Abstract
The research described here is focused upon studying the activation of mice peritoneal macrophages when submitted to in vitro effects of Tityus serrulatus scorpion venom and its major toxic peptides. Several functional events were analysed, such as: cytotoxicity, spreading, extent of phagocytosis, vacuole formation and changes of internal calcium concentration. Among the main results observed, when macrophages are subjected to the effects of soluble venom of Tityus serrulatus scorpion venom, a partially purified fraction (FII) or a pure toxin (Ts1), are an increment in the percentage of phagocytosis and vacuole formation, a decrement of the spreading ability, accompanied by oscillations of internal calcium concentration. The net results demonstrate that scorpion venom or its major toxins are effective stimulators of macrophage activity; the effect of whole soluble venom or partially purified fractions is due to the toxic peptides, seen here clearly with Ts1. The possible involvement of Na+-channels in these events is discussed. A basic understanding of the underlying molecular mechanisms responsible for macrophage activation should serve as a foundation for novel drug development aimed at modulating macrophage activity.
Keywords: calcium, macrophage, Na+-channel, scorpion toxin, spreading, vacuole formation
Introduction
It is known that animal venoms contain different components capable of affecting the immune system. For example, they can cause an inflammatory response syndrome [1–4]. Macrophages are highly specialized cells that ingest and sequester a wide variety of foreign bodies and cell debris; however, the exact cellular mechanism by which they engulf such particles is incompletely known. These cells play an important role in the immune response and in the host defence system.
In Brazil the scorpion Tityus serrulatus (TSV) is considered one of the most dangerous species to humans and is responsible for many clinical cases of envenomation in the southeast region of this country (reviewed in [5]). Its venom exhibits a wide range of biological properties due to the presence of toxic peptides that affect excitable and non-excitable tissues (reviewed in [6]). Earlier publications have reported the effects on the peripheral nervous system, where they enhance the release of neurotransmitters [7], but specific effects have also been described on the gating mechanism of ion-channels, such as Na+-channels [8,9].
Although the effects of TSV venom on the immune response have been studied in vivo[10,11] and in vitro[12–14], there is little information on the effects of TSV on the macrophage-related host defence system. To elucidate the effect of TSV and its major toxic fraction (FII), as well as that of a pure toxin (Ts1) in this system, macrophage activation was measured using four different parameters: phagocytic activity, cellular spreading, vacuole formation and intracellular calcium concentration.
When macrophages contact a foreign agent or sense a particular stimulus, they might become activated and this process occurs by different pathways [15–17]. Mediators are synthesized or their relative concentration modified, causing abnormal cellular functions [18,19]. With respect to their heterogeneity, macrophages respond to a series of down- and up-regulation of several cellular processes [20]. As described recently, several agents have been used to promote macrophage activation. This includes the use of lipopolysaccharide (LPS) [21], snake venom [2,3,22] and scorpion venom [12–14]. Treatment of macrophages with a variety of chemical compounds can lead to the formation of many vacuoles into the cytoplasm [23,24]. The fusion of the lysosomal compartment with vacuoles containing these compounds is a fundamental mechanism by which macrophage can kill microbes [23,24]. Phagocytosis [25], vacuolization of the cytoplasm and fusion with endosomes and lysosomes are among the morphological changes that can be observed [26–28].
Several of the events mentioned in the above section are regulated (activated or inhibited) by the presence of calcium, an important second messenger of cells. Oscillations on the level of calcium are important factors in the regulation of cellular viability and proper function of all internal processes taking place inside cells.
This study was designed to compare the effect of the soluble venom from T. serrulatus, an enriched toxic fraction (FII, containing several peptides) and the major pure toxin (Ts1) on macrophage activation in vitro. The effects of these toxins on internal calcium concentration were studied using confocal microscopy. The fluorophore (FLUO3) was used for the determination of the internal calcium variations. Because Ts1 was shown to be the most active agent that causes the events observed, and as it is known that Ts1 modifies the gating mechanism of Na+-channels, the involvement of Na+-channels in the activation mechanisms of macrophages is thought to be possibly involved.
Materials and methods
Chemical reagents and buffers
Fetal calf serum (FCS), RPMI-1640 medium, horseradish peroxidase and LPS serotype 0111:B4 were purchased from Sigma (St Louis, MO, USA). FLUO3 was obtained from Molecular Probes, now Invitrogen (Carlsbad, CA, USA).
Scorpion venom and toxin purification
The venom from the scorpion T. serrulatus (TSV) was a gift from the Instituto Butantan, São Paulo, Brazil. The venom was fractionated essentially as described by Possani et al.[29]. Three different venom preparations were used for the present work: the whole soluble venom, the Sephadex G-50 fraction II (FII) and the pure toxin Ts1. A short description of the procedures used can also be found in our previous publication [14].
Animals
BALB/c female mice (15–20 g) maintained in the Animal House Facilities of our Institute of Biotechnology were used throughout the study to obtain the peritoneal macrophages. Mice were handled and used according to the animal welfare committee of the Autonomous University of the State of Morelos, and conform to the rules of the International Society on Toxicology guidelines (see Toxicon 30: 1–12, 1992).
Stimulation of mice peritoneal macrophages
Groups of mice from BALB/c were killed and their cells were harvested by peritoneal lavage [30]. The isolation and preparation of the in vitro culture of macrophages have been described in our previous publication [14]. These cells were exposed to 50 µg/ml of soluble TSV, or FII and/or Ts1 in RPMI-1640 containing 10% FCS and incubated at 37°C in a humidified atmosphere of 5% carbon dioxide (CO2) for various time-intervals. The various parameters studied and reported here with these macrophages are described below.
Cytotoxicity assay
Macrophage cells maintained in RPMI-1640 medium supplemented with 10% FCS seeded at 1 × 106 cells/ml on to 96-well plates, control and/or treated cells with 50 µg/ml of soluble TSV, or FII and/or Ts1 were incubated at 37°C in a 5% CO2 atmosphere. After different incubation times, the supernatants were removed and the remaining living cells assessed by fixing and staining with crystal violet (0·2% in 20% methanol). Absorbance was measured in each well by reading at 620 nm in a microplate reader. Cytotoxicity was calculated according to Ruff and Gifford [31] and the cell viability is reported.
Functional status assays
Phagocytosis assays
Binding and phagocytosis of yeast were analysed in vitro after the addition of fresh FCS, as described by Zebedee et al.[32]. Briefly, 1 × 106 cells/ml were seeded in triplicate on 24-well tissue culture plates containing slides and cultured in RPMI-1640 plus 10% FCS. After incubating at 37°C for 2 h in humidified 5% CO2, cells slides were rinsed in normal saline to remove non-adherent cells. One group of the adherent cells was exposed to 50 µg/ml of soluble TSV, or FII and/or Ts1. Another group of adherent cells were exposed to 5 µg/ml of LPS. After various intervals, opsonized yeasts, at an effector-to-target ratio of 1:5, were added to macrophage cultures for varying periods of time at 37°C. Unattached yeasts were removed by washing wells three times with phosphate-buffered saline (PBS). The media were aspirated and the cells were fixed with methanol for 20 min at room temperature and washed three times with PBS and stained with safranine for 40 s. The fixed slides were mounted with coverslips and were then examined using a microscope at 40 × magnification. Three coverslips per experimental condition were used to determine the percentage of phagocytic cells. The percentage was determined by counting the number of macrophages with internalized yeast.
Spreading
The macrophage-spreading assays were performed as described previously by Arruda et al.[33]. Briefly, 1 × 106 cells/ml were seeded in triplicate on 24-well plates containing slides to assess cell adhesion and cultured in RPMI-1640 plus 10% FCS. After incubation at 37°C for 2 h in humidified 5% CO2, cells slides were rinsed in normal saline to remove non-adherent cells. The adherent cells were exposed to 50 µg/ml of soluble TSV, or FII and/or Ts1. After different time-periods, the cells were fixed with methanol and then stained with crystal violet for 1 min. Slides were mounted with coverslips and examined by light microscopy at 40 × magnification. Spread cells from three different areas of each of the triplicate wells were counted after being incubated with TSV, FII or Ts1 and reported as the percentage over the total number of cells examined.
Vacuolization assay
Peritoneal macrophages were obtained and maintained as described above. For the vacuolization assay the macrophages were incubated with the RPMI-1640 medium supplemented with 5% FCS and 1 mM/ml NH4Cl [34] and exposed to 50 µg/ml TSV, FII or Ts1. The plates were incubated for different time-periods at 37°C with 5% CO2. For detection of vacuole formation more than 100 cells were inspected with an inverted microscope at 40 × magnification and the cell cultures were stained with 0·05% neutral red solution for 5 min. The cells were washed with PBS containing 0·2% bovine serum albumin (BSA), 70% ethanol and 0·37% hydrochloric acid (HCl). The percentage of vacuolated cells was determined by counting the number of macrophages vacuolated.
Cell labelling and intracellular calcium measurements
Macrophages were extracted and cultivated as described above, using 12 mm round cover glasses. Once the adherent macrophages were obtained, normal culture media were substituted with RMI-1640 media supplemented with 10% FCS, 10 µM FLUO3-AM (cell permeant 1 mM dimethylsulphoxide (DMSO) solution molecular probes cat no. F-14218), 0·01% Pluronic F-127 (molecular probes cat. no. P-6866) and incubated for 30 min at 37°C in a humidified 5% CO2. After incubation with FLUO3 cells were washed four times with RMI-1640 media supplemented with 10% FCS medium and were ready for intracellular Ca2+ concentration measurements.
Data acquisition was performed using a Zeiss LSM 510 META confocal microscope. Cells were transferred to a recording/perfusion chamber (Warner instruments cat. no. RC-25, Hamden, CT, USA); the cell culture media were substituted with PBS. Macrophages were then exposed to the venom or toxins in PBS, and all subsequent measurements were conducted in the presence of the venom or toxins in PBS at the final concentrations described above. Intracellular Ca2+ concentration was measured using the 488 nm line of an argon laser and emission was recorded using a long-pass 505 nm filter; normal transmitted light images (Nomarsky interference) were recorded simultaneously using a second photomultiplier. Images were captured every minute with a resolution of 1024 × 1024 pixels in an 8-bit greyscale (256 shades of grey).
Fluorescence quantitation was performed using the public domain image analysis software suite ImageJ version 1·33u (Wayne Rasband, National Institutes of Health; http//rsb.info.nih.gov/ij/java1.5.q_13). Briefly, average pixel intensities were measured for 10 cells per experiment per frame using circular marquees that matched closely each cell diameter. Thus, the values reported are the results of simultaneous measurements using 10 cells per frame. An equivalent area of the background was also measured per frame. In order to reduce background noise due to vibration and other possible factors, the background average pixel intensity was subtracted from the cellular average pixel intensity for each frame (corrected average cellular pixel intensity). All data were normalized, using as reference the first frame of the corrected average cellular pixel intensity of the control (no toxin added) and the corrected average cellular pixel intensity of the experimental conditions. Data are expressed as arbitrary fluorescence units.
Statistical analyses
Data are expressed as the mean ± standard deviation. Statistical analyses were performed by Student's t-test and the level of significance was set at P < 0·05.
Results
Effect of venom and its major toxins on macrophage viability
The effects of TSV, fraction FII, and pure toxin Ts1 were analysed and their cytotoxicity was measured. Groups of mice were used for macrophage collection, which were then exposed in vitro to 50 µg/ml of soluble venom and its major toxins and incubated under the same conditions for different time-periods. All macrophages culture exposed to the activating agents from 12 up to 120 h presented around 5% of lyses. That is, cell survival is high, as can be observed in Fig. 1.
Fig. 1.
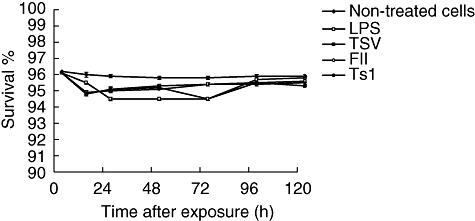
Effect of crude venom and its major toxins on macrophage viability. Groups of BALB/c female mice were killed and their peritoneal macrophages collected and divided into three groups: (a) non-treated cells (black diamonds), (b) macrophages exposed to 5 µg/ml of lipopolysaccharide (LPS) (white squares) and (c) macrophage cultures exposed to 50 µg/ml of Tityus serrulatus (TSV) (black squares), partially purified fraction (FII) (white circles) or a pure toxin (Ts1) (black circles). After different times of incubation of the cultured peritoneal macrophages, the survival percentage was measured. Each point represents the mean value of samples from four experiments in different groups of five mice. Statistical differences between the treatments were P < 0·05.
Effect of TSV and its major toxins on functional status of macrophages
To evaluate the functional status of mouse macrophages, independent cultures were exposed to 50 µg/ml TSV, FII and Ts1 and compared with those exposed to LPS. A group of non-treated cells was conducted in parallel as control. The percentage of two parameters were measured: phagocytosis and spreading.
Phagocytosis
The percentage of macrophages with internalized yeast is shown in Fig. 2. The internalized yeast also began to appear in control macrophages on macrophages exposed to soluble venom and its major toxins 15 min after treatment. The groups of macrophages exposed to TSV, FII, Ts1 and LPS showed a significant increase in the phagocytosis, as a function of exposure time up to 24 h (Fig. 2). For the entire time-period the percentages of macrophages with internalized yeast were similar among the groups of cells exposed to venom and its toxins and significantly higher (P < 0·001) when compared with non-treated cells or subjected to LPS (Fig. 2).
Fig. 2.
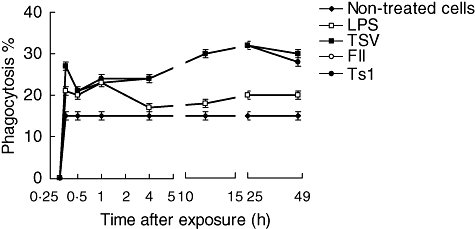
Percentage of phagocytosis. Peritoneal macrophages were obtained and seeded in tissue culture plates containing coverslips, and stimulated in vitro with 50 µg/ml of soluble Tityus serrulatus (TSV), partially purified fraction (FII) or pure toxin (Ts1) or 5 µg/ml of lipopolysaccharide (LPS), as described in Material and methods. Three coverslips per experimental condition were used to determine the percentage of phagocytic cells. Each point represents the mean ± standard deviation value of samples from four experiments carried out on different groups each consisting of five mice. Statistical differences between the expositions were P < 0·001.
Spreading
The spreading test is based on the ability of activated macrophages to adhere to plastic and to spread. Up to 75% of normal macrophages, without any treatment, adhere to the plate and appear to spread (Fig. 3). However, they do not become activated. During the first 60 min of macrophages exposure to 50 µg/ml TSV, FII or Ts1 a smaller percentage of spreading was observed (approximately 50%), compared with the untreated cells (Fig. 3); however, the macrophages become clearly activated because there is an increment in the percentage of phagocytosis (see Fig. 2). These data are supported further by the results shown in Figs 4 and 5, where only the treated cells are higher vacuolated. Among groups of macrophages exposed to venom and its major toxins, the percentage of spreading was practically identical to the LPS-activated macrophages.
Fig. 3.
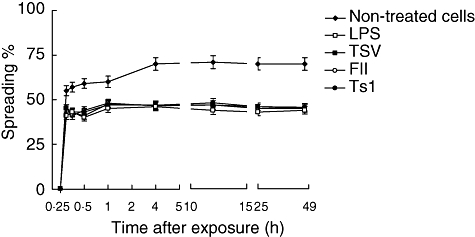
Percentage of spreading. Peritoneal macrophages were obtained and seeded in tissue culture plates containing coverslips and stimulated in vitro. Each point represents the mean ± standard deviation value of samples from four experiments carried out on different groups each consisting of five mice. Statistical differences between the treatments were P < 0·001.
Fig. 4.
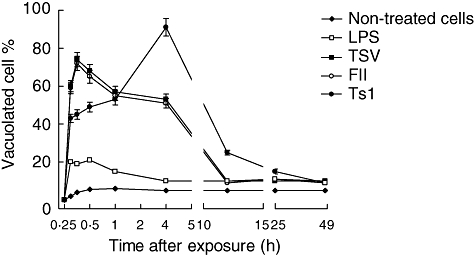
Vacuole formation. Macrophages were obtained and exposed to Tityus serrulatus (TSV), partially purified fraction (FII) or pure toxin (Ts1), as described previously. Vacuoles formation was observed 2 h after exposition. The cells were stained and examined by light microscopy at 40 × magnification. Symbols are as follows: non-treated cells (diamonds), lipopolysaccharide (LPS) (open squares), TSV (closed squares), FII (closed circles) and Ts1 (open circles).
Fig. 5.
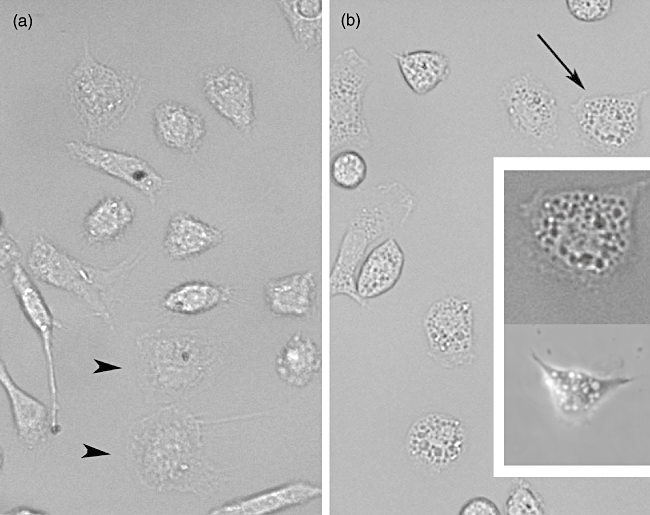
Macrophage morphology and vacuolization. (a) Untreated cells. Black arrowheads show macrophages adhered and spread but not activated; note the small amount of vacuolization. (b) Macrophages exposed to the effect of pure toxin (Ts1). Cells were observed 2 h after addition of the toxin. Arrow indicates a cell (upper insert) whose image was enhanced to show the great number of vacuoles produced; lower insert shows a macrophage 4 h after Ts1 treatment. For both panels (a and b) Normarsky images were obtained at 40× magnification.
Induction of vacuoles in TSV, FII or Ts1-stimulated peritoneal macrophages
Normal morphological shape of a given cell is usually taken as a good indication of the functional state of the cell. It is known that drugs and/or microorganisms, under certain circumstances, can modify its morphology by an internal vacuolization process [35,36].
To determine whether scorpion venom and its major toxins induced vacuoles in peritoneal macrophages, experiments were conducted in the presence of 50 µg/ml of TSV, FII and Ts1 observed up to 48 h exposure. The percentage of vacuolization is shown in Fig. 4. The non-treated cells and those treated with LPS show a slight degree of vacuolization (approximately 5%). Macrophages submitted to venom and toxin treatment, however, showed a significant increment (P < 0·001) in the total number of vacuolated cells (up to 80%). The vacuolization process begins after 15 min exposure to TSV or toxins. The kinetics is different depending on the different agents used. For TSV- and FII-treated macrophages the effect is higher up to the first 30 min. In contrast, the macrophages exposed to Ts1 showed a maximum effect after 4 h of the onset of the experiment (approximately 90%). Two main morphological features were observed: formation of large vacuoles (see Fig. 5) and modification of the round shape of macrophages and spreading. Figure 5a shows non-treated cells. The picture was taken 2 h after macrophage treatment, because it corresponds to the half-time (50%) needed for maximal vacualization. In Fig. 5b we can observe vacuolated macrophage; the inset (upper cell) shows the same cell indicated by the arrow, with higher magnification and contrast, whereas the bottom picture shows another cell 4 h after treatment. TSV and FII have a maximum at shorter time (from 15 to 30 min), whereas Ts1 have a maximum at 4 h (see inset bottom cell). At 2 h after the onset of the experiment all treated conditions have approximately the same value of vacuolization.
Intracellular calcium oscillations
Exposure of the macrophages to FII and Ts1 induces oscillations of intracellular Ca2+ that has a period of about 20 min. The effect of the toxins is very rapid and also induces morphological changes. The macrophages contract when the amount of intracellular Ca2+ is high and relax when it reaches a minimum level of the divalent cation, but the macrophages never detach from the substrate. After a contraction/relaxation cycle the macrophages tend to become rounder (Figs 6 and 7). As mentioned previously, the amount of vacuolization grows during the first hours of exposure (Fig. 4) and, interestingly, the vacuoles become more refringent when the Ca2+ levels are high. The intracellular Ca2+ appears clearly to be localized in defined domains of the cytoplasm, in or near the places where more vacuoles are localized. The effects of FII and Ts1 are practically indistinguishable. They induce Ca2+ cycles with the same period and magnitude. It remains to be shown whether other peptides present in FII also cause the same effect as Ts1.
Fig. 6.
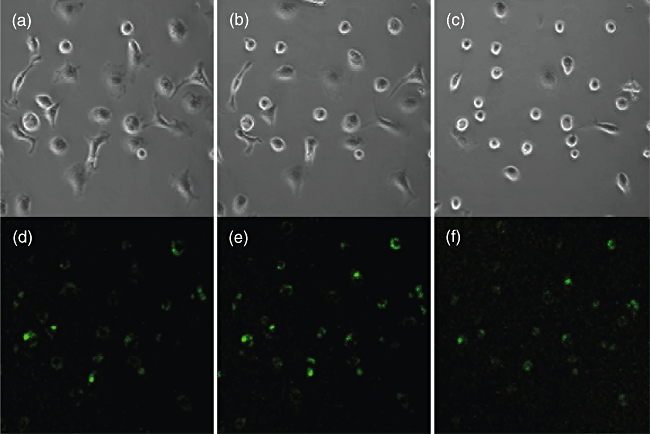
Typical effect of Tityus serrulatus (TSV) or toxins on macrophages. Time lapse transmitted light images (Normarsky interference) exposed to TSV scorpion venom. (a) Time 0; (b) 30 min; (c) 45 min after addition of purified pure toxin (Ts1). Intracellular Ca2+ concentration (FLUO3 fluorescence) of the same cells at the same time-points (d, e, f). Note the increment and decrement of the Ca2+ signal and the changes in macrophage morphology.
Fig. 7.
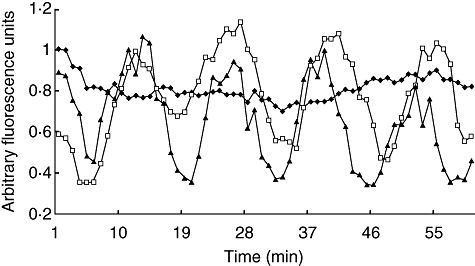
Calcium oscillation. Time-lapse quantitization of intracellular Ca2+ concentration in macrophages treated with a partially purified venom fraction (fraction II) and purified pure toxin (Ts1). Vehicle control (phosphate-buffered saline): filled diamonds; FII: empty squares; purified Ts1: filled triangles. Note that the addition of vehicle lowers the intensity of the control at time 0 but it then becomes considerably stable, compared with the intracellular Ca2+ fluctuations observed in the toxin-treated cells.
Discussion
As mentioned in the introductory section, T. serrulatus is a dangerous scorpion found in Southeast Brazil, where it is responsible for most clinically important human accidents. Its venom consists of a complex mixture of several toxins that exhibit various biological activities. One of the most important components of this venom is Ts1, initially called toxin gamma, which is a 61 amino acid long peptide stabilized by four disulphide bridges [37].Ts1 is the major component of FII that can be separated by gel filtration through Sephadex G-50 [29]. The structure and function of highly purified Ts1 was performed. X-ray crystallographic data show that it folds according to a αβ-cysteine stabilized structural motif [38]. Electrophysiological experiments conducted with Ts1 show that its primary targets are Na+-channels of excitable cells [8], where they cause an anomalous depolarization; Na+-channels under the effect of Ts1 open at more negative membrane potentials. This pharmacological effect is described in the literature as a beta-effect, found in many peptides from scorpion venom of the genus Centruroides, in contrast to the alpha-effect found initially in peptides isolated from scorpions of the genus Androctonus (see review [39]). In earlier experiments conducted by our group it was shown that Ts1 activates macrophages and stimulates the liberation of interleukins (IL)-1a, IL-1b, IL-6 and tumour necrosis factor (TNF) (see [14]).
In the present study the experiments were designed in order to discern the functional status of macrophages exposed to TSV, FII and pure Ts1. Application of this venom and toxins at the concentration of 50 µg/ml to macrophages in in vitro cell cultures showed that they have a very low cytotoxic effect (Fig. 1). The macrophages survived reasonably well up to 120 h of culture. However, the application of the venom and toxins modify the functional status of these cells. Figures 2–4 show clearly that several parameters are altered. Formation of vacuoles and phagocytic activity is increased, compared with control samples (see example in Fig. 5). It is known that macrophage activation results in the increment of metabolic rates, stimulates the secretion of growth factors, cytokines and inflammatory mediators and enhances phagocytotic activity [22]. Administration of venom and toxins increase macrophage activation, as shown in Fig. 2. The macrophages exhibit different phenotypes which are related mainly to their cell morphology, surface antigen expression and function. Until now, a limited number of data have been available concerning the morphological and biochemical aspects related to the cells activated by TSV, FII and Ts1. This study shows that the soluble venom and major toxins of T. serrulatus causes morphological changes in the shape of the macrophages. Some major morphological changes were observed: formation of large vacuoles (see Fig. 5), modification of the round shape and spreading. Spreading of macrophages has been considered as a marker of cellular activation and differentiation [40]. Here it is shown that by venom and toxin exposure macrophages decrease their ability to adhere and spread on coverslips, an effect that was reduced significantly (P < 0·001).
Because similar effects were observed when assaying TSV (soluble whole venom), FII (a subfraction with similar molecular weight components) and Ts1 (a highly purified peptide), we can assume that most effects described here are due to the action of this toxin (Ts1).
Because Ts1 causes an important morphological change of the macrophages, and these are due to cytoskeleton reorganization, it was assumed that the second messenger Ca2+ could be implicated in this process. For this reason, in the present work the measurement of variations on the level of internal calcium was verified by means of the calcium sensitive fluorophore FLUO3 using a real-time confocal imaging system. As shown clearly in Fig. 6, there are obvious morphological changes over time (0, 30 and 45 min after onset of the experiment) that seem to correlate well with intracellular calcium oscillations. This was demonstrated for macrophages under the effect of FII and pure toxin Ts1. The action of both agents is practically identical. Figure 7 shows the profile of variations on internal calcium concentration as a function of time. The plotted values are the mean fluorescence of 10 specific cells per frame analysed. A cyclic oscillation is observed for macrophages intracellular Ca2+ concentration. As it was demonstrated earlier that Ts1 shifts to more negative potential the opening of voltage-dependent Na+ channels [8], it is assumed that these channels might be involved in its molecular mechanism of action. The rationale behind the series of events observed are thought to start by the opening of Na+-channels at more negative potentials, which causes depolarization of the macrophages, facilitating Ca2+ entrance into the cells. In fact, there is a clear increment in the levels of internal calcium, as shown in Fig. 6e. The presence of voltage-gated sodium channels (subtype Nav1·5) in macrophages has been demonstrated recently by Carrithers et al.[41]. Further evidence of the specificity of Ts1 in recognizing this subtype of channel was demonstrated a long time ago by Renaud et al.[42], measuring the effect of Ts1 (at that time called toxin gamma of T. serrulautus) on the tetrodotoxin-resistant Na+ channel of mammalian cardiac cells. It is now well known that Nav1·5 is one of the main subtypes of Na+-channels in heart tissue (see also [8] for the effect of Ts1 in cardiac cells). Taken together, all this information leaves little doubt that the main effect of Ts1 in macrophages begins by modifying Nav1·5 channel permeability, thus impairing the ion-channel balance of these cells, among which are Ca2+ ions. The level of calcium concentration is oscillatory, as shown in Fig. 7. Our data indicate that the time–course of each oscillatory event is about 20 min. We have observed these repetitive events for several hours. However, the intracellular Ca2+ oscillations are likely to induce permanent changes in the macrophage physiology. The periodic uptake of Ca2+ by the mitochondria is likely to induce apoptosis in the long term (for reviews see [43,44]). This effect could be enhanced by the secretion of TNF, which is known to be a powerful apoptosis-inducing factor, and it was shown to be the case in our earlier in vitro study [14]. Furthermore, the overall energy balance of the macrophage can be affected as a great deal of energy is used in the removal of Ca2+ from the cytoplasm. The macrophage morphological changes also correlate with Ca2+ peaks and valleys in Fig. 7; the vacuoles became more refringent and the cell seemed to contract when Ca2+ was high, whereas when Ca2+ concentration was low the cells tended to become rounder. These changes suggest massive reorganization of the cytoskeleton.
In conclusion, most of the events described here are due very probably to toxins such as Ts1 present in this venom. There is an additional point worth discussing: experimental animals, including humans, stung by scorpions of the species T. serrulatus might present severe physiological complications [45]. Depending upon the prompt application of serotherapy, the prognostic outcome of the intoxication process can be overcome and the individual life can be saved. However, if the time elapsed between the accident and the therapeutic application of an efficient anti-venom is delayed for more than 2 h, the survival of the victim can be compromised. We are confident that studies such the ones reported here will help in understanding the molecular events that follow the long-term effect of this kind of envenomation. Here we have described some of the cellular and molecular physiological events occurring with this macrophage model. Additional experimentation with other tissues should provide insights for more effective treatment or for the development of new drugs useful in scorpion sting therapy.
Acknowledgments
The authors are indebted to Fredy I. V. Coronas for technical support during toxin purification and Andrés Saraleguí for support at the ‘Unidad Confocal’ of the Biotechnology Institute. This work was supported partially by grants from UAEMOR-02-01 of the Secretaria de Educación Pública (SEP-PROMEP) to V. L.P., grant SEP-CONACyT 48646-Q and P49498-Q from the Mexican Government given to L. D. P. and E. R. respectively.
References
- 1.Lomonte B, Gutierrez JM, Romero M, Nunez J, Tarkowski A, Hanson LA. An MTT-based method for the in vivo quantification of myotoxic activity of snake venoms and its neutralization by antibodies. J Immunol Methods. 1993;161:231–7. doi: 10.1016/0022-1759(93)90299-m. [DOI] [PubMed] [Google Scholar]
- 2.Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998;7:339–46. doi: 10.1080/09629359890866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petricevich VL, Teixeira CF, Tambourgi DV, Gutierrez JM. Increments in serum cytokine and nitric oxide levels in mice injected with Botrhops asper and Bothrops jararaca snake venom. Toxicon. 2000;38:1253–66. doi: 10.1016/s0041-0101(99)00227-5. [DOI] [PubMed] [Google Scholar]
- 4.Petricevich VL. Cytokine and nitric oxide production following severe envenomation. Curr Drug Targets Inflamm Allergy. 2004;3:325–32. doi: 10.2174/1568010043343642. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa AK, Caricati CP, Lima MLSR, et al. Antigenic cross-reactivity among the venoms from several species of Brazilian scorpions. Toxicon. 1994;32:989–98. doi: 10.1016/0041-0101(94)90377-8. [DOI] [PubMed] [Google Scholar]
- 6.Becerril B, Marangoni S, Possani LD. Toxins and genes isolated from scorpions of the genus Tityus: a review. Toxicon. 1997;35:821–35. doi: 10.1016/s0041-0101(96)00198-5. [DOI] [PubMed] [Google Scholar]
- 7.Freire-Maia I, Campos JA. Pathophysiology and treatment of scorpion poisoning. In: Ownby CL, Odell GV, editors. Natural toxins: characterization, pharmacology and therapeutics. Oxford: Pergamon Press; 1989. pp. 139–59. [Google Scholar]
- 8.Yatani A, Kirsh GE, Possani LD, Brown AM. Effects of two new world scorpion toxins on single channel and whole cell cardiac sodium channels. Am J Physiol. 1988;254:H443–51. doi: 10.1152/ajpheart.1988.254.3.H443. Heart Cir Physiol 23. [DOI] [PubMed] [Google Scholar]
- 9.Kirsh GE, Skattebol A, Possani LD, Brown A. Modification of Na+ channel gating by an alpha scorpion toxin from Tityus serrulatus. J Gen Physiol. 1989;93:67–83. doi: 10.1085/jgp.93.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magalhães MM, Pereira ME, Amaral CF, et al. Serum levels of cytokines in patients envenomated by Tityus serrulatus scorpion sting. Toxicon. 1999;37:1155–64. doi: 10.1016/s0041-0101(98)00251-7. [DOI] [PubMed] [Google Scholar]
- 11.Petricevich VL, Peña CF. The dynamics of cytokine of nitric oxide secretion in mice injected with Tityus serrulatus scorpion venom. Mediators Inflamm. 2002;11:173–80. doi: 10.1080/09622935020138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petricevich VL. Effect of Tityus serrulatus venom on cytokine production and the activity of murine macrophages. Mediators Inflamm. 2002;11:23–31. doi: 10.1080/09629350210308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petricevich VL, Lebrun I. Immunomodulation effects of the Tityus serrulatus venom on murine macrophage functions in vitro. Mediators Inflamm. 2005;24:39–49. doi: 10.1155/MI.2005.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petricevich VL, Hernández Cruz A, Coronas FIV, Possani LD. Toxin gamma from Tityus serrulatus scorpion venom plays an essential role in immunomodulation of macrophages. Toxicon. 2007;50:666–75. doi: 10.1016/j.toxicon.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 16.Martin FA, Rojas-Diaz D, Luis-Garcia MA, Gonzalez-Mora JL, Castellano MA. Simultaneous monitoring of nitric oxide, oxyhemoglobin and deoxyhemoglobin from small areas of the rat brain by in vivo visible spectroscopy and a least-square approach. J Neurosci Methods. 2004;140:75–80. doi: 10.1016/j.jneumeth.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–26. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 19.Jedynak M, Siemiatkowski A. The role of monocytes/macrophages and their cytokines in the development of immunosuppression after severe injury. Pol Merkuriuz Lek. 2002;13:238–41. [PubMed] [Google Scholar]
- 20.Arandjelovic S, Bogic M, Raskovic S. The role of mononuclear phagocytes and dendritic cells in allergic inflammation. Srp Arh Celok Lek. 1998;126:46–53. [PubMed] [Google Scholar]
- 21.Nacife VP, Soeiro M de N, Gomes RN, D'Avila H, Castro-Faria Neto HC, Meirelles M de N. Morphological and biochemical characterization of macrophages activated by carrageenan and lipopolysaccharide in vivo. Cell Struct Funct. 2004;29:27–34. doi: 10.1247/csf.29.27. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez Cruz A, Mendonca RZ, Petricevich VL. Crotalus durissus terrificus venoms interferes with morphological, functional, and biochemical changes in murine macrophage. Mediators Inflamm. 2005;14:349–59. doi: 10.1155/MI.2005.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkin M, Ardí WG, Orr HC, Lachman AB. Induction in vitro by autonomic drugs of cytoplasmic vacuoles in ascites tumor cells. J Natl Cancer Inst. 1962;28:187–201. [PubMed] [Google Scholar]
- 24.Seglem PO, Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976;100:276–80. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- 25.Reaven EP, Axline SG. Subplasmalemmal microfilaments and microtubules in resting and phagocytozing cultivated macrophages. J Cell Biol. 1973;59:12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luzio JP, Poupon V, Lindsay MR, Mullock BM, Piper RC, Pryor RR. Membrane dynamics and the biogenesis of lysosomes. Mol Membr Biol. 2003;20:141–54. doi: 10.1080/0968768031000089546. [DOI] [PubMed] [Google Scholar]
- 27.Scout CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–52. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- 28.Vieira M, Dutra JM, Carvalho TM, Cunha-e-Silva NL, Souto-Padron T, Souza W. Cellular signaling during the macrophage invasion by Trypanosoma cruzi. Histochem Cell Biol. 2002;118:491–500. doi: 10.1007/s00418-002-0477-0. [DOI] [PubMed] [Google Scholar]
- 29.Possani LD, Martin BM, Mochca-Morales J, Svendsen I. Purification and chemical characterization of the major toxins from the venom of the Brazilian scorpion Tityus serrulatus Lutz and Mello. Carlsberg Res Commun. 1981;46:195–205. [Google Scholar]
- 30.Cohn ZA, Benson B. The differentiation of mononuclear phagocytes: morphology, cytochemistry, and biochemistry. J Exp Med. 1965;121:153–70. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruff MR, Gifford GE. Purification and physicochemical characterization of rabbit tumor necrosis factor. J Immunol. 1980;125:1671–7. [PubMed] [Google Scholar]
- 32.Zebedee SL, Koduri RK, Muherjee J. Mouse–human immunoglobulin G1 chimeric antibodies with activities against Cryptococcus neoformans. Antimicrob Agents Chemother. 1994;38:1507–14. doi: 10.1128/aac.38.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arruda MS, Richini VB, Oliveira SM, Vilani-Moreno FR. Experimental murine mycobacteriosis: evaluation of the functional activity of alveolar macrophages in thalidomide-treated mice. Braz J Med Biol Res. 2004;37:485–92. doi: 10.1590/s0100-879x2004000400005. [DOI] [PubMed] [Google Scholar]
- 34.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10670–5. [PubMed] [Google Scholar]
- 35.Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–88. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 37.Possani LD, Martin BM, Svendsen I, Rode GS, Erickson BW. Scorpion toxins from Centruroides noxius and Tityus serrulatus: primary structures and sequence comparison by metric analysis. Biochem J (Lond) 1985;229:739–50. doi: 10.1042/bj2290739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polikarpov I, Sanches M, Marangoni S, Toyama MH, Teplyakov A. Crystal structure of neurotoxin Ts1 from Tityus serrulatus provides insights into the specificity and toxicity of scorpion toxins. J Mol Biol. 1999;290:175–84. doi: 10.1006/jmbi.1999.2868. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez de la Vega RC, Possani LD. Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure–function relationships and evolution. Toxicon. 2005;46:831–44. doi: 10.1016/j.toxicon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Ogura M, Kitamura M. Oxidant stress incites spreading of macrophages via extracellular signal-regulated kinases and p38 mitogen-activated protein kinase. J Immunol. 1998;161:3569–74. [PubMed] [Google Scholar]
- 41.Carrithers MD, Dib-Hajj S, Carrithers LM, et al. Expression of the voltage-gated sodium channel Nav1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol. 2007;178:7822–32. doi: 10.4049/jimmunol.178.12.7822. [DOI] [PubMed] [Google Scholar]
- 42.Renaud JF, Fosset M, Schweitz H, Lazdunski M. The interaction of polypeptide neurotoxins with tetrodotoxin-resistant Na+ channels in mammalian cardiac cells. Correlation with inotropic and arrhythmic effects. Eur J Pharmacol. 1986;120:161–70. doi: 10.1016/0014-2999(86)90536-4. [DOI] [PubMed] [Google Scholar]
- 43.Szabadkai G, Rizzuto R. Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett. 2004;567:111–5. doi: 10.1016/j.febslet.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 44.Putney JW, Thomas AP. Calcium signaling: double duty for calcium at the mitochondrial uniporter. Curr Biol. 2006;16:19R812–15. doi: 10.1016/j.cub.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishikawa AK, Caricati CP, Lima MLSR, et al. Antigenic cross-reactivity among the venoms from several species of Brazilian scorpions. Toxicon. 1994;32:989–98. doi: 10.1016/0041-0101(94)90377-8. [DOI] [PubMed] [Google Scholar]


