Abstract
In this study, we examined the role of nitric oxide (NO) in controlling vascular integrity mediated by vascular endothelial (VE)-cadherin in chronic inflammation. Periapical granulomas were analysed for the expression of inducible NO synthase (iNOS) and VE-cadherin, and more iNOS expression than VE-cadherin was shown. Human umbilical vein endothelial cells (HUVECs) were stimulated with proinflammatory cytokines and lipopolysaccharide extracted from Porphyromonas gingivalis and it induced iNOS expression, whereas it reduced VE-cadherin expression, compared with negative controls. On the other hand, pre-incubation with 1400W, an iNOS-specific inhibitor, markedly reduced iNOS expression in stimulated HUVECs and restored VE-cadherin expression to its control level, suggesting that vascular integrity was modulated in conjunction with the reduction of NO. Immunocytochemistry confirmed the functional role of NO in cultured HUVEC monolayers with or without 1400W. These data are consistent with a hypothesis suggesting that NO could attenuate VE-cadherin-mediated vascular integrity in human chronic inflammation.
Keywords: endothelial cells, nitric oxide, periapical granulomas, VE-cadherin, 1400W
Introduction
Vascular endothelial (VE) activation is an early step in leucocyte–endothelial adhesion and a hallmark of inflammatory processes [1]. The VE is important in cell recruitment because adhesion of leucocytes to vascular endothelial cells (ECs) in the bloodstream is a crucial step in inflammation and immunity [2]. Lymphocyte circulation and diapedesis are controlled by ECs, and in vivo and in vitro studies have shown that ligands on ECs affect circulating lymphocytes [3]. These proinflammatory effects provoke disruption of the EC tight junction and enhance endothelial permeability. VE-cadherin, a homophilic adhesion molecule localized in endothelial adherens junctions, is particularly important in cell to cell adhesion and inflammation [4] because it modulates vascular permeability of the endothelial monolayer in inflammatory tissues [5,6]. Although this process has been well described, the mechanisms of vascular integrity in chronic inflammation are only partially understood.
Periapical periodontitis is an infectious disease that causes substantial tissue damage and resorption of supporting bone around the root apex [7]. The periapical granulomas are generally polymicrobial, with many different anaerobic bacteria in the root canal systems, and histologically exhibit granulomatous tissues rich in blood vessels. Therefore, it is suitable for the analysis of vascular immune systems in chronic inflammation. Nitric oxide (NO) is a free radical that mediates cytotoxic effects against host tissues and cells [8–10], and plays a vital role in the regulation of inflammation and immunity [11,12]. The association between VE-cadherin and inducible NO synthase (iNOS) in human chronic inflammation has been shown [13]. In chronic inflammation, cytokine-mediated endothelial signalling results in leucocyte transmigration into locally inflamed areas through intercellular gaps within the endothelium [2,14,15]. Furthermore, Kubes et al.[16] have demonstrated that inhibition of NO synthesis decreases microvascular permeability in feline small intestine. On the basis of these findings, we hypothesized that VE-cadherin-mediated vascular integrity might be controlled by NO in chronic inflammation.
To elucidate our hypothesis, we examined human granulomatous tissues obtained surgically from inflamed periapical lesions, and analysed for iNOS and VE-cadherin expression. In addition, human umbilical vein endothelial cells (HUVECs) were examined to determine the role of NO in vascular integrity.
Materials and methods
Preparation of human samples
Twenty-eight chronic periapical periodontitis patients (11 male; ages 23–79 years) referred to the Department of Endodontics, Nihon University Dental Hospital, were examined. The experimental protocol was approved by the Ethics Committee of the Nihon University School of Dentistry, based on the Declaration of Helsinki. Periapical lesions were obtained at the time of surgical treatment of periapical periodontitis and divided into two portions. One portion was prepared for paraffin sections, followed by haematoxylin and eosin (H&E) stains. The other portion was prepared for frozen tissue sections and analysed for iNOS and VE-cadherin expression.
Quantitative analysis of inflammatory infiltrates
To determine the association between disease severity and VE-cadherin or iNOS expression, inflammatory infiltrates (macrophages, lymphocytes, neutrophils, plasma cells, fibroblasts and ECs) were counted using H&E-stained specimens in three consecutive microscopic fields at ×200 magnification under a light microscope.
Two-colour immunofluorescence image analysis
The localization of iNOS- and VE-cadherin-expressing ECs in periapical granulomas was analysed by two-colour immunofluorescence image analysis, as described previously [13]; however, additional quantitative analysis was performed in this study. Positive cells were examined using a fluorescence microscope (Eclipse E600; Nikon, Tokyo, Japan) and the number of ECs immunoreacting with each antibody were counted in five different fields of vision per section at a magnification of × 200.
Extraction of lipopolysaccharide from Porphyromonas gingivalis (Pg-LPS)
The P. gingivalis FDC 381 was grown anaerobically in brain heart infusion broth (Difco, Detroit, MI, USA) with bovine serum (5%), haemin (5 µg/ml) and vitamin K3 (1 µg/ml) at 37°C in an anaerobic chamber (Model 1024; Forma Scientific, Marietta, OH, USA) for 2 days. Bacterial cells were collected by centrifugation, washed three times with pyrogen-free water and lyophilized. Lipopolysaccharide (LPS) was extracted from lyophilized cells using the hot phenol/water method [17], and the crude extract was purified using repeated ultracentrifugation at 100 000 g for 3 h. Finally, samples were treated with nuclease P1 (Yamasa Shoyu, Chiba, Japan) and lyophilized [18].
Induction and inhibition of iNOS expression in HUVECs
The HUVECs purchased from Lonza Walkersville, Inc. (Basel, Switzerland) were maintained according to the manufacturer's instructions. Third-passage cells were seeded onto six-well tissue-culture plates (BD Falcon, Franklin Lakes, NJ, USA) at a density of 1 × 104 cells/cm2, and were stimulated with a combination of 0 or 20 µg/ml of Pg-LPS and 0 or 50 ng/ml of interferon (IFN)-γ, tumour necrosis factor (TNF)-α or interleukin (IL)-1β (R&D Systems, Inc., Minneapolis, MN, USA) for 20 h. After incubation, the supernatants were harvested and the cells were collected using trypsin-ethylenediamine tetraacetic acid. The number of viable cells was determined using a cell-counting kit (Wako Fine Chemicals, Osaka, Japan).
For the inhibition of iNOS expression, HUVECs were pre-incubated with or without 1400W (N-[3-(aminomethyl)benzyl] acetamidine·2HCl; Biomol International LP, Plymouth Meeting, PA, USA) for 1·5 h. The cells were then stimulated with a combination of Pg-LPS and IFN-γ, TNF-α or IL-1β as described above.
Measurement of iNOS and VE-cadherin protein in cultured HUVECs
Enzyme-linked immunosorbent assay (ELISA) was performed to measure the amount of iNOS and VE-cadherin protein in cultured HUVECs. ELISA kits for iNOS or VE-cadherin were purchased from R&D Systems or Kamiya Biomedical Co. (Seattle, WA, USA) respectively. The manufacturers' instructions were followed.
RNA extraction and real-time polymerase chain reaction analysis
Total RNA was extracted from frozen tissue sections of periapical granulomas or cultured HUVECs using 1 ml of Trizol Reagent (Invitrogen, CA, USA). The complementary DNA was amplified in 25-µl reaction mixtures containing SYBR Premix Ex Taq (Takara Bio, Inc., Shiga, Japan), polymerase chain reaction (PCR) primers for iNOS or VE-cadherin (20 µM each of sense and anti-sense). The primer sequences were as follows (shown 5′→3′): iNOS, GAACCGAGGGTACATGCTGGA (reverse) and ACCAGTACGTTTGGCAATGGAGA (forward); VE-cadherin, ACGTCTCCTGTCTCTGCATGC (reverse) and GGCAAGATCAAGTCAAGCGTG (forward); and glyceraldehydes-3-phosphate dehydrogenase (GAPDH), GACAAGCTTCCCGTTCTCAG (reverse) and GAGTCAACGGATTTGGTCGT (forward). The assays were performed using a Smart Cycler (Cepheid, Sunnyvale, CA, USA) and gene expression levels were normalized by dividing the calculated values for the mRNA samples by those of GAPDH mRNA.
Immunocytochemistry of EC monolayers treated with iNOS inhibitors
Third-passage HUVECs were seeded at a density of 1 × 104 cells/cm2 on chamber slides (Nalge Nunc International, Rochester, NY, USA) coated with fibronectin (10 µg/ml, Sigma, St Louis, MO, USA); 1400W was pre-incubated, followed by stimulation with Pg-LPS and IFN-γ, TNF-α or IL-1β for 20 h. The cultured HUVECs on the slides were analysed for iNOS and VE-cadherin expression using immunocytochemistry.
Statistical analysis
All data are expressed as means ± standard deviation. spss version 15·0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Differences between iNOS and VE-cadherin expression in periapical granulomas or cultured HUVECs were analysed using the Mann–Whitney U-test or by one-way analysis of variance followed by a Student–Newman–Keuls test respectively. Differences were considered significant at P < 0·05. The associations between iNOS or VE-cadherin protein expression and the number of inflammatory infiltrates in the granulomatous tissues were determined using the correlation coefficient (R).
Results
Histological evaluation of periapical lesions
Paraffin sections of H&E-stained periapical lesions were evaluated histologically using Nair's definition of radicular cysts [7]. Of the 28 periapical lesions, 20 exhibited granulomatous tissues rich in blood vessels and infiltrated with a large number of inflammatory cells. No epithelial cells were observed in any views of these samples, and the specimens were diagnosed as periapical granulomas (Fig. 1a). The other eight samples were diagnosed as radicular cysts, with complete epithelial lining (Fig. 1b); however, the epithelial cells express a large amount of iNOS mRNA expression and make more patient-to-patient variation in the expression levels. Therefore, the radicular cysts were excluded from this study.
Fig. 1.
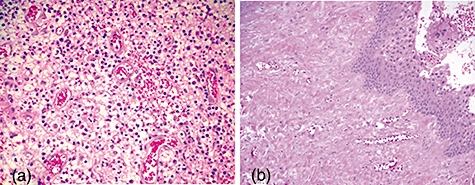
Histological examination of periapical lesions with haematoxylin and eosin stains. (a) Periapical granulomas, which were rich in blood vessels. (b) Radicular cysts with epithelium-lined tissues. Original magnification: ×100 (a) or ×50 (b).
Expression of iNOS and VE-cadherin in periapical granulomas
In two-colour immunofluorescence image analysis, ECs were identified based on fluorescent nuclear counterstaining using 4,6-diamine-2-phenylindole (DAPI) and morphological features (Fig. 2a). The resulting immunostaining demonstrated strong immunoreactivity for the iNOS antibody in ECs and in the other inflammatory cells (Fig. 2b), whereas the VE-cadherin polyclonal antibody reacted only with ECs (Fig. 2c). Merging the immunofluorescence images demonstrated that ECs expressed VE-cadherin and iNOS simultaneously (Fig. 2d). No positive staining was observed for negative controls lacking both antibodies (Fig. 2e, f).
Fig. 2.
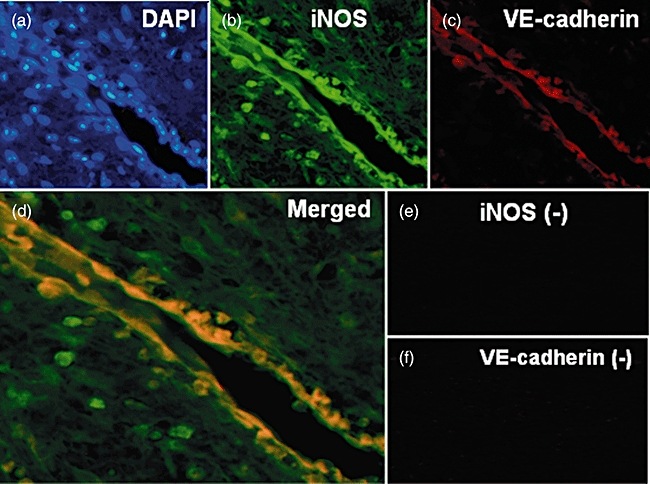
Two-colour immunofluorescence image analysis of periapical granulomas. (a) Nuclear counterstains using 4,6-diamine-2-phenylindole. (b) Inducible nitric oxide synthase (iNOS) monoclonal antibody. (c) Vascular endothelial (VE)-cadherin polyclonal antibody. (d) Merged images. (e) Negative controls for iNOS. (f) Negative controls for VE-cadherin. Original magnification: ×100 (a–c, e, f) and ×200 (d).
The mean percentage of iNOS- or VE-cadherin-expressing ECs in each patient was determined relative to the number of DAPI-positive ECs (Fig. 3a). For eight patients, these values were 15·3 ± 4·5 or 8·7 ± 3·7% respectively (Fig. 3b), and were significantly different.
Fig. 3.
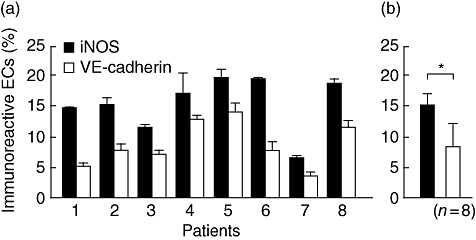
Quantitative analysis of two-colour immunofluorescence image analysis of periapical granulomas. The number of immunoreactive endothelial cells (ECs) for vascular endothelial (VE)-cadherin or inducible nitric oxide synthase (iNOS) antibodies was divided by that of 4,6-diamine-2-phenylindole-positive ECs. (a) iNOS- and VE-cadherin-positive ECs of eight patients. (b) Mean percentages of immunoreactive ECs in eight patients. *P < 0·05.
Real-time PCR analysis for periapical granulomas revealed that the levels of iNOS and VE-cadherin expression varied among patients (Fig. 4, upper panel); however, iNOS mRNA expression was higher than that of VE-cadherin in all samples. When the associations between the expression level of iNOS or VE-cadherin and the number of inflammatory infiltrates in the granulomatous tissues were examined (Fig. 4, lower panel), the correlation coefficients (R) for iNOS- or VE-cadherin-expressing ECs were 0·929 or 0·760 respectively, and both mRNA expressions demonstrated significant correlation (P < 0·01) with the number of inflammatory infiltrates.
Fig. 4.
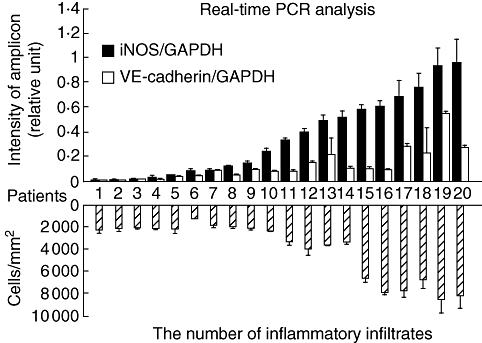
Real-time polymerase chain reaction analysis for inducible nitric oxide synthase (iNOS) and vascular endothelial (VE)-cadherin mRNA expression (an upper panel) and the number of inflammatory infiltrates (a lower panel) in periapical granulomas. Intensity of iNOS and VE-cadherin mRNA expression was normalized by that of glyceraldehydes-3-phosphate dehydrogenase. The number of inflammatory infiltrates was counted using paraffin sections stained with haematoxylin and eosin. The correlation coefficient (R) of 0·929 (iNOS-expressing ECs) or 0·760 [VE-cadherin-expressing endothelial cells (ECs)] was calculated, and both mRNA expressions demonstrated significant correlation (P < 0·01) with the number of inflammatory infiltrates.
Expression of iNOS and VE-cadherin in cultured HUVECs and the effects of iNOS inhibitors
Among various combinations of Pg-LPS and proinflammatory cytokines, Pg-LPS with either IFN-γ, TNF-α or IL-1β, or Pg-LPS with the combination of two cytokines, did not exhibit a marked difference between iNOS and VE-cadherin expression (data not shown). On the other hand, Pg-LPS with all the cytokines demonstrated a significant difference; therefore, we cultured HUVECs with this combination for the following studies. As shown in Fig. 5, stimulated HUVECs increased iNOS protein expression, compared with non-stimulated controls, whereas pre-incubation with 1400W markedly decreased iNOS expression. On the other hand, stimulation decreased VE-cadherin protein levels compared with non-stimulated controls. Pre-incubation with 1400W caused a statistically significant increase in the amount of VE-cadherin protein compared with stimulated cells not pretreated with 1400W.
Fig. 5.
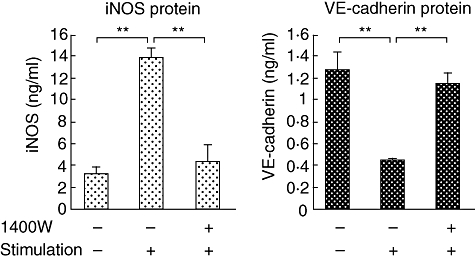
Measurement of inducible nitric oxide synthase and vascular endothelial (VE)-cadherin protein levels by cultured human umbilical vein endothelial cells (HUVECs) using enzyme-linked immunosorbent assay. HUVECs were pre-incubated with or without 1400W, followed by the stimulation with the combination of Porphyromonas gingivalis lipopolysaccharide, interferon-γ, tumour necrosis factor-α and interleukin-1β for 20 h. **P < 0·01.
Moreover, as shown in Fig. 6, incubation of the cells with the Pg-LPS/inflammatory cytokine combination markedly up-regulated iNOS mRNA expression compared with untreated controls, and pre-incubation with 1400W reduced iNOS mRNA expression significantly. Cell stimulation significantly down-regulated VE-cadherin mRNA expression compared with untreated controls; however, pre-incubation with 1400W restored VE-cadherin mRNA expression.
Fig. 6.
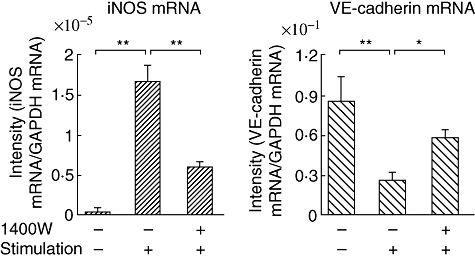
Real-time polymerase chain reaction analysis for inducible nitric oxide synthase and vascular endothelial-cadherin mRNA expression by human umbilical vein endothelial cells pre-incubated with or without 1400W. The stimulation was then performed using the combination of Porphyromonas gingivalis lipopolysaccharide, interferon-γ, tumour necrosis factor-α and interleukin-1β for 20 h. *P < 0·05; **P < 0·01.
Effects of 1400W on VE-cadherin organization in EC monolayers
To determine the functional role of 1400W in vascular permeability, the effects of Pg-LPS/inflammatory cytokine stimulation and 1400W pretreatment on HUVECs seeded in chamber slides were examined as shown in Fig. 7. In non-stimulated HUVECs, VE-cadherin expression was clearly visible on the cell surface, whereas no iNOS immunoreactivity was observed. Stimulation of the cells increased iNOS expression and decreased VE-cadherin expression. Pre-incubation of the cells with 1400W before stimulation substantially reduced iNOS expression, while it increased expression of VE-cadherin at EC junctions. Interestingly, pre-incubation with 1400W also increased the monolayer density, suggesting that resistance to permeability was restored. Thus, VE-cadherin and iNOS had opposite expression profiles in HUVECs.
Fig. 7.
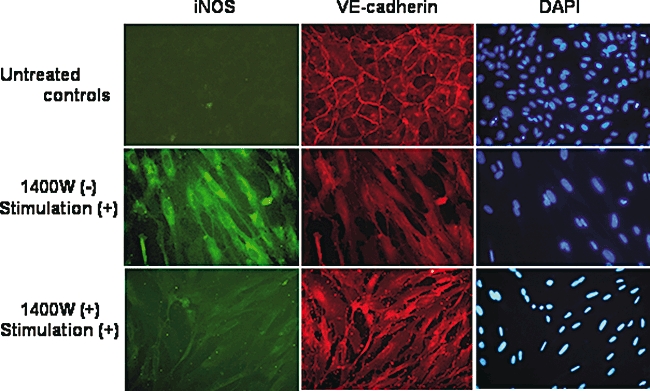
Effects of 1400W on vascular endothelial (VE)-cadherin organization in human umbilical vein endothelial cell (HUVEC) monolayers in chamber slides. HUVECs were pre-incubated with or without 1400W, followed by the stimulation with the combination of Porphyromonas gingivalis lipopolysaccharide, interferon-γ, tumour necrosis factor-α and interleukin-1β for 20 h. Immunocytochemistry was performed using anti-nitric oxide synthase monoclonal and VE-cadherin polyclonal antibodies. Nuclear counterstain was performed using 4,6-diamine-2-phenylindole.
Discussion
In this study, we performed a quantitative analysis of iNOS and VE-cadherin expression in ECs in periapical granulomas. Two-colour immunofluorescence image analysis demonstrated that some ECs have a capability to express both of these proteins, but the number of cells expressing iNOS was significantly higher than the number expressing VE-cadherin. In addition, real-time PCR analysis demonstrated higher expression of iNOS mRNA than that of VE-cadherin mRNA. Thus, at both the protein and mRNA levels, iNOS expression is more prevalent than VE-cadherin expression in periapical granulomas.
Levels of iNOS and VE-cadherin mRNA expression in the periapical granuloma samples varied from patient to patient, despite the fact that all patients were asymptomatic and of similar clinical status. To examine the reason for the patient-to-patient variation in expression levels, we determined the correlation coefficients for the association between mRNA expression and the number of inflammatory infiltrates in periapical granulomas. The results indicated that the number of inflammatory infiltrates is associated more closely with the level of iNOS expression than with the level of VE-cadherin expression, consistent with the accumulation of iNOS-expressing cells in inflamed lesions [19]. Another possible explanation is that the strength of stimuli, such as LPS, released by Gram-negative anaerobes and inflammatory cytokines might differ slightly among patients. Negative controls for periapical granulomas may help understanding of the meaning of the difference between iNOS and VE-cadherin expression; however, periapical granulomas were pathological lesions, and suitable negative controls could not be obtained.
The P. gingivalis is a recognized pathogen within the group of Gram-negative anaerobic rods [20]. Although it has been isolated from periodontal pockets, it has also been found in human dental root canal systems and periapical abscesses [21,22]. The primary virulence factors of P. gingivalis include LPS, fimbriae, proteases, phospholipase, alkaline and acid phosphatases, and cytotoxic metabolites [20,23,24]. P. gingivalis induces tissue destruction and abscesses in murine models, and augments cytokine release from human periodontal ligament cells [25,26]. Thus, this microorganism could be a major pathogen of periapical periodontitis [27].
Because the association between NO and VE-cadherin in managing vascular integrity in chronic inflammation was still unclear, we next examined the association between iNOS and VE-cadherin expression in cultured HUVECs. When unstimulated HUVECs were analysed, less iNOS expression than VE-cadherin was found. However, after the cells were stimulated with P. gingivalis LPS combined with inflammatory cytokines, iNOS expression increased significantly, whereas VE-cadherin expression decreased significantly, indicating opposite expression profiles for iNOS and VE-cadherin. Interestingly, gingipains (toxic proteases) from P. gingivalis cleave VE-cadherin in ECs, causing them to lose their cell adhesion properties [28], and also to induce EC detachment and apoptosis. Thus, our observations are consistent with a role for P. gingivalis in the pathogenicity of ECs in periapical granulomas.
To determine the role of NO in VE-cadherin expression in ECs, we applied 1400W to the cultured HUVECs. This compound is a highly specific inhibitor of iNOS, with at least a 5000-fold higher selectivity for iNOS than for endothelial NOS (eNOS) [29]. ELISA and real-time PCR analyses demonstrated that pre-incubation of the cells with 1400W inhibited iNOS expression significantly, whereas pre-incubation with 1400W increased VE-cadherin expression significantly, suggesting that the reduction of NO synthesis up-regulates VE-cadherin expression by HUVECs.
In a study by Murohara et al.[30], the inhibition of endogenous NO formation by Nω-nitro-l-arginine methyl ester (L-NAME), a non-selective NO synthase inhibitor, did not affect the expression of VE-cadherin in cultured HUVECs. Our study differed in that we focused on iNOS, whereas they analysed the role of eNOS, which is constitutively expressed rather than inducible [31]. Thus, NO synthesized by eNOS may not affect VE-cadherin expression in cultured ECs.
To determine the functional role of iNOS specific inhibitors in vascular integrity, we also analysed the effects of 1400W on VE-cadherin organization in HUVEC monolayers using chamber slides. Strong immunoreactivity against VE-cadherin antibody was seen clearly in the intercellular junctions of non-stimulated monolayers, whereas no iNOS immunoreactivity was observed. However, when the cells were stimulated, iNOS expression was enhanced and VE-cadherin expression was reduced markedly. Pre-incubation of the cells with 1400W increased the density of VE-cadherin-expressing ECs and restored permeability at the EC junctions while reducing iNOS expression. Thus, NO inhibition demonstrated clearly that NO can control VE-cadherin organization at junctions in the HUVEC monolayer.
Recently, inhibition of NO synthesis was shown to trigger anti-inflammatory responses [32–34]. Therefore, iNOS-specific inhibitors could be considered for clinical use in pharmacological anti-inflammation therapies. The findings in this study raise the possibility that 1400W could be used in the treatment of chronic inflammatory diseases such as periapical periodontitis. Further studies using animal models are required to assess this possibility conclusively.
Taken together, we conclude that the increase in NO in local inflammation promotes the loss of VE-cadherin and could augment the migration of leucocytes from the bloodstream, prolonging and exaggerating local inflammation.
Acknowledgments
We thank Dr M. Tamura for assistance with the preparation of LPS from P. gingivalis. This study was supported by grants from Grant-in-Aid for Scientific Research (C) for 2006 (no. 16591923) and 2008 (no. 20592240), and from Nihon University Individual Research Grant for 2007 and 2008.
References
- 1.Haubner F, Lehle K, Münzel D, et al. Hyperglycemia increases the levels of vascular cellular adhesion molecule-1 and monocyte-chemoattractant-protein-1 in the diabetic endothelial cell. Biochem Biophys Res Commun. 2007;360:560–5. doi: 10.1016/j.bbrc.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–96. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Orlova VV, Economopoulou M, Lupu F, et al. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell–cell contacts. J Exp Med. 2006;203:2703–14. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corada M, Liao F, Lindgren M, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–84. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 5.Hermant B, Bibert S, Concord E, et al. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem. 2003;278:14002–12. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]
- 6.Navaratna D, McGuire PG, Menicucci G, et al. Proteolytic degradation of VE-cadherin alters the blood–retinal barrier in diabetes. Diabetes. 2007;56:2380–7. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- 7.Nair PN. Review: new perspectives on radicular cysts: do they heal? Int Endod J. 1998;31:155–60. doi: 10.1046/j.1365-2591.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- 8.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biologic activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 9.Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–8. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laroux FS, Pavlick KP, Hines IN, et al. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113–18. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 11.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;8:1397–406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 12.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–18. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 13.Hama S, Takeichi O, Hayashi M, et al. Co-production of vascular endothelial cadherin and inducible nitric oxide synthase by endothelial cells in periapical granuloma. Int Endod J. 2006;39:179–84. doi: 10.1111/j.1365-2591.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 14.Gál I, Bajnok E, Szántó S, et al. Visualization and in situ analysis of leukocyte trafficking into the ankle joint in a systemic murine model of rheumatoid arthritis. Arthritis Rheum. 2005;52:3269–78. doi: 10.1002/art.21532. [DOI] [PubMed] [Google Scholar]
- 15.McGettrick HM, Lord JM, Wang KQ, et al. Chemokine- and adhesion-dependent survival of neutrophils after transmigration through cytokine-stimulated endothelium. J Leukoc Biol. 2006;79:779–88. doi: 10.1189/jlb.0605350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubes P. Nitric oxide-induced microvascular permeability alterations: a regulatory role for cGMP. Am J Physiol. 1993;265:H1909–15. doi: 10.1152/ajpheart.1993.265.6.H1909. [DOI] [PubMed] [Google Scholar]
- 17.Westphal O, Jann K. Bacterial lipopolysaccharide. Extractions with phenol-water and further application of the procedure. In: Whister RL, editor. Methods in carbohydrate chemistry. 5th. New York: Academic Press; 1965. pp. 89–91. [Google Scholar]
- 18.Ogawa T. Chemical structure of lipid A from Porphyromonas (Bacteroides) gingivalis lipopolysaccharide. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 19.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 20.Sundqvist G. Pathogenicity and virulence of black-pigmented Gram-negative anaerobes. FEMS Immunol Med Microbiol. 1993;6:125–38. doi: 10.1111/j.1574-695X.1993.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 21.Fouad AF, Barry J, Caimano M, et al. PCR-based identification of bacteria associated with endodontic infections. J Clin Microbiol. 2002;40:3223–31. doi: 10.1128/JCM.40.9.3223-3231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JFJr S, Rocas IN, Oliveira JC, et al. Detection of putative oral pathogens in acute periradicular abscesses by 16S rDNA-directed polymerase chain reaction. J Endod. 2001;27:164–7. doi: 10.1097/00004770-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, Metzger D, Leeman S, et al. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci USA. 2006;103:13777–82. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davey M, Liu X, Ukai T, et al. Bacterial fimbriae stimulate proinflammatory activation in the endothelium through distinct TLRs. J Immunol. 2008;180:2187–95. doi: 10.4049/jimmunol.180.4.2187. [DOI] [PubMed] [Google Scholar]
- 25.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behaviour. Science. 2007;317:666–70. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 26.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacinto RC, Gomes BP, Shah HN, et al. Incidence and antimicrobial susceptibility of Porphyromonas gingivalis isolated from mixed endodontic infections. Int Endod J. 2006;39:62–70. doi: 10.1111/j.1365-2591.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- 28.Sheets SM, Potempa J, Travis J, et al. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect Immun. 2005;73:1543–52. doi: 10.1128/IAI.73.3.1543-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fink R, Dikalov S, Fink N. ESR techniques for the detection of nitric oxide in vivo as an index of endothelial function. Pharmacol Rep. 2006;58(Suppl.):8–15. [PubMed] [Google Scholar]
- 30.Murohara T, Witzenbichler B, Spyridopoulos I, et al. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999;19:1156–61. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- 31.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;1:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 32.Badn W, Hegardt P, Fellert MA, et al. Inhibition of inducible nitric oxide synthase enhances anti-tumour immune responses in rats immunized with IFN-γ-secreting glioma cells. Scand J Immunol. 2007;65:289–97. doi: 10.1111/j.1365-3083.2007.01901.x. [DOI] [PubMed] [Google Scholar]
- 33.Ohsugi S, Iwasaki Y, Takemura Y, et al. An inhaled inducible nitric oxide synthase inhibitor reduces damage of Candida-induced acute lung injury. Biomed Res. 2007;28:91–9. doi: 10.2220/biomedres.28.91. [DOI] [PubMed] [Google Scholar]
- 34.Tang Q, Svensson CI, Fitzsimmons B, et al. Inhibition of spinal constitutive NOS-2 by 1400W attenuates tissue injury and inflammation-induced hyperalgesia and spinal p38 activation. Eur J Neurosci. 2007;25:2964–72. doi: 10.1111/j.1460-9568.2007.05576.x. [DOI] [PubMed] [Google Scholar]


