other developmental considerations of thirty-eight possible variants of the DiGeorge anomaly. Am J Med Genet Suppl. 1987;3:43–66. doi: 10.1002/ajmg.1320280508. [DOI] [PubMed] [Google Scholar]
- 11.Jawad AF, McDonald-Mcginn DM, Zackai E, Sullivan KE. Immunologic features of chromosome 22q11·2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) J Pediatr. 2001;139:715–23. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- 12.Miller J F. Inmunological functions of the thymus. Lancet. 1961;2:748–9. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 13.Wells WJ, Parkman R, Smogorzewska E, Barr M. Neonatal thymectomy: does it effect immune function? J Thorac Cardiovasc Surg. 1998;115:1041–6. doi: 10.1016/S0022-5223(98)70403-9. [DOI] [PubMed] [Google Scholar]
- 14.Eysteinsdottir JH, Freysdottir J, Haraldsson A, et al. The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life. Clin Exp Immunol. 2004;136:349–55. doi: 10.1111/j.1365-2249.2004.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halnon NJ, Jamienson B, Plunkett M, Kitchen CMR, Pham T, Krogstad P. Thymic function and impaired maintenance of peripheral T cell populations in children with congenital heart disease and surgical thymectomy. Pediatr Res. 2005;57:42–8. doi: 10.1203/01.PDR.0000147735.19342.DE. [DOI] [PubMed] [Google Scholar]
- 16.Madhok AB, Chandrasekran A, Parnell V, Gandhi M, Chowdhury D, Pahwa S. Levels of recent thymic emigrant cells decrease in children undergoing partial thymectomy during cardiac surgery. Clin Diagn Lab Immunol. 2005;12:563–5. doi: 10.1128/CDLI.12.5.563-565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torfadottir H, Freysdottir J, Skaftadottir I, Haraldsson A, Sigfusson G, Ogmundsdottir HM. Evidence for extrathymic T cell maturation after thymectomy in infancy. Clin Exp Immunol. 2006;145:407–12. doi: 10.1111/j.1365-2249.2006.03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol. 2004;34:999–1010. doi: 10.1002/eji.200324478. [DOI] [PubMed] [Google Scholar]
- 19.Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–97. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 20.Ye P, Kirschner DE. Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants. J Immunol. 2002;169:4968–79. doi: 10.4049/jimmunol.168.10.4968. [DOI] [PubMed] [Google Scholar]
- 21.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–76. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 22.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin 7 in T cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 23.Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood. 1996;88:1887–94. [PubMed] [Google Scholar]
- 24.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1 mediated T-cell depletion: implications for T cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 25.García-Pérez MA, Paz-Artal E, Corell A, et al. Mutations of CD40 ligand in two patients with hyper-IgM syndrome. Immunobiology. 2003;207:285–94. doi: 10.1078/0171-2985-00241. [DOI] [PubMed] [Google Scholar]
- 26.Geha RS, Notarangelo LD, Casanova JL, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120:776–94. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–9. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 28.Markert ML, Devlin BH, Alexieff MJ, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109:4539–47. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sempowski G, Thomasch J, Gooding M, et al. Effect of thymectomy on human peripheral blood T cell pools in myasthenia gravis. J Immunol. 2001;166:2808–17. doi: 10.4049/jimmunol.166.4.2808. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR. 1994;44:1–19. [Google Scholar]
- 31.Mackall CL, Hackim FT, Gress RE. T-cell regeneration: all repertoires are not created equal. Immunol Today. 1997;18:245. doi: 10.1016/s0167-5699(97)81664-7. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood. 2002;99:2851–8. doi: 10.1182/blood.v99.8.2851. [DOI] [PubMed] [Google Scholar]
- 33.Laky K, Lefrancois L, von Freeden-Jeffry V, Murray R, Puddington L. The role of IL-7 in thymic and extrathymic development of TCRγδ cells. J Immunol. 1998;161:707–13. [PubMed] [Google Scholar]
- 34.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–71. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 35.Poulin JF, Viswanathan MN, Harris JM, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–86. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goronzy JJ, Weyand CM. T cell development and receptor diversity during age. Curr Opin Immunol. 2005;17:468–75. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]


