Abstract
Probiotics are promoted as being beneficial to health and positive effects on the immune system have been reported. Beneficial immune effects have been attributed to several mechanisms, including stimulating T helper 1 (Th1) immunity. To explore the effects of the probiotic Bifidobacterium animalis on Th1- and Th2-mediated immune responses, two different animal models representing either Th1- or Th2-mediated immune responses were used: a rat model for experimental autoimmune encephalomyelitis (EAE) (Th1) and a mouse model for respiratory allergy induced by ovalbumin (OVA) (Th2). B. animalis administration started when the mice or rats were 2 weeks old. Respiratory allergy or EAE were induced when the animals were 6–7 weeks old. In the allergy model, B. animalis modestly reduced the number of infiltrating eosinophils and lymphocytes in the lungs, but no effects on allergen-specific serum immunoglobulin E levels were found. Cytokine profiles assessed after culturing spleen cells with the mitogen concanvalin A (ConA) showed that B. animalis skewed the Th1/Th2 balance towards Th1 in females. However, allergen-induced cytokine production in females was not affected by B. animalis. In males, B. animalis significantly decreased ConA-induced interleukin-13 and a trend towards lower levels of OVA-induced Th2 cytokines. In the EAE model, B. animalis significantly reduced the duration of clinical symptoms by almost 2 days in males and improved the body weight gain during the experimental period compared with the control group. Our data show that B. animalis reduced several immune parameters in the allergy as well as in the autoimmunity model.
Keywords: allergy, autoimmunity, Bifidobacterium animalis, probiotics
Introduction
The number of commercially available products that are supplemented with probiotics is rising. Dairy products that contain probiotics are sold in every supermarket and probiotic food supplements can be purchased in pharmacies or via the internet. For infants, infant formulas containing probiotics are also currently available. Probiotics are defined as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’[1]. Beneficial effects on the gut [2,3] and the immune system [4,5] are reported. The beneficial effects of probiotics on the immune system are strain-dependent [6].
It is thought that probiotics are able to prevent or alleviate allergic diseases. Evidence for this from human studies is scarce, although some promising results have been published. Recent studies showed that Bifidobacterium longum BB536 improved several subjective medical symptoms and also a slight reduction in pollen-specific immunoglobulin (Ig)E in patients suffering from rhinitis triggered by Japanese cedar pollen [7–9]. The efficacy of probiotics in the prevention or alleviation of atopic eczema in early childhood has been investigated most extensively. A recent meta-analysis has shown that the evidence for efficacy of probiotics is more convincing in prevention than in treatment of atopic eczema [10]. Prevention of atopic eczema was demonstrated in atopic infants who received Lactobacillus GG during the first 6 months of their life [11–13]. In contrast, in a study with a similar experimental setup Lactobacillus acidophilus did not prevent development of atopic eczema at 6 and 12 months, but increased sensitization significantly against several allergens at 12 months [14]. Although enhanced sensitization does not necessarily mean that more allergic disorders will develop in these infants, it does demonstrate that immune effects are not beneficial per se.
Currently, there are several infant formulas on the market that are supplemented with probiotics. However, it is questionable if the administration of these probiotics will have health benefits, e.g. prevention of allergy. Furthermore, several safety issues apply to this vulnerable group. Possibly, infants might be a susceptible group when considering immunomodulatory compounds, because the immune system is still developing. In a mouse model for respiratory allergy, it has been shown that the developing immune system is more susceptible to immunomodulation by L. casei Shirota (LcS). When the mice received LcS during lactation the pulmonary inflammatory response induced by ovalbumin (OVA) was aggravated, whereas LcS did not influence this in adult mice [15]. In addition, the fact that certain probiotics are known to stimulate T helper type 1 (Th1) immunity, which has been suggested as one of the mechanisms by which they can suppress Th2-mediated allergic diseases [16–20], might be an additional safety issue. Excessive immunostimulation might aggravate or induce Th1-mediated immune responses, e.g. autoimmune diseases. Currently, only limited publications are available that have studied the effects of probiotics on autoimmunity and only a few studies in rodents have been published. These studies show that in Th1-mediated immune responses the effects of probiotics are also strain-dependent. LcS increased the duration of clinical symptoms in a rat model for experimental autoimmune encephalomyelitis (EAE) [15,21]. In a murine EAE model it was shown that the outcome of EAE was dependent upon the cytokine profile induced by different probiotic strains. L. casei induced immunoregulatory cytokines and improved EAE, while L. reuteri induced proinflammatory cytokines and aggravated EAE [22].
To investigate further the effects of probiotics on Th1- and Th2-mediated immune responses, the probiotic Bifidobacterium animalis was administered from lactation phase onwards and effects on EAE and respiratory allergy were studied.
Methods
Bacteria
The B. animalis was a kind gift of Numico Research (Wageningen, the Netherlands). B. animalis was cultured at Numico Research and delivered as a freeze-dried powder that was stored at −80°C until use. Suspensions for oral gavage were prepared freshly on the day of gavage by dissolving the powder in saline at a concentration of 5 × 109 colony-forming units (CFU) per ml.
Animals
Pregnant BALB/c mice were obtained from our own breeding colony. Mice were bred specific pathogen-free and kept under conventional conditions. Mice received Hope Farms chow pellets (Woerden, the Netherlands) and water ad libitum. The breeding colony of the animals was prescreened/monitored for endogenous pathogenic viruses and bacteria and was found negative.
Pregnant-specific pathogen-free Lewis rats (LEW/HanHsD) were obtained from Harlan (Horst, the Netherlands). Rats were fed Hope Farms chow pellets and water ad libitum.
The experimental setup of the studies was examined and agreed upon by the Institutional Animal Ethics Committee.
Experimental design respiratory allergy
To study the effects of B. animalis early after birth, young suckling BALB/c mice were used. After birth, the pups were randomized and cross-suckled between the dams. Each nest contained the same number of pups with an equal male : female ratio. Oral administration of B. animalis started when the mice were 2 weeks old. Mice received 1 × 109 CFU B. animalis or saline alone (controls) daily in a volume of 200 µl, except for the first week when this dose was administered in 100 µl. At weaning (21 days after birth) mice were taken away from their mothers and housed in the experimental groups. Mice were sensitized and challenged with OVA (eight females and eight males per experimental group), as described earlier [23], with some minor modifications. Sensitization of the mice started when they were 6 weeks old (day 0). Mice were sensitized on days 0 and 14 by intraperitoneal injection with 10 µg OVA (grade V; Sigma Aldrich, St Louis, MO, USA) adsorbed onto 2·25 mg aluminium hydroxide (AlumInject; Pierce, Rockford, IL, USA) in saline or with saline alone. Mice were challenged on days 35, 38 and 41 by inhalation of OVA or saline aerosols in a plexiglass exposure chamber for 20 min. Aerosols were generated by nebulizing a solution with 10 mg/ml OVA in saline or saline alone using a nebulizer. At day 43, mice were killed and blood was collected, clotted and serum was obtained for determination of OVA-specific antibodies. Spleens were collected and single cell suspensions were prepared and cultured for cytokine measurements.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed by flushing the lungs with 1 ml sterile phosphate-buffered saline . BAL fluid was centrifuged at 400 gfor 10 min. Cell pellets were used for determination of total cell numbers and cytospin preparations. Cytospins were stained with May–Grünwald–Giemsa and on each preparation 400 cells were counted. Supernatants obtained for cytokine measurement were stored at −80°C.
Preparation and culturing of single-cell suspension of the spleen for cytokine production
Spleens were collected and single-cell suspensions were prepared under aseptic conditions by pressing the spleen through a sterile 70 µm nylon cell strainer. Cells were washed in RPMI-1640 (Gibco, Life Technologies, Breda, the Netherlands) with 5% heat-inactivated fetal calf serum (FCS) (PAA, Linz, Austria), 100 U/ml penicillin and 100 µg/ml streptomycin (standard medium) (10 min, 4°C, 300 g) and resuspended in 10 ml standard medium with 10% FCS. The concentration of the cell suspensions was adjusted to 2 × 106 cells/ml. Spleen cells (100 µl/well; 2 × 105 cells) were stimulated with 5 µg/ml concanavalin A (ConA; 100 µl/well) for 48 h or with OVA (100 µg/ml) for 96 h. Supernatants were collected for cytokine measurements.
The OVA-specific IgE enzyme-linked immunosorbent assay
The OVA-specific IgE titres in sera were determined by enzyme-linked immunosorbent assay, as described previously [15].
Bioplex for cytokines
The Th1 and Th2 cytokines were measured in BAL fluid and in supernatants of spleen cells that were cultured with ConA or OVA. Cytokine levels were detected with a Bioplex 5-plex cytokine assay kit that could detect interleukin (IL)-4, IL-5, IL-10, IL-13 and interferon (IFN)-γ (Biorad Life Science, Hercules, CA, USA), according to the manufacturer's instructions. Cytokine measurements were performed on a Luminex® (Biorad Life Science) and Luminex software was used to calculate the amount of cytokines (in pg/ml supernatant).
Experimental design EAE
After birth, the Lewis rats were randomized and cross-suckled between the dams. Each nest contained the same number of pups with an equal male : female ratio. Oral administration of B. animalis started when the rats were 2 weeks old. Rats received 1 × 109 CFU daily in a volume of 200 µl. Control rats received 200 µl saline daily. At weaning (21 days after birth) rats were taken away from their mothers and housed in the experimental groups.
Acute EAE was induced at the age of 7 weeks, as described previously [24]. Rats (eight females and eight males per experimental group) were injected subcutaneously in the left ankle with an emulsion containing 20 µg guinea pig myelin basic protein (MBP; Sigma), 500 µg Mycobacterium tuberculosis type H37RA (Difco, Detroit, MI, USA), 50 µl complete Freund's adjuvant (Difco) supplemented with saline (0·9% NaCl) to reach a volume of 100 µl and females were dosed with 80 µl of the same solution. The dose for immunization with MBP is dependent upon body weight, which was lower in females at the age of 7 weeks. Control rats (n = 4 females and n = 4 females per control) were not immunized and received either B. animalis or saline. After induction of EAE body weights were recorded daily. Neurological signs were also scored daily and graded from 1 to 5; 0: no clinical signs; 0·5: loss of tonicity in distal half of tail; 1: flaccid tail; 1·5: unsteady gait; 2: partial hind limb paralysis; 2·5: complete hind limb paralysis; 3: paralysis of the complete lower part of the body up to the diaphragm; 4: paraplegia; and 5: death because of EAE. Rats were killed 27 days after induction of EAE.
Statistical analysis
In both the allergy and the EAE experiments, both sexes were included in the experiments, because at initiation B. animalis administration was performed in litter. Previously, it has been demonstrated that females are more susceptible for sensitization with OVA [25] and immunization with MBP [26,27]. In addition, immunization with MBP was based upon body weight and as females were lighter than males they were immunized with less MBP, because overdosing can be lethal. Statistical analysis was therefore performed separately in males and females.
Statistical analysis was performed with spss software (SPSS Inc., Chicago, IL, USA). Data are presented as means with standard errors. The significance level was set at P = 0·05. When data were not normally distributed they were log10-transformed prior to analysis. To determine statistically significant differences in cell numbers in lung lavage fluid, a one-way analysis of variance was used. The Levene test was used for homogeneity of variance. When the variance was not equal, the Games–Howell test was used as a post hoc test, otherwise Bonferroni's post hoc test was used. Statistical differences of clinical symptoms and duration of symptoms in the EAE experiment between the experimental groups that were immunized with EAE were determined with a one-tailed Mann–Whitney U-test.
Results
Effects of B. animalis on respiratory allergy
Inflammatory response and cytokines in the lungs
In both male and female mice that were sensitized and challenged with OVA an increase of total cell numbers in BAL fluid was found, which was caused predominantly by a significant increase of eosinophils (Table 1). Previously, it was shown that females were more susceptible to sensitization with OVA [15,25], but in this experiment there was no gender difference in the number of cells infiltrating the lungs. In males, the number of lymphocytes was increased significantly in sensitized mice, but not in sensitized mice that received B. animalis. In sensitized females and males that received B. animalis lower numbers of total cells, eosinophils and lymphocytes were observed, but none of these reductions were significant.
Table 1.
Number of cells in lung lavage fluid.
| Group | n | Totala | Macrophages | Eosinophils | Lymphocytes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | s.e. | Mean | s.e. | Mean | s.e. | Mean | s.e. | ||
| Females | |||||||||
| Control | 4 | 0·381 | 0·089 | 0·316 | 0·083 | 0·005 | 0·0005 | 0·104 | 0·051 |
| Control + B. animalis | 4 | 0·319 | 0·121 | 0·277 | 0·086 | 0 | 0 | 0·038 | 0·032 |
| OVA | 8 | 0·632 | 0·060 | 0·405 | 0·059 | 0·109* | 0·042 | 0·110 | 0·036 |
| OVA + B. animalis | 8 | 0·389 | 0·064 | 0·306 | 0·059 | 0·045** | 0·015 | 0·069 | 0·010 |
| Males | |||||||||
| Control | 4 | 0·241 | 0·027 | 0·214 | 0·015 | 0 | 0 | 0·025 | 0·019 |
| Control + B. animalis | 4 | 0·343 | 0·155 | 0·277 | 0·086 | 0 | 0 | 0·038 | 0·032 |
| OVA | 8 | 0·620* | 0·103 | 0·416 | 0·076 | 0·059* | 0·028 | 0·135* | 0·036 |
| OVA + B. animalis | 8 | 0·410 | 0·071 | 0·330 | 0·055 | 0·024** | 0·088 | 0·052 | 0·018 |
Mean values were significantly different from those of the control group: *P < 0·05;
P < 0·01.
Cell numbers are expressed in 106 cells; data were log-transformed before statistical analysis. To determine statistical significance a one-way analysis of variance with Bonferroni's post hoc test or the Games–Howell (when variance was not equal in the Levene's test) post hoc test was used. B., Bifidobacterium; n, number of mice per group; OVA, ovalbumin; s.e., standard error.
Several cytokines were assessed in the BAL fluid. IL-10 could not be detected and levels of IFN-γ and IL-4 could be detected in sensitized males and females, but were very low. IL-13 was not affected by sensitization and challenge in females, whereas in males IL-13 levels were higher (not significant). No effects of B. animalis on IL-13 levels were observed. In males and females, IL-5 was increased significantly after sensitization and challenge. Administration of B. animalis did not affect the increase in IL-5 (data not shown).
The OVA-specific serum IgE
OVA-specific IgE levels were detected only in sensitized mice and not in vehicle-treated controls. In both sensitized males and females, OVA-specific IgE titres were not affected by B. animalis treatment (data not shown).
Cytokine production by spleen cells stimulated with ConA
Spleen cells from sensitized females produced more Th2 cytokines after ex vivo stimulation with ConA than sensitized males. After in vitro stimulation with the mitogen, ConA spleen cells from female mice that were sensitized with OVA produced significantly more IL-10. No statistically significant differences were observed for IL-4, IL-5, IL-13 and IFN-γ production (Table 2). B. animalis did not affect the increased IL-10 production. B. animalis induced a trend towards higher IFN-γ levels in control as well as in sensitized females. Furthermore, in control mice B. animalis reduced IL-4 significantly.
Table 2.
Cytokine production by spleen cells stimulated with concanavalin A (ConA).
| Group | n | IL-4a | IFN-γ | IL-5 | IL-10 | IL-13 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.e. | Mean | s.e. | Mean | s.e. | Mean | s.e. | Mean | s.e. | ||
| Females | |||||||||||
| Control | 4 | 113 | 8·7 | 149 | 16 | 9·1 | 1·7 | 9·9 | 0·83 | 45 | 5·6 |
| Control + B. animalis | 4 | 81* | 6·7 | 439 | 129 | 8·2 | 2·2 | 8·9 | 1·7 | 44 | 11 |
| OVA | 8 | 121 | 14 | 176 | 21 | 21 | 5·9 | 20* | 3·0 | 62 | 11 |
| OVA + B. animalis | 8 | 134 | 7·6 | 328 | 69 | 18 | 3·3 | 22* | 3·2 | 74 | 11 |
| Males | |||||||||||
| Control | 4 | 50 | 7·8 | 168 | 39 | 2·4 | 0·89 | 4·3 | 1·1 | 16 | 3·5 |
| Control + B. animalis | 4 | 57 | 12 | 244 | 73 | 3·6 | 0·32 | 6·2 | 1·2 | 21 | 4·8 |
| OVA | 8 | 114** | 10 | 197 | 13 | 15* | 2·9 | 18*** | 2·2 | 45** | 4·3 |
| OVA + B. animalis | 8 | 83 | 8·5 | 175 | 35 | 9·4* | 1·7 | 13** | 0·28 | 29† | 3·6 |
Mean values were significantly different from those of the control group: *P < 0·05;
P < 0·01;
P < 0·001.
Mean values were significantly different from those of the ovalbumin (OVA) group.
Cytokines are expressed in pg/ml supernatant. Data were log-transformed before statistical analysis. To determine statistical significance a one-way analysis of variance with Bonferroni's post hoc test or the Games–Howell (when variance was not equal in the Levene's test) post hoc test was used. B., Bifidobacterium; IFN, interferon; IL, interleukin; n, number of mice per group; s.e., standard error.
In males that were sensitized with OVA ConA-induced production of IL-4, IL-5, IL-10 and IL-13 was increased, whereas IFN-γ was not. B. animalis significantly lowered the increased IL-13 levels (Table 2).
The effects of B. animalis on Th2 cytokines is predominantly observed in males. We cannot explain this difference, but it might be a consequence of the difference in the amount of cytokines that is produced in response to ConA. The production of cytokines in females is higher than in males and it is possible that the immunomodulatory effects of B. animalis are only moderate and are detected more easily in males.
Cytokine production by spleen cells stimulated with OVA
Spleen cells were stimulated with OVA to detect allergen-induced cytokine production. In sensitized females IL-4, IL-5, IL-10 and IL-13 were all detectable. OVA-specific production of IFN-γ was very low. B. animalis did not influence these cytokine levels (data not shown).
Figure 1 shows cytokine levels in sensitized males. In sensitized males levels of OVA-specific IL-4, IL-5, IL-10 and IL-13 were detected, whereas IFN-γ levels were very low and probably reflect background levels. B. animalis induced a trend towards lower Th2 cytokine levels.
Fig. 1.
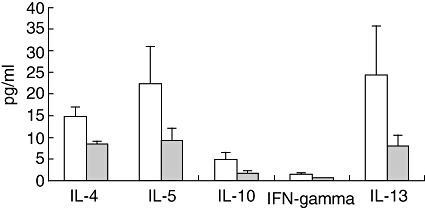
Male mice were sensitized and challenged with ovalbumin (OVA) and received either saline (white bars) or Bifidobacterium animalis (grey bars). Cytokine production was assessed after ex vivo stimulation of spleen cells with 100 µg/ml OVA for 96 h. Cytokines were assessed with a bioplex T helper type 1 (Th1)/Th2 kit on a Luminex® and expressed as mean ± standard error in pg/ml supernatant.
Effects of B. animalis on EAE
Body weight
In Fig. 2 the relative gain in body weight after immunization of female (Fig. 2a) and male (Fig. 2b) Lewis rats is shown. Growth is normal in both male and female control rats (not shown).
Fig. 2.
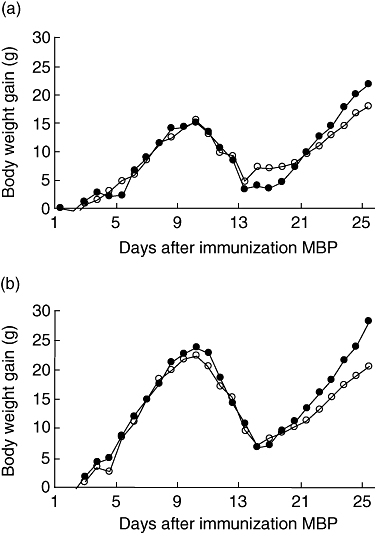
Female (a) and male (b) rats were immunized with myelin basic protein and received a daily oral gavage with saline (open circles) or Bifidobacterium animalis (closed circles). Body weight gain is expressed as the increase in body weight in grams from the day of immunization. Mean values were significantly different from the saline group: *P < 0·05.
Weight loss was observed in all rats that were immunized with MBP. In females that did not receive probiotics this occurred from days 13 to 16 and in females that received B. animalis from days 12 to 16. At day 17, rats began to gain weight again. The body weight gain during the total experiment was 31 (±3·6) g in females that received B. animalis and 24·9 (±2·2) g in controls. This difference approached significance (P = 0·084). In immunized males, weight loss was observed from days 12 to 17 in the probiotic and the control groups. The body weight gain during the experiment was significantly (P = 0·029) higher in males that received B. animals (54·8 ± 5·0 g) than in males that received the vehicle (41·6 ± 3·9 g).
Clinical symptoms
All rats that were immunized with MBP developed EAE. The first clinical symptom that appeared was loss of tonicity of the tail. In Table 3 clinical parameters of EAE are summarized. In females the day of onset and CDI were not influenced by B. animalis. The duration of symptoms was somewhat shorter in females that received B. animalis, but this was not significant. In males onset and cumulative disease index were not affected by B. animalis. The duration of symptoms, however, was almost 2 days shorter in males that receive B. animalis (P = 0·006). This is also illustrated in Fig. 3, in which a time–course of the mean clinical score is shown. The curve of males that received B. animalis is shifted to the left (Fig. 3b). In females no shift in the curve was observed. We have analysed whether the significant difference in duration was also observed when males and females were grouped and analysed together. The duration of clinical symptoms for all rats that received the vehicle was 7·1 days [standard deviation (s.d.) 2·1] and for rats that received B. animalis 5·8 days (s.d. 1·5). This difference was statistically significant (P = 0·017).
Table 3.
Effects of Bifidobacterium animalis on clinical parameters of experimental autoimmune encephalomyelitis (EAE).
| Group | n | Disease onseta (days) | Duration of symptomsb (days) | CDIc | ||
|---|---|---|---|---|---|---|
| Mean | s.e. | Mean | s.e. | |||
| Females | ||||||
| EAE + saline | 4 | 14·4 | 0·6 | 6·8 | 1·1 | 4·5 |
| EAE + B. animalis | 4 | 13·6 | 0·3 | 6·0 | 0·7 | 4·9 |
| Males | ||||||
| EAE + saline | 4 | 13·4 | 0·4 | 7·4 | 0·6 | 6·1 |
| EAE + B. animalis | 4 | 13·9 | 0·6 | 5·5** | 0·3 | 5·9 |
Disease onset is the first day that clinical signs were observed in a treatment group;
the duration of symptoms is the mean of the total number of days clinical symptoms were a treatment group;
CDI (cumulative disease index) is the sum of the cumulative daily scores per group divided by the number of days that the clinical symptoms were observed in this group. All data are expressed as mean ± standard error (s.e.), except for the CDI. Significantly different from the EAE + saline group:
P < 0·01. n: number of rats per group.
Fig. 3.
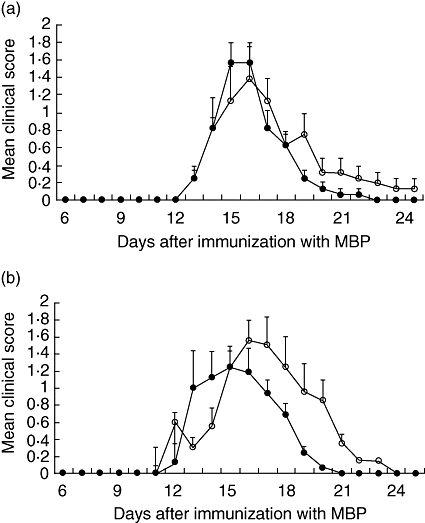
Female (a) and male (b) rats were immunized with myelin basic protein and received a daily oral gavage with saline (open circles) or Bifidobacterium animalis (closed circles). Time–course of the mean clinical score per day (±standard error) is shown.
Discussion
The results presented in this paper demonstrate that administration of B. animalis has immune effects in rodent models for autoimmunity as well as allergy. In the model for respiratory allergy the most pronounced effects were observed on the pulmonary inflammatory response and on IL-13 production by spleen cells stimulated ex vivo with ConA, but not IgE. In several animal models for allergy, in which other probiotic strains were used, it was demonstrated that a reduction in allergen-specific IgE was accompanied by a shift towards Th1 responses [5,17,28]. Our data suggest that B. animalis shifted the Th1/Th2 balance towards Th1 in non-sensitized mice, whereas in sensitized mice a reduction of ConA-induced IL-13 and a trend towards lower OVA-specific Th2 cytokines was observed, without an increase of IFN-γ. It has been demonstrated previously that effects of probiotics are known to depend upon the immune status of an individual, e.g. differential effects were observed in healthy individuals compared with patients which were allergic to milk [29].
In the autoimmunity model, which is an acute disease model in which all rats recuperate, B. animalis shortened the duration of clinical symptoms significantly and had a positive effect on total body weight gain. Hence, animals that receive B. animalis recover faster than controls. The mechanisms by which B. animalis induced these beneficial effects on EAE are unclear, but do suggest an involvement of regulatory T cells, which have been shown to be involved in immune effects of probiotics [30,31]. It has been demonstrated previously that effects of probiotics on EAE in mice were strain-dependent, either stimulating or suppressing the clinical symptoms. Cytokine profiles induced in the gut correlated with the effects on EAE. Strains that induced proinflammatory cytokines in the gut aggravated the cumulative disease burden, whereas strains that induced regulatory cytokines reduced this [22]. L. casei suppressed collagen-induced arthritis by down-regulating Th1 and proinflammatory cytokines and up-regulating the immunoregulatory cytokine IL-10 [32]. Hence, probiotics are able to induce immune effects via different mechanisms, including immunoregulation. In this study we could not find any effects of B. animalis on MBP-specific production of Th1 and Th2 cytokines by spleen cells (data not shown).
It might be possible that other mechanisms, rather than stimulating Th1 or regulatory T cells, are involved in the effects of probiotics on allergy and autoimmunity. The recently discovered subset of T cells, Th17 cells, are important in inflammatory responses, including autoimmunity. The key cytokine produced by Th17 cells is IL-17, which induces the production of proinflammatory cytokines such as IL-6 and TNF-α[33]. It has been shown that Th17, and not Th1 cells, are pathogenic cells in EAE [34]. The role of Th17 cells in allergy is less clear, but they are involved in the influx of neutrophilic granulocytes in an experimental model for asthma [35]. At present there is limited information on effects of probiotics on Th17 cells. L. casei suppressed experimental arthritis by reducing proinflammatory cytokines, including Th17 [32]. Possibly, suppression of this newly discovered subset of T cells by probiotics might explain effects observed in different experimental models that all involve inflammatory responses, i.e. autoimmunity, colitis and allergy. The precise mechanisms that are involved are not clear and will be the subject of further research.
In conclusion, we have shown that B. animalis has suppressive effects on both allergic as well as autoimmune responses. It is important to note that these immune effects cannot be translated directly into a health benefit for humans. However, animal models are well suited to give an indication of the immunomodulatory properties and mechanisms. To gain more insight into the efficacy and safety of a probiotic strain in a certain population, e.g. infants, well-designed human trials are necessary.
Acknowledgments
We would like to acknowledge the Food and Consumer Product Safety Authority (VWA, Den Haag, the Netherlands) for financial support. For supplying the probiotic B. animalis and critical reading of the manuscript, we would like to thank Dr Johan Garssen and Dr Jan Knol from Danone Nederland B.V. We acknowledge Gerty Schreibelt from VU Medical Centre, Amsterdam, for supplying the protocol for EAE induction. For technical assistance we would like to thank Liset de la Fonteyne, Yvonne Wallbrink and Bert Verlaan from the Laboratory for Health Protection Research of the National Institute for Public Health and the Environment. We thank Hans Strootman, Dirk Elberts and Piet van Schaaik from the PMP department of the Dutch Vaccine Institute or biotechnical support in the animal experiments.
References
- 1.FAO/WHO. Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario: Canada; 2002. [Google Scholar]
- 2.Madsen KL. The use of probiotics in gastrointestinal disease. Can J Gastroenterol. 2001;15:817–22. doi: 10.1155/2001/690741. [DOI] [PubMed] [Google Scholar]
- 3.Penner R, Fedorak RN, Madsen KL. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol. 2005;5:596–603. doi: 10.1016/j.coph.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Ezendam J, van Loveren H. Probiotics: immunomodulation and evaluation of safety and efficacy. Nutr Rev. 2006;64:1–14. doi: 10.1111/j.1753-4887.2006.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzaki T, Chin J. Modulating immune responses with probiotic bacteria. Immunol Cell Biol. 2000;78:67–73. doi: 10.1046/j.1440-1711.2000.00887.x. [DOI] [PubMed] [Google Scholar]
- 6.Madsen K. Probiotics and the immune response. J Clin Gastroenterol. 2006;40:232–4. doi: 10.1097/00004836-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Xiao JZ, Kondo S, Yanagisawa N, et al. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol Int. 2007;56:67–75. doi: 10.2332/allergolint.O-06-455. [DOI] [PubMed] [Google Scholar]
- 8.Xiao JZ, Kondo S, Yanagisawa N, et al. Effect of probiotic Bifidobacterium longum BB536 in relieving clinical symptoms and modulating plasma cytokine levels of Japanese cedar pollinosis during the pollen season. A randomized double-blind, placebo-controlled trial. J Invest Allergol Clin Immunol. 2006;16:86–93. [PubMed] [Google Scholar]
- 9.Xiao J-Z, Kondo S, Yanagisawa N, et al. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clin Exp Allergy. 2006;36:1425–35. doi: 10.1111/j.1365-2222.2006.02575.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116–21. e11. doi: 10.1016/j.jaci.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 12.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–71. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 13.Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–21. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Ezendam J, van Loveren H. Lactobacillus casei Shirota administered during lactation increases the duration of autoimmunity in rats and enhances lung inflammation in mice. Br J Nutr. 2008;99:83–90. doi: 10.1017/S0007114507803412. [DOI] [PubMed] [Google Scholar]
- 16.Hessle C, Hanson LA, Wold AE. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–82. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. The effect of oral feeding of Lactobacillus casei strain Shirota on immunoglobulin E production in mice. J Dairy Sci. 1998;81:48–53. doi: 10.3168/jds.S0022-0302(98)75549-3. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–62. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murosaki S, Yamamoto Y, Ito K, et al. Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J Allergy Clin Immunol. 1998;102:57–64. doi: 10.1016/s0091-6749(98)70055-7. [DOI] [PubMed] [Google Scholar]
- 20.Pohjavuori E, Viljanen M, Korpela R, et al. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy. J Allergy Clin Immunol. 2004;114:131–6. doi: 10.1016/j.jaci.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Baken KA, Ezendam J, Gremmer ER, et al. Evaluation of immunomodulation by Lactobacillus casei Shirota: immune function, autoimmunity and gene expression. Int J Food Microbiol. 2006;112:8–18. doi: 10.1016/j.ijfoodmicro.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Maassen CB, van Holten JC, Balk F, et al. Orally administered Lactobacillus strains differentially affect the direction and efficacy of the immune response. Vet Q. 1998;20(Suppl.)(3):S81–3. [PubMed] [Google Scholar]
- 23.Smit JJ, van Loveren H, Hoekstra MO, Nijkamp FP, Bloksma N. Influence of the macrophage bacterial resistance gene, Nramp1 (Slc11a1), on the induction of allergic asthma in the mouse. FASEB J. 2003;17:958–60. doi: 10.1096/fj.02-0985fje. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks JJ, Alblas J, van der Pol SM, van Tol EA, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med. 2004;200:1667–72. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melgert BN, Postma DS, Kuipers I, et al. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35:1496–503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 26.Bebo BF, Schuster JC, Vandenbark AA, Offner H. Gender differences in experimental autoimmune encephalomyelitis develop during the induction of the immune response to encephalitogenic peptides. J Neurosci. 1998;52:420–6. doi: 10.1002/(SICI)1097-4547(19980515)52:4<420::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 28.Sashihara T, Sueki N, Ikegami S. An analysis of the effectiveness of heat-killed lactic acid bacteria in alleviating allergic diseases. J Dairy Sci. 2006;89:2846–55. doi: 10.3168/jds.S0022-0302(06)72557-7. [DOI] [PubMed] [Google Scholar]
- 29.Pelto L, Isolauri E, Lilius EM, Nuutila J, Salminen S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin Exp Allergy. 1998;28:1474–9. doi: 10.1046/j.1365-2222.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- 30.Feleszko W, Jaworska J, Rha RD, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 31.Chapat L, Chemin K, Dubois B, Bourdet-Sicard R, Kaiserlian D. Lactobacillus casei reduces CD8+ T cell-mediated skin inflammation. Eur J Immunol. 2004;34:2520–8. doi: 10.1002/eji.200425139. [DOI] [PubMed] [Google Scholar]
- 32.So J-S, Kwon H-K, Lee C-G, et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol. 2008;45:2690–9. doi: 10.1016/j.molimm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 34.Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS) Allergol Int. 2008;57:115–20. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 35.Hellings PW, Kasran A, Liu Z, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]


