Abstract
We wanted to evaluate whether testing for anti-phosholipid antibodies other than anti-cardiolipin (aCL) and anti-beta-2 glycoprotein I (aβ2GPI) immunoglobulin (Ig)G and IgM identifies patients with recurrent pregnancy loss (RPL) who may be positive for anti-phospholipid syndrome (APS). In a cross-sectional study comprising 62 patients with APS, 66 women with RPL, 50 healthy blood donors and 24 women with a history of successful pregnancies, we tested IgM and IgG antibodies to phosphatidic acid, phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl glycerol, phosphatidyl inositol and phosphatidyl serine with and without beta-2 glycoprotein I (β2GPI) from a single manufacturer as well as aCL and aβ2GPI antibodies. Diagnostic accuracies of individual and combined anti-phospholipid (aPL) assays were assessed by computing sensitivities, specificities, positive predictive values and negative predictive values together with their 95% confidence intervals. There was a general trend for increased sensitivities in the presence of β2GPI co-factor with significant effect for certain specificities. The overall combined sensitivity of the non-recommended aPL assays was not significantly higher than that of the aCL and aB2GPI tests. Multiple aPL specificities in RPL group is not significantly different from controls and therefore of no clinical significance.
Keywords: anti-phospholipid antibodies (cardiolipin/beta-2 glycoprotein), anti-phospholipid syndrome, autoantibodies, pregnancy loss
Introduction
Anti-phospholipid syndrome (APS) is an acquired thrombophilic disease characterized by thrombosis and/or pregnancy-related morbidity associated with anti-phospholipid (aPL) antibodies [1]. The laboratory criteria for the diagnosis of definite APS now include anti-beta-2 glycoprotein 1 (aβ2GP1) immunoglobulin (Ig)G and IgM antibodies, as well as anti-cardiolipin (aCL) IgG and IgM and lupus anti-coagulant (LA) assays [2–4]. However, autoantibodies to several other phospholipids and molecules associated with the coagulation pathways have been suggested to be of diagnostic utility in some patients with clinical features of APS [5–9]. Of particular interest to us is whether or not anti-phosphatidic acid (aPA), anti-phosphatidyl choline (aPC), anti-phosphatidyl ethanolamine (aPE), anti-phosphatidyl glycerol (aPG), anti-phosphatidyl inositol (aPI) or anti-phosphatidyl serine (aPS) are of clinical significance in APS associated with recurrent pregnancy loss (RPL).
Negatively charged PL antibodies such as aPI and aPS have been demonstrated previously to show significant association with aCL antibodies [10]. Some investigators have suggested that testing for aPL antibodies other than LA and aCL may help to identify women with RPL with clinical features of APS who may benefit from treatment [11,12]. However, the clinical relevance of these antibodies in the routine work-up of patients with RPL has been disputed [13,14]. In another study of thrombosis associated with systemic lupus erythematosus, no improvement in diagnosis performance was observed when aPL antibodies other than aCL and LA [15] were tested. Furthermore, the requirements for detecting aPL antibodies such as aPS remain controversial [4]. With the inclusion of aβ2GP1 IgG and IgM antibodies to the laboratory assays in evaluating APS, the rationale for additional aPL antibodies testing in RPL remains to be investigated. To address the clinical significance and diagnostic accuracies of several aPL antibodies in APS associated with RPL, we tested aPA, aPC, aPE, aPG, aPI IgG and IgM antibodies with and without β2GP1 as co-factor in four distinct groups.
Materials and method
Study groups
For this study, serum samples from 62 confirmed APS patients, 66 women with RPL, 50 healthy blood donors (HBD) and 24 women with a history of successful pregnancies (WSP) were investigated. Of the 202 participants, 10 were males, with five each in the APS and HBD groups. A diagnosis of APS was made based on the revised International Consensus Statement for definite APS [4]. All patients with APS were repeatedly positive for LA, aCL or aβ2GPI (IgG and IgM) antibodies. All met the clinical criteria for either pregnancy morbidity or arterial or venous thrombosis as defined by the International Consensus Guidelines for the diagnosis of APS [4].
All patients with RPL had been seen at either the University of Utah or LDS Hospital in Salt Lake City and all had at least three consecutive pregnancy losses. All had testing for LA, aCL and aβ2GPI (IgG and IgM) antibodies to exclude APS. All were also offered testing for other known and suspected causes of RPL, including testing for Factor V Leiden, the prothrombin G20210 mutation, thyroid stimulating hormone, assessment of the luteal phase by luteal phase progesterone levels or endometrial biopsy and assessment of the intrauterine cavity either by hysterosalpingography or sonohysterography. Some women with RPL had karyotypes along with their male partners. Of the original 88 patients in this group, 22 were excluded for not fulfilling the criteria for RPL. None of the women included was positive for any of the potential abnormalities assessed. Serum samples for the APS and RPL patients were collected between July 2003 and October 2006 and healthy controls between October 2003 and February of 2007. All samples were stored at −80°C until used.
Anti-phospholipid antibody testing
The LA was detected according to the guidelines of the International Society on Thrombosis and Haemostasis [2]. At initial diagnosis, tests for aCL were performed using either a previously published ‘in-house’ assay [13] employing serum standards derived from the Antiphospholipid Standardization Laboratory [16,17] or aCL kit from Inova Diagnostics (San Diego, CA, USA). All subjects were tested for aβ2GPI using kits from Inova. The cut-offs of the aCL and aβ2GPI assays were determined by the manufacturer (Inova). Using a percentile-based method to establish reference intervals, aCL antibody values greater than 20 IgG anti-phospholipid unit (GPL) or 20 IgM anti-phospholipid unit (MPL) were deemed positive. With this cut-off value of 20 GPL or MPL, 98·6% and 98.8% of the healthy population (n = 488) was found to be negative for aCL IgG and IgM antibodies respectively (product inserts for aCL IgG and IgM assays; Inova). In addition, aCL antibody values between 20–79 GPL or MPL were considered moderately positive and levels above 80 GPL or MPL were interpreted as strongly positive. With respect to the aβ2GPI assays, values greater than 20 standard IgG aB2GPI unit (SGU) or 20 standard IgM aB2GPI unit (SMU) were reported to be positive as recommended by the manufacturer (Inova). A study group of 203 healthy individuals (aged 18–65 years) was used to develop percentile-based cut-off values for both aβ2GPI IgG and IgM assays. With this system, more than 99% of the reference population had SGU less than 20 while 97.5% of this group had SMU less than 20 [18].
For the non-recommended aPL IgG and IgM assays from Aesku Diagnostics (Wendelsheim, Germany), we evaluated six different PL antigenic specificities. These included assays for aPA, aPC, aPE, aPG, aPI and aPS antibodies. The phosphatidic acid was obtained from egg yolk, phosphatidyl choline, phosphatidyl ethanolamine and phosphatidyl serine were all obtained from bovine brain, while phosphatidyl glycerol and phosphatidyl inositol were extracted from egg yolk lecithin and bovine liver respectively (personal communication, Dr Pfeiffer Sascha, Aesku Diagnostics). To assess the requirement for PL-specific recognition, Nunc Maxisorp plates made negatively charged by radiation and were coated with the indicated phospholipid with or without beta-2 glycoprotein I (β2GPI) purified from human plasma as co-factor. For these aPL assays, a cut-off greater than 15 U/ml was considered positive as recommended by the manufacturer. The cut-off was established using serum samples from 100 HBD and calculated based on mean ± 3 standard deviations (Aesku Diagnostics). All calibrators (measured in U/ml with a range from 0 to 300) were produced using human serum tested for human immunodeficiency virus and hepatitis B. Based on the manufacturer's product inserts, these calibrators where not calibrated from any known standard. Negative and positive control materials were provided by the manufacturer for each of the aPL antibody kits. Patients’ sera were diluted in sample buffer containing Tris-buffered saline (TBS), sodium chloride (NaCl), bovine serum albumin and less than 0·1% sodium azide. All washes were performed with wash buffer containing TBS, NaCl, Tween 20 and less than 0·1% sodium azide. Experienced laboratory personnel using procedures recommended by the manufacturers performed all testing within 8 weeks.
Confidentiality
The study of these aPL antibodies in subjects and controls was approved by the Institutional Review Board of the University of Utah, Salt Lake City.
Statistical analysis
Statistical analyses were performed using sas software, version 9·1 of the SAS System (copyright© 2002–2003; SAS Institute, Inc., Cary, NC, USA). To characterize the clinical accuracy of each marker, we computed sensitivities, specificities, positive predictive values (PPVs) and negative predictive values (NPVs) together with their 95% confidence intervals (CI) [19]. To assess the effect of co-factor in the diagnostic accuracy of each marker and for all other statistical comparisons, we used the r software package, version 2·5 (copyright© 2007; The R Foundation for Statistical Computing).
Results
Study participants and profile of recommended aPL markers
Of an original cohort of 75 patients with APS, we excluded 13 subjects after re-evaluation of clinical data and application of the revised International Consensus criteria for the diagnosis of definite APS [4]. Patients in the APS group had thrombosis and/or met obstetric criteria for APS and tested positive for at least one of the standard tests on two separate occasions at least 12 weeks apart. Sixteen of the 57 (28·1%) female APS patients had suffered pregnancy morbidity only. All women in the RPL group had three or more consecutive pregnancy losses. Table 1 shows the age, sex and aPL antibody profile of the recommended diagnostic assays in the study cohort. Of the 45·8% of APS patients who were positive for LA, 29·6% were LA positive only. LA activity was not tested in individuals in the HBD and WSP groups; however, they were all screened for aCL and aβ2GP1 IgG and IgM antibodies.
Table 1.
Profiles of recommended anti-phospholipid (aPL) antibodies in the different study groups.
| Demographics and aPL profile | Groups | |||
|---|---|---|---|---|
| APS (n = 62) | RPL (n = 66) | HBD (n = 50) | WSP (n = 24) | |
| Age (years) | 35·7 ± 11·5 | 33·1 ± 5·7 | 31·3 ± 11·9 | 34·0 ± 8·5 |
| Sex (female/male) | 57/5 | 66/0 | 45/5 | 24/0 |
| aPL antibody profile | ||||
| LA positive* | 27 | 0 | n.d. | n.d. |
| IgG aCL positive† | 46 | 0 | 1 | 0 |
| IgG aβ2GP1 positive† | 36 | 2 | 1 | 0 |
| LA or IgG aCL or aβ2GP1 positive | 60 | 2 | 2 | 0 |
| IgM aCL positive† | 16 | 2 | 1 | 1 |
| IgM aβ2GP1 positive† | 22 | 0 | 0 | 0 |
| IgM aCL or aβ2GP1 positive† only | 45 | 2 | 1 | 1 |
27/59 APS patients tested for aCL (LA).
Medium or high positive results, according to the manufacturer, on two occasions at least 12 weeks apart. Age, mean ± standard deviation; n.d., not performed; APS, anti-phospholipid syndrome; RPL, recurrent pregnancy loss; HBD, healthy blood donor; WSP, women with histories of successful pregnancies; aCL, anti-cardiolipin; aβ2GP1, anti-beta-2 glycoprotein I; LA, lupus anti-coagulant.
Diagnostic performance of aPL antibodies with and without β2GPI co-factor
The percentage of positive samples in each group for aPA, aPC, aPE, aPG, aPI and aPS IgM and IgG antibodies based on a cut-off greater than 15 U/ml is shown in Fig. 1a and b. For all antibodies tested, levels were significantly higher (data not shown) and more prevalent in the APS group compared with the RPL, HBD and WSP groups. The prevalence of these antibodies did not differ significantly between the RPL and controls for the same specificities in the presence of co-factor, except for aPA IgG (Fig. 1a and b).
Fig. 1.
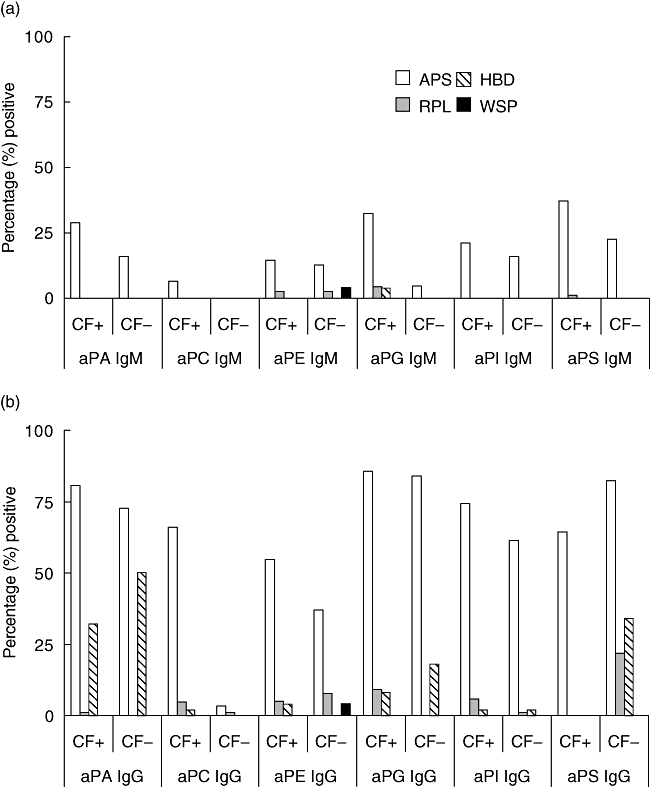
Recognition of anti-phospholipid (aPL) antibodies is specificity-dependent upon beta-2 glycoprotein I (β2GPI) co-factor (CF) for detection. Phospholipid-specific assays were designed with (CF+) or without (CF−) β2GPI CF and assessed for antibody reactivity in all the study participants. The bars represent the percentage of positive individuals in each group.
To determine the clinical significance of aPL antibody testing in the APS and RPL groups, we assessed the individual sensitivities and specificities of the markers evaluated (with their corresponding 95% CI) with and without β2GPI (Table 2a and b). Based on the presence of β2GPI co-factor, we observed a trend of increased sensitivities for all specificities except aPS IgG, especially in the APS group. In this group, the aPA (IgM), aPC (IgM and IgG) and aPG (IgM) demonstrated significantly higher sensitivities (P < 0·05) in the presence of co-factor than their counterparts without β2GP1. The sensitivity of the aPS IgG assays in the absence of β2GPI was increased significantly, although with a compromise in specificity. This effect was most prominent in the RPL group, where the percentage of responders is higher at 25·8% without co-factor versus 0% with co-factor (Fig. 1a). The absence of co-factor reduced significantly the PPV of the aPS IgG and the NPV for aPC IgG in the APS group (P < 0·05) (data not shown). The PPV and NPV in the APS group for the other specificities and their respective isotypes were not affected significantly.
Table 2.
Effect of beta-2 glycoprotein I (β2GPI) co-factor on assay-specific diagnostic performance (APS) and assay-specific diagnostic performance (RPL).
| Assay | Sensitivity (95% CI), % | Specificity (95% CI), % | ||
|---|---|---|---|---|
| + β2GPI | − β2GPI | + β2GPI | − β2GPI | |
| (a) APS | ||||
| aPA-IgM | 29·0 (18·2–42) | 16·2 (8·0–27·6) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPC-IgM | 6·4 (1·8–15·8) | 1·6 (0·0–8·6) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPE-IgM | 17·8 (9·2–29·6) | 13·0 (5·8–23·8) | 100·0 (95·2–100·0) | 98·6 (92·6–100·0) |
| aPG-IgM | 32·2 (21–45·4) | 4·8 (1–13·4) | 97·2 (90·6–99·6) | 100·0 (95·2–100·0) |
| aPI-IgM | 21·0 (11·6–33·2) | 16·2 (8–27·6) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPS-IgM | 38·8 (26·6–52) | 22·6 (13–35) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPA-IgG | 80·6 (68·6–89·6) | 74·2 (61·6–84·4) | 77·0 (65·8–86·0) | 86·4 (76·6–93·4) |
| aPC-IgG | 66·2 (53–77·6) | 3·2 (0·4–11·2) | 98·6 (92·6–100·0) | 100·0 (95·2–100·0) |
| aPE-IgG | 56·4 (43·2–69) | 35·4 (23·8–48·6) | 97·2 (90·6–99·6) | 96·0 (88·6–99·2) |
| aPG-IgG | 85·4 (74·2–93·2) | 85·4 (74·2–93·2) | 94·6 (86·8–98·6) | 86·4 (76·6–93·4) |
| aPI-IgG | 74·2 (61·6–84·4) | 61·2 (48–73·4) | 98·6 (92·6–100·0) | 98·6 (92·6–100·0) |
| aPS-IgG | 64·6 (51·4–76·2) | 83·8 (72·4–92)* | 100·0 (95·2–100·0) | 75·6 (64·4–85)* |
| (b) RPL | ||||
| aPA-IgM | 0·0 (0·0–5·4) | 0·0 (0·0–5·4) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPC-IgM | 0·0 (0·0–5·4) | 0·0 (0·0–5·4) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPE-IgM | 1·6 (0–8·2) | 3 (0·4–10·6) | 100·0 (95·2–100·0) | 98·6 (92·6–100·0) |
| aPG-IgM | 4·6 (1–12·8) | 0·0 (0·0–5·4) | 97·2 (90·6–99·6) | 100·0 (95·2–100·0) |
| aPI-IgM | 0·0 (0·0–5·4) | 0·0 (0·0–5·4) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPS-IgM | 1·6 (0–8·2) | 0·0 (0·0–5·4) | 100·0 (95·2–100·0) | 100·0 (95·2–100·0) |
| aPA-IgG | 1·6 (0–8·2) | 0·0 (0·0–5·4) | 77·0 (65·8–86·0) | 86·4 (76·6–93·4) |
| aPC-IgG | 4·6 (1–12·8) | 1·6 (0–8·2) | 98·6 (92·6–100·0) | 100·0 (95·2–100·0) |
| aPE-IgG | 6·0 (1·6–14·8) | 9·0 (3·4–18·8) | 97·2 (90·6–99·6) | 96·0 (88·6–99·2) |
| aPG-IgG | 10·6 (4·4–20·6) | 0·0 (0·0–5·4) | 94·6 (86·8–98·6) | 86·4 (76·6–93·4) |
| aPI-IgG | 6·0 (1·6–14·8) | 1·6 (0·0–8·2) | 98·6 (92·6–100·0) | 98·6 (92·6–100·0) |
| aPS-IgG | 0·0 (0·0–5·4) | 25·8 (15·8–38·0)* | 100·0 (95·2–100·0) | 75·6 (64·4–85)* |
95% CI, 95% confidence interval for the calculated sensitivities and specificities for each of the indicated antibody marker in the presence (+) and absence (−) of β2GPI.
Denotes significant difference between assays at P < 0·05. Results for APS and RPL groups are shown using control data from the healty blood donor (HBD) and women with histories of successful pregnancies (WSP) groups. aPA, anti-phosphatidic acid; aPC, anti-phosphatidyl choline; aPE, anti-phosphatidyl ethanolamine; aPG, anti-phosphatidyl glycerol; aPI, anti-phosphatidyl inositol; aPS, anti-phosphatidyl serine; Ig, immunoglobulin.
Clinical and diagnostic relevance of multiple aPL testing in APS and RPL
To assess the relevance of multiple aPL antibodies testing in APS, we computed the individual and combined sensitivities, specificities, PPVs and NPVs of the recommended assays (aCL and aβ2GP1 IgM and IgG only) to all non-recommended aPL antibodies tested (Table 3). Thesecomparisons were limited to aPL markers in the presence of β2GP1 as co-factor. The combined sensitivity was calculated based on the number of APS or RPL patients who tested positive for one or more of the markers shown while the combined specificity was estimated based on the number HBD and WSP who were negative for the aPL antibodies indicated. No significant difference in the individual or combined sensitivities, specificities, PPVs and NPVs was observed between the recommended and non-recommended assays in the APS group (Table 3). In the RPL group, however, we observed significant differences (P < 0·05) in the individual or combined sensitivities of the recommended assays and non-recommended aPL tests. In addition, the PPV of all the Aesku aPL assays was significantly higher than that for either the aCL or aCL and aβ2GP1 (Table 3). The increased combined sensitivity of the non-recommended aPL antibodies in the RPL group was, however, compromised by a decreased in specificity. Moreover, the prevalence of these non-standard aPL antibodies was not significantly different in the RPL and the control groups (Fig. 1a and b).
Table 3.
Individual and combined diagnostic performance of anti-phospholipid (aPL) antibody assays.
| Assays | Sensitivity (95% CI)*, % | Specificity (95% CI)*, % | PPV (95% CI)*, % | NPV (95% CI)*, % |
|---|---|---|---|---|
| APS group | ||||
| aCL | 83·8 (72·4–92·0) | 87·8 (78·2–94·2) | 85·2 (73·8–93·0) | 86·6(76·8–93·4) |
| aβ2GPI | 69·4 (56·4–80·4) | 96·0 (88·6–99·2) | 93·4 (82·2–98·6) | 78·8 (69·0–86·8) |
| aCL and β2GPI | 87·0 (76·2–94·2) | 85·2 (75·0–92·4) | 83·0 (71·8–91·2) | 88·8 (79·0–95·0) |
| aPL Aesku | 92·0 (82·2–97·4) | 70·2 (61·0–81·6) | 72·2 (61·0–81·6) | 91·2 (80·8–97·0) |
| RPL group | ||||
| aCL | 3·0 (0·4–10·6)† | 87·8 (78·2–94·2) | 18·2 (2·2–51·8)‡ | 50·4 (41·4–59·4) |
| aβ2GPI | 4·6 (1·0–12·8)† | 96·0 (88·6–99·2) | 50·0 (11·8–88·2) | 53·0 (44·2–61·6) |
| aCL and β2GPI | 6·0 (1·6–14·8)† | 85·2 (75·0–92·4) | 26·6 (7·8–55·2)‡ | 50·4 (41·4–59·4) |
| aPL Aesku | 39·4 (27·6–52·2)† | 70·2 (58·6–80·4) | 54·2 (39·2–68·6)‡ | 56·6 (45·8–66·8) |
95% CI for the calculated individual and combined sensitivities, specificities, positive predictive values (PPVs) and negative predictive values (NPV) for the recommended (without LA) and all non-recommended aPL assays in the anti-phospholipid syndrome (APS) or recurrent pregnancy loss (RPL) groups compared with the healthy blood donor (HBD) and women with histories of successful pregnancies (WSP) controls.
Denotes significant differences (P < 0·05) between the sensitivities of aCL or anti-beta-2 glycoprotein I (aβ2GPI) or aCL and β2GPI versus all aPL Aesku.
Shows significant differences (P < 0·05) in the PPV between aCL or aCL and β2GPI versus all aPL Aesku (RPL groups only).
CI, confidence interval.
Discussion
The presence of persistent LA activity or antibodies against either aCL or β2GPI of IgG and/or IgM isotype is currently the cornerstone for a laboratory diagnosis of APS. However, other PL-specific antibodies are sought frequently by clinicians with patients with features of APS who test negative for the recommended aPL assays. In this study, we have investigated if testing other aPL antibodies improves the diagnostic performance of APS in women with three or more consecutive pregnancy losses. In addition, we evaluated the requirement of β2GPI as co-factor on the diagnostic performance of aPL assays tested. Our results show that the use of β2GPI as co-factor generally improves the sensitivity with significant effects for certain PL-specificities. No significant effect on assay specificities was observed except for the aPS assay. The overall combined sensitivity of the non-recommended aPL assays investigated in this study was not significantly higher than that of the aCL and aB2GPI tests. This finding alone demonstrates the clinical redundancy of these non-recommended assays in routine practice.
Our analyses of these non-recommended aPL assays in terms of sensitivity and specificity showed PL-specific and/or isotype-dependent clinical relevance. These differences may reflect the incomplete understanding of assay conditions necessary to detect these antibodies as well as the role of these molecules in disease pathogenesis. In terms of understanding the requirement for detecting these aPL antibodies in vitro, β2GP1 may not be the only co-factor necessary for the detection of all the aPL antibodies tested. Although it is an established co-factor for anionic PL antibodies [20,21], zwitterionic PL specificities are also known to be β2GP1-independent. For example, autoantibodies to phosphatidylethanolamine (PE) are known to be kininogen-dependent and -independent [7]. Indeed, the presence or absence of β2GP1 did not affect significantly the sensitivity and specificity of the aPE assay independent of isotype antibody. With respect to the aPS assays, the significant differences in diagnostic performance with and without co-factor for the same set of patients point to the need for an in-depth understanding for the role of these molecules in the diagnosis of APS. In the case of the RPL group, the absence of co-factor in the aPS IgG assay increased the prevalence of responders with significant loss of specificity. In addition, we could not document a consistent diagnostic utility for both the IgM and IgG isotypes. Using aPS assays in the absence of β2GP1, one group of investigators has reported diagnostic utility for this phospholipid antibody in patients with RPL who test negative for aCL and LA [12]. Although it could be argued that the patient serum could serve as a source of co-factor, some investigators have reported heterogeneity in the levels of β2GP1 because of polymorphisms [22,23]. There is also evidence that detection of antibody responses to phosphatidylserine–prothrombin complex (PS/PT) rather than to phosphatidylserine alone may be of diagnostic value for APS [9,24]. In addition, annexin 5 has also been reported to be marker of APS [25].
Our results from the combined diagnostic performances of the standard and non-standard aPL assays substantiate the redundancy and, therefore, lack of clinical utility of testing the latter routinely in the evaluation of APS and RPL. First, these antibodies are almost always present in individuals who tested positive for LA, aCL and/or aβ2GP1 antibodies. Secondly, when these non-standard aPL antibodies occur in isolation, their prevalence is not significantly different from that in normal individuals. Lastly, the individual sensitivities of these assays are not significantly higher than the aCL and aβ2GP1antibodies.
The assay performance of aPL antibodies greatly impacts the diagnosis of APS [17,26,27]. Very few studies, if any, have actually used an unbiased screening approach that may provide answers to the diagnostic impact of aPL antibodies and their correlation with antigenic specificity, cross-reactivity or with titres. Our APS and RPL patient material was not completely unselected, as we chose to study sera with known results for the currently recommended diagnostic markers. The low prevalence of aCL and aβ2GP1 IgG and IgM antibodies in the two sets of healthy controls indicate that selection bias was not a large confounding effect. The question of whether it is better to err on the false-negative rather than on the false-positive side remains unanswered here. However, our previous study on the diagnostic accuracies of non-recommended aPL antibodies with kits from two different manufacturers did not reveal any clinical relevance for this study cohort [28]. Moreover, in splitting the results of the different aPL antibodies tested by clinical groups APS, RPL, HBD and WSP, as shown in Fig. 1, we have demonstrated that the prevalence of these antibodies in the RPL group do not differ significantly from the controls.
Our analyses have been limited by the lack of crucial experimental data, such as the detailed composition of the different buffers and the method of coating the various PLs, factors which are all known to affect the outcome of PL antibody recognition and/or detection. Because this information is proprietary, we cannot comment further on how this may influence the results obtained in our study. In addition, whether or not the conditions used in the design of the aPL assays described in this study affects the outcome observed here is highly debatable. Standardization of assays and further studies may be required to assess further the clinical relevance of these aPL assays tested here. Our findings, however, demonstrate a current lack of clinical utility in the routine use of the aPL antibody assays investigated in this study as diagnostic tools in women with RPL who may be at risk for APS.
Acknowledgments
We thank Jurhee Rice (Clinical Coordinator of the APS Study, University of Utah) and Jamie Sorensen (Associated Regional and University Pathologists Institute for Clinical and Experimental Pathology, Salt Lake City, Utah) for help with recruiting of patients, clinical data and sample processing. We also thank Amit R. Phansalkar and Andrew Wilson for help with statistical analysis. All kits for this study were provided free of charge by Inova Diagnostics (San Diego, CA, USA) and Aesku Diagnostics (Wendelsheim, Germany). This work was supported by funds from the ARUP Institute for Clinical and Experimental Pathology.
References
- 1.Roubey RA. Autoantibodies to phospholipid-binding plasma proteins: a new view of lupus anticoagulants and other ‘antiphospholipid’ autoantibodies. Blood. 1994;84:2854–67. [PubMed] [Google Scholar]
- 2.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995;74:1185–90. [PubMed] [Google Scholar]
- 3.Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome. Arthritis Rheum. 1999;42:1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 5.Fleck RA, Rapaport SI, Rao LV. Antiprothrombin antibodies and the lupus anticoagulant. Blood. 1988;72:512–9. [PubMed] [Google Scholar]
- 6.Oosting JD, Derksen RH, Bobbink IW, Hackeng TM, Bouma BN, de Groot PG. Antiphospholipid antibodies directed against a combination of phospholipids with prothrombin, protein C, or protein S: an explanation for their pathogenic mechanism? Blood. 1993;81:2618–25. [PubMed] [Google Scholar]
- 7.Sugi T, McIntyre JA. Autoantibodies to phosphatidylethanolamine (PE) recognize a kininogen–PE complex. Blood. 1995;86:3083–9. [PubMed] [Google Scholar]
- 8.Galli M, Beretta G, Daldossi M, Bevers EM, Barbui T. Different anticoagulant and immunological properties of anti-prothrombin antibodies in patients with anti-phospholipid antibodies. Thromb Haemost. 1997;77:486–91. [PubMed] [Google Scholar]
- 9.Nojima J, Kuratsune H, Suehisa E, Iwatanti Y, Kanakura Y. Acquired activated protein C resistance associated with IgG antibodies against beta2-glycoprotein I and prothrombin as a strong risk factor for venous thromboembolism. Clin Chem. 2005;51:545–52. doi: 10.1373/clinchem.2004.043414. [DOI] [PubMed] [Google Scholar]
- 10.Gharavi AE, Harris EN, Asheron RA, Hughes GR. Anticardiolipin antibodies: isotype distribution and phospholipid specificity. Ann Rheum Dis. 1987;46:1–6. doi: 10.1136/ard.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laroche P, Berard M, Rouquette AM, Desqruelle C, Boffa MC. Advantage of using both anionic and zwitterionic phospholipid antigens for the detection of antiphospholipid antibodies. Am J Clin Pathol. 1996;106:549–54. doi: 10.1093/ajcp/106.4.549. [DOI] [PubMed] [Google Scholar]
- 12.Franklin RD, Kutteh WH. Antiphospholipid antibodies (APA) and recurrent pregnancy loss: treating a unique APA positive population. Hum Reprod. 2002;17:2981–5. doi: 10.1093/humrep/17.11.2981. [DOI] [PubMed] [Google Scholar]
- 13.Branch DW, Silver R, Pierangeli S, van Leeuwen I, Harris EN. Antiphospholipid antibodies other than lupus anticoagulant and anticardiolipin antibodies. Obstet Gynecol. 1997;89:549–5. doi: 10.1016/s0029-7844(97)00065-3. [DOI] [PubMed] [Google Scholar]
- 14.Fialova L, Mikulikova L, Matous-Malbohan I, Benesova O, Zwinger A. Prevalence of various antiphospholipid antibodies in pregnant women. Physiol Res. 2000;49:299–305. [PubMed] [Google Scholar]
- 15.Bertolaccini ML, Roch B, Amengual O, Atsumi T, Khamashta MA, Hughes GR. Multiple antiphospholipid tests do not increase the diagnostic yield in antiphospholipid syndrome. Br J Rheumatol. 1998;37:1229–32. doi: 10.1093/rheumatology/37.11.1229. [DOI] [PubMed] [Google Scholar]
- 16.Harris EN, Pierangeli S, Birch D. Anticardiolipin wet workshop report. Fifth International Symposium on antiphospholipid antibodies. Am J Clin Pathol. 1994;101:616–24. doi: 10.1093/ajcp/101.5.616. [DOI] [PubMed] [Google Scholar]
- 17.Pierangeli SS, Stewart M, Silva LK, Harris EN. An antiphospholipid wet workshop: 7th International Symposium on Antiphospholipid Antibodies. J Rheumatol. 1998;25:156–60. [PubMed] [Google Scholar]
- 18.Lewis S, Keil LB, Binder WL, DeBari VA. Standardized measurement of major immunoglobulin clas (IgG, IgA, and IgM) antibodies to β2 glycoprotein I in patients with antiphospholipid antibodies. J Clin Lab Anal. 1998;12:293–7. doi: 10.1002/(SICI)1098-2825(1998)12:5<293::AID-JCLA8>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 20.Galli M, Comfurius P, Maassen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–47. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 21.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–24. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamboh MI, Wagenknecht DR, McIntyre JA. Heterogeneity of the apolipoprotein H*3 allele and its role in affecting the binding of apolipoprotein H (beta 2-glycoprotein I) to anionic phospholipids. Hum Genet. 1995;95:385–8. doi: 10.1007/BF00208960. [DOI] [PubMed] [Google Scholar]
- 23.Mehdi H, Aston CE, Sanghera DK, Hamman RF, Kamboh MI. Genetic variation in the apolipoprotein H (beta2-glycoprotein I) gene affects plasma apolipoprotein H concentrations. Hum Genet. 1999;105:63–71. doi: 10.1007/s004399900089. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi A, Hayashi T, Chino Y, et al. Significance of antiprothrombin antibodies in patients with systemic lupus erythematosus: clinical evaluation of the antiprothrombin assay and the antiphosphatidylserine/prothrombin assay, and comparison with other antiphospholipid antibody assays. Mod Rheumatol. 2006;16:158–64. doi: 10.1007/s10165-006-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozic B, Irman S, Gaspersic N, Kveder T, Rozman B. Antibodies against annexin A5: detection pitfalls and clinical associations. Autoimmunity. 2005;38:425–30. doi: 10.1080/08916930500288356. [DOI] [PubMed] [Google Scholar]
- 26.Favaloro EJ, Silvestrini R. Assessing the usefulness of anticardiolipin antibody assays: a cautious approach is suggested by high variation and limited consensus in multilaboratory testing. Am J Clin Pathol. 2002;118:548–57. doi: 10.1309/JAMH-GDQ6-6BYK-DW6J. [DOI] [PubMed] [Google Scholar]
- 27.Reber G, Schousboe I, Tincani A, et al. Inter-laboratory variability of anti-beta2-glycoprotein I measurement. A collaborative study in the frame of the European Forum on Antiphospholipid Antibodies Standardization Group. Thromb Haemost. 2002;88:66–73. [PubMed] [Google Scholar]
- 28.Tebo AE, Jaskowki TD, Phansalkar AR, Litwin CM, Branch DW, Hill HR. Diagnostic Performance of Phospholipid-specific Assays for the Evaluation of Antiphospholipid Syndrome. Am J Clin Pathol. 2008;129:870–5. doi: 10.1309/6MPULFBL24FM9B50. [DOI] [PubMed] [Google Scholar]


