Abstract
Hypericum perforatum (Hp) has been used medicinally to treat a variety of conditions including mild-to-moderate depression. Recently, several anti-inflammatory activities of Hp have been reported. An ethanol extract of Hp was fractionated with the guidance of an anti-inflammatory bioassay (lipopolysaccharide (LPS)-induced prostaglandin E2 production (PGE2)), and four constituents were identified. When combined together at concentrations detected in the Hp fraction to make a 4 component system, these constituents (0.1 µM chlorogenic acid, 0.08 µM amentoflavone, 0.07 µM quercetin, and 0.03 µM pseudohypericin) explained the majority of the activity of the fraction when activated by light, but only partially explained the activity of this Hp fraction in dark conditions. One of the constituents, light-activated pseudohypericin, was necessary, but not sufficient to explain the reduction in LPS-induced PGE2 of the 4 component system. The Hp fraction and the 4 component system inhibited lipoxygenase and cytosolic phospholipase A2, two enzymes in the PGE2-mediated inflammatory response. The 4 component system inhibited the production of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α), and the Hp fraction inhibited the anti-inflammatory cytokine interleukin-10 (IL-10). Thus, the Hp fraction and selected constituents from this fraction showed evidence of blocking pro-inflammatory mediators but not enhancing inflammation-suppressing mediators.
Keywords: Hypericum perforatum, anti-inflammatory, Prostaglandin E2, RAW 264.7, pseudohypericin, flavonoids
5) Introduction
The synthesis of prostaglandins plays a critical role in normal physiological processes as well as acute and chronic inflammatory states (Dubois et al., 1998; Portanova et al., 1996) and the key enzymes involved in prostaglandin biosynthesis are prostaglandin endoperoxide synthases, also known as cyclooxygenases. Cyclooxygenase-1 (COX-1) is responsible for housekeeping functions such as maintenance of gastric mucosa (Smith et al., 1996). Cyclooxygenase-2 (COX-2) is induced by lipopolysaccharide (LPS) to produce prostaglandins, of which prostaglandin E2 (PGE2) is one of the main mediators of inflammation (Minghetti et al., 1999; O’Sullivan et al., 1992). Cytosolic phospholipase A2 (cPLA2) releases arachidonic acid, the substrate for COX and lipoxygenase (LOX) enzymes, from membrane phospholipids.
Cytokines mediate the inflammatory response in a complex manner, during its early, middle, and late stages. Tumor necrosis factor-α (TNF-α), an early pro-inflammatory cytokine, is involved in the pathogenesis of many inflammatory diseases and can regulate the growth, proliferation, and viability of leukocytes (Aggarwal, 2000; Calamia, 2003). Interleukin-10 (IL-10), an anti-inflammatory cytokine predominant in the later phases of inflammation, is a potent inhibitor of macrophage function, and IL-10 can block the synthesis of TNF-α and can inhibit COX-2 induction (Niiro et al., 1995; de Waal-Malefyt et al., 1991). Preparations that can modulate one or many of the mediators of inflammation may be useful for the treatment of inflammatory diseases.
Hypericum perforatum (Hp) contains unusual compounds such as hypericin, pseudohypericin, and hyperforin, as well as compounds present throughout the plant kingdom (Bilia et al., 2002). Raso et al. (2002) found that giving 100 mg/kg of Hp extract by gavage to mice two times daily significantly reduced COX-2 protein levels in peritoneal macrophages. Hp extracts and many of the constituents in these extracts (light-activated pseudohypericin, flavonoid compounds, hyperforin) reduced LPS-induced PGE2 production in RAW 264.7 macrophages (Hammer et al., 2007). Furthermore, Hp extracts exhibited light-independent reductions in LPS-induced PGE2, but pseudohypericin significantly decreased LPS-induced PGE2 at 1 and 2 µM only in light-activated conditions and 20 µM hypericin increased PGE2 with and without LPS only in light-activated conditions. This data established that although individual constituents like pseudohypericin and hypericin needed activation by light to produce an effect on PGE2, confirming previously reported light-activated bioactivities of hypericin (Bilia et al., 2002; Carpenter et al., 1991), Hp extracts did not differ in light-activated and dark conditions, contrary to previously reported bioactivities (Bilia et al., 2002; Schmitt et al., 2006a; Schmitt et al., 2006b). Other compounds like the flavonoids and caffeic acid derivatives did not differ in light-activated and dark treatments (Hammer et al., 2007). It is vital to understand the effects of isolated active constituents as well as combinations of active constituents to relate the bioactivity of the constituents to the bioactivity of extracts (Spinella, 2002). Of 10 potentially bioactive constituents tested, only the concentration of pseudohypericin detected in the Hp extracts (0.2 to 1 µM) was above the level of pure constituent (1 µM) needed to observe a significant reduction in PGE2 production in RAW 264.7 mouse macrophages (Hammer et al., 2007). However, pseudohypericin’s presence in the extracts did not appear to account for the activity of the extracts, suggesting that the interactions of the constituents may be important (Hammer et al., 2007).
Bioactivity-guided fractionation was used to identify constituents present in an Hp ethanol extract that may be responsible for anti-inflammatory activity of the extract. Our hypothesis was that flavonoid compounds were contributing to the anti-inflammatory activity of the Hp extracts, along with other constituents that may interact with the flavonoids. To test this hypothesis, we used a strategy intended to enrich the fractions in flavonoids, and evaluated the fractions for a reduction in LPS-induced PGE2 production. To compare our anti-inflammatory results to a known compound, we used concentrations of quercetin exceeding the levels found in Hp extracts and that have previously been shown to inhibit inflammatory endpoints of interest as a positive control.
6) Results/Discussion
The bioactivities of Hp fractions from four rounds of iterative fractionations are presented in Table 1. The original Hp ethanolic extract significantly inhibited LPS-induced PGE2 production in RAW 264.7 mouse macrophages at both 10 and 20 µg/ml. There was a significant reduction in cell viability associated with the 20 µg/ml dose of the Hp extract, although the reduction in PGE2 (46% of PGE2 control) could not be fully explained by this decreased cell viability data (58% of cell viability control). This original Hp extract was fractionated using ethanol, chloroform, or hexane into three fractions (1A: ethanol, 1B: hexane, and 1C: chloroform). The most active fraction from the first round of fractionation at 10 µg/ml was fraction 1C when compared with other fractions; 36% of the PGE2 production compared to control and 74% of cell viability compared to control. Subfractionation of fraction 1C by column chromatography with a solvent series of chloroform (CHCl3), acetonitrile (CH3CN), and methanol (MeOH) led to 4 fractions (2A, 2B, 2C, 2D), of which, 10 µg/ml of fraction 2C most significantly decreased PGE2 as compared to control (44% of PGE2 control, 96% of cell viability control) and was the most active of the second round fractions at 10 µg/ml. Fraction 2C was further sub-fractionated using column chromatography with 1:1 CH3CN:CHCl3 to 1:1 MeOH: CH3CN (3A, 3B, 3C, 3D, 3E, 3F). Of the third round fractions, fraction 3A significantly decreased PGE2 (22% of PGE2 control, 85% of cell viability control) at a concentration as low as 10 µg/ml. Fraction 3A was further sub-fractionated using column chromatography with a step gradient from 10% CH3CN:CHCl3 to 100% MeOH into 7 fractions (4A, 4B, 4C, 4D, 4E, 4F, 4G). The most active fraction from the last round of fractionation was fraction 4F (58% of PGE2 control, 101% of cell viability control) at 2 µg/ml; however, the reduction in PGE2 was not statistically significant.
Table 1.
Reduction in LPS-induced PGE2 and cell viability of Hp fractions and subfractions in RAW 264.7 mouse macrophages. Mean percent of LPS-induced PGE2 level as compared to media + LPS + DMSO control (95% confidence intervals) and mean percent of cell viability as compared to media + DMSO control-treated cells ± standard error of Hp fractions n=8 for treatments. PGE2 and cell viability data represents light-activated and dark treatments combined as there was no significant difference between the treatments. The concentration of 10 µg/ml was chosen to compare fractions from each round of fractionation; fractions from rounds 3 and 4 were assayed at the highest concentration possible based on the amount of DMSO that can be added onto the cells. Fractions in the culture media without LPS did not significantly affect the concentration of PGE2 as compared to the media + DMSO control. Addition of LPS to the culture media + DMSO control increased the level of PGE2 20–38 fold over media + DMSO control alone (0.1 ±0.05 ng/ml for media + DMSO, 2.7 ± 0.5 ng/ml for media + DMSO + LPS). Quercetin (10 µM) positive control significantly inhibited PGE2 production (11(8–16) % of PGE2 control)
| Fractionation | Treatment | (µg/ml) | PGE2 Percent of Control | Cell Viability Percent of Control |
|---|---|---|---|---|
| Original Ethanol Extract | Extract | 20 | 43 (28–67)* | 58 ± 14* |
| 10 | 46 (21–98)* | 97 ± 16 | ||
| Fractionation Round 1 | Fraction 1A | 24 | 25 (25–53)* | 55 ± 18* |
| Extract into 1A–1C | 10 | 63 (63–100) | 80 ± 17 | |
| Fraction 1B | 22 | 16 (7–93)* | 74 ± 13 | |
| 10 | 76 (21–97) | 108 ± 28 | ||
| Fraction 1C | 10 | 36 (23–27)* | 74 ± 17 | |
| Fractionation Round 2 | Fraction 2A | 40 | 40 (37–100) | 46 ± 20* |
| Fraction 1C into subfractions 2A–2D | 10 | 59 (80–100) | 96 ± 12 | |
| Fraction 2B | 76 | 14 (6–29)* | 46 ± 20* | |
| 40 | 42 (40–100)* | 67 ± 15 | ||
| 10 | 100 (87–108) | 113 ± 21 | ||
| Fraction 2C | 40 | 8 (4–18)** | 40 ± 11* | |
| 10 | 44 (27–71)* | 96 ± 2 | ||
| Fraction 2D | 40 | 17 (7–40)* | 36 ± 3* | |
| 10 | 49 (20–100) | 71 ± 6 | ||
| Fractionation Round 3 | Fraction 3A | 10 | 22 (10–51)** | 85 ± 1 |
| Subfraction 2C into subfraction 3A–3F | Fraction 3B | 13 | 82 (34–100) | 99 ± 3 |
| Fraction 3C | 5 | 89 (40–100) | 93 ± 5 | |
| Fraction 3D | 4 | 80 (33–100) | 101 ± 5 | |
| Fraction 3E | 4 | 97 (90–115) | 88 ± 2 | |
| Fraction 3F | 4 | 36 (24–100) | 99 ± 6 | |
| Fractionation Round 4 | Fraction 4A | 2 | 100 (74–100) | 109 ± 8 |
| Subfraction 3A into subfractions 4A–4F | Fraction 4B | 4 | 100 (51–100) | 104 ± 11 |
| Fraction 4C | 3 | 100 (64–100) | 115 ± 21 | |
| Fraction 4D | 1 | 100 (45–100) | 93 ± 23 | |
| Fraction 4E | 0.8 | 100 (50–100) | 107 ± 15 | |
| Fraction 4F | 0.7 | 58 (20–100) | 101 ± 7 | |
| Fraction 4G | 0.8 | 63 (43–100) | 98 ± 5 | |
p-value < 0.05 as compared to control.
p-value < 0.001 as compared to control.
The concentrations of 10 constituents were quantified in the original Hp extract and the four most active fractions (1C, 2C, 3A, 4F) are shown in Table 2. The most abundant constituents in the original Hp extract were hyperforin (12.5 µM), chlorogenic acid (6.1 µM), rutin (2.7 µM), and hyperoside (1.6 µM) (Table 2). After the first round of fractionation, the concentrations of all the constituents in fraction 1C were at or below 1 µM. It is possible that agents that suppressed the inhibition of PGE2 production were removed in the earlier stages of fractionation since the concentration of putative active constituents decreased successively from the extract to fraction 1C and then to fraction 2C. In addition, unknowns comprised a larger portion of the later subfractions because the concentration of constituents decreased as the fractionation progressed, although activity remained about the same and was even greater from fraction 2C to 3A. The ratios of the 4 putative bioactive constituents in the fraction seemed to follow the pattern: greatest amount of chlorogenic acid, followed by roughly equal amounts of quercetin and amentoflavone, and the least amount of pseudohypericin (Table 2 figure legend). Ratio analysis of the levels of the four constituents in the extract and active fractions suggested that the greatest activity was obtained when the levels of chlorogenic acid, quercetin, and amentoflavone were approximately the same and that these concentrations were two to three times higher than pseudohypericin, as seen with fraction 3A. Additionally, the lowest activity was seen when only chlorogenic acid and pseudohypericin were detected, as seen with fraction 4F. Although compounds such as hypericin may have non-reversibly adsorbed to the silica gel column, results from the PGE2 assay confirmed that at least one fraction was active from each round as the fractionation progressed. Additionally, flavonoids were compounds of particular interest in this fractionation and in previous studies, hypericin was shown to increase PGE2 production in LPS-induced RAW 264.7 mouse macrophages (Hammer et al., 2007).
Table 2.
Constituents identified and quantified (µM) in 10 µg/ml of Hp extracts, fractions and subfractions. Constituents identified and quantified by LC-MS-UV analysis.
| Extract | Fraction 1C | Fraction 2C | Fraction 3Aa | Fraction 4F | |
|---|---|---|---|---|---|
| Chlorogenic Acid | 6.1 ± 0.34d | 1.0 ± 0.1c | 0.3 ± 0.01c | 0.1 ± 0.11b | 1.4 ± 0.003b |
| Rutin | 2.7 ± 1.7c | Detected | Detected | - | - |
| Hyperoside | 1.6 ± 1.5c | 0.2 ± 0.002b | 0.03 ± 0.01b | - | - |
| Isoquercitrin | 0.3 ± 0.1b | Detected | Detected | - | - |
| Quercitrin | 0.03 ± 0.01a | 0.1 ± 0.01b | 0.02 ± 0.04b | - | - |
| Quercetin | 0.2 ± 0.009b | 0.1 ± 0.009b | 0.02 ± 0.3b | 0.07 ± 0.2a | - |
| Amentoflavone | 0.2 ± 0.05b | 0.09 ± 0.2ab | 0.02 ± 0.4ab | 0.08 ± 0.2ab | - |
| Pseudohypericin | 0.2 ± 0.28b | 0.04 ± 0.008a | 0.001 ± 0.06a | 0.03 ± 0.04a | 0.01 ± 0.001a |
| Hypericin | 0.1 ± 0.03a | Detected | Detected | Detected | - |
| Hyperforin | 12.5 ± 0.5e | 0.02 ± 0.003a | Detected | Detected | - |
Identified compounds from fraction 3A provided the basis for the 4 component system. Ten metabolites were quantified for the original extract and each of the active fractions and subfractions. n=3 for each. The data is represented as mean concentration of constituents detected in 10 µg/ml extract or fraction ± standard error. This concentration was chosen to facilitate comparison of levels of constituents between extracts and fractions. “Detected” indicates detection by the MS; however the amount was too low for quantification with standard curves generated by the UV absorption. “-“ represents constituents not detected by the MS.
Mean values within each column with different superscript letters were significantly differenta<b<c<d<e (p<0.05) and values with more than one letter were not significantly different than means sharing either of the letters. Ratios of chlorogenic acid: quercetin: amentoflavone: pseudohypericin in the extract and fractions are: extract, 30.5:1:1:1; fraction 1C, 25:2.5:2.3:1; fraction 2C, 300:20:20:1, fraction 3A, 3.3:2.3:2.7:1; fraction 4F, 140:0:0:1.
Since fraction 3A was significantly active in the PGE2 assay and from the later rounds of fractionation, experiments were conducted to determine if combining its putative bioactive constituents (chlorogenic acid, amentoflavone, quercetin, and pseudohypericin) into a 4 component system at the amount detected in fraction 3A could explain the reduction in PGE2 by fraction 3A. These constituents were also studied together as a 4 component system at ten times and one hundred times the amount detected in fraction 3A. None of the four constituents alone reduced PGE2 in light-activated or dark conditions (Table 3). Combinations of the four constituents revealed that combinations without pseudohypericin were not effective at reducing PGE2. Two-way and three-way combinations with pseudohypericin seemed to explain some of the light-activated activity of the Hp fraction, however; not to as great of an extent as the 4 component system. The combination of all four constituents (34% of PGE2 control, 101% of cell viability control) was sufficient to explain the anti-inflammatory activity of fraction 3A (12% of PGE2 control, 85% of cell viability control) in light-activated conditions. Furthermore, this combination of constituents was even more effective at reducing PGE2 in light-activated than dark conditions. Hyperforin and hypericin were not added to the 4 component system because they were only detected in the fraction and were not able to be quantified using standard curves of the pure compound. However, later experiments determined that adding 0.01 µM or 0.001 µM hyperforin to the 4 component system did not change the reduction in PGE2 associated with the system (data not shown) and hypericin increased the production of PGE2 and had significant cytotoxicity associated with low doses in the RAW 264.7 macrophage cells (Hammer et al., 2007). Thus, the 4 component system explained the light-activated activity of the Hp fraction but not the dark activity and pseudohypericin was necessary for the light-activated activity.
Table 3.
Reduction in PGE2 and cell viability by combinations of the putative bioactive constituents identified in Fraction 3A. Mean percent of LPS-induced PGE2 level as compared to media + LPS + DMSO control (95% confidence intervals) and mean percent of cell viability as compared to media + DMSO control-treated cells ± standard error of constituents identified in fraction 3A. n=8 for anti-inflammatory treatments; n=8 for cytotoxicity treatments. Q=0.07 µM quercetin, A= 0.08 µM amentoflavone, CA= 0.2 µM chlorogenic acid, PH= 0.03 µM pseudohypericin. Cytotoxicity data represents light-activated and dark treatments combined as there was no difference between light-activated versus dark treatments. Constituents in the culture media without LPS did not affect the concentration of PGE2 as compared to the media + DMSO control. Addition of LPS to the culture media + DMSO control increased the level of PGE2 10–34 fold over the media + DMSO control alone (0.07 ±0.03 ng/ml for media + DMSO, 1.6 ± 0.4 ng/ml for media + DMSO + LPS). Quercetin (10 µM) positive control significantly inhibited PGE2 production (11 (8–16) % of control)
| PGE2 Percent of light-activated control |
PGE2 Percent of dark control |
Cell Viability Percent of Control |
|
|---|---|---|---|
| Fraction 3A | 12 (7–18)* | 32 (3–48)* | 85 ± 4 |
| Q | 100 (98–100) | 99 (96–105) | 86 ± 16 |
| A | 103 (96–106) | 104 (99–110) | 92 ± 8 |
| CA | 115 (100–127) | 122 (104–128) | 103 ± 18 |
| PH | 103 (98–109) | 100 (98–114) | 76 ± 11 |
| Q + A | 90 (78–100) | 92 (86–98) | 116 ± 22 |
| CA + Q | 100 (71–100) | 100 (91–104) | 112 ± 16 |
| CA + A | 88 (80–99) | 100 (76–108) | 99 ± 7 |
| PH + Q | 61 (39–85)* | 98 (79–104)$ | 87 ± 5 |
| PH + A | 51 (16–84)* | 83 (84–100)$ | 96 ± 13 |
| PH + CA | 93 (71–100) | 102 (96–108) | 104 ± 15 |
| CA + Q + A | 95 (86–100) | 99 (79–114) | 80 ± 24 |
| Q + A + PH | 50 (17–80)* | 78 (85–99)$ | 113 ± 17 |
| CA + Q+ PH | 69 (35–89)* | 78 (50–98) | 94 ± 13 |
| CA + A + PH | 65 (36–96)* | 74 (69–197) | 101 ± 8 |
| CA, A, Q, PH | 34 (29–36)* | 78 (49–86)$ | 101 ± 10 |
| 10x CA, A, Q, PH | 31 (24–35)* | 68 (38–79)$ | 102 ± 7 |
| 100x CA, A, Q, PH | 11 (5–17)* | 53 (31–59)*$ | 95 ± 12 |
p-value < 0.05 as compared to control.
significant difference between light-activated and dark treatments.
Since the four constituents together seemed to best account for the reductions in PGE2 of the fraction, further explorations compared only the 4 component system with the Hp fraction to determine if comparable synergy existed in other endpoints. To assess the reduction in PGE2 associated with fraction 3A and the 4 component system, COX-1 and COX-2 protein levels (Figure 1) and enzyme activities (Figure 2) were examined. LPS-treated groups are shown in Figure 1 and treatments without LPS are described in the legend for COX-1 and COX-2 protein levels. No change in COX-1 protein level was detected among treatments without the addition of LPS and COX-1 protein levels were indistinguishable in the LPS and non-LPS treated controls. No change in COX-1 protein level was detected with the fractions or 4 component system when LPS was added (Figures 1a and 1b). COX-2 protein level was increased with the addition of LPS to the culture media and there was no change in COX-2 protein level when the treatments were added without LPS (Figure 1). The positive control, 100 µM quercetin, significantly decreased the LPS-induced COX-2 protein level, as described in the Figure 1 legend. COX-2 protein was reduced when fraction 3A and 100x the 4 component system were included in light-activated conditions (Figured 1a and 1c), but not in dark treatment conditions (Figure 1 legend). Fractions 1C and 2C did not reduce LPS-induced COX-2 protein levels in light-activated conditions (Figures 1a and 1c), further confirming the PGE2 data showing that fraction 3A was the most anti-inflammatory among the active fractions.
Figure 1.
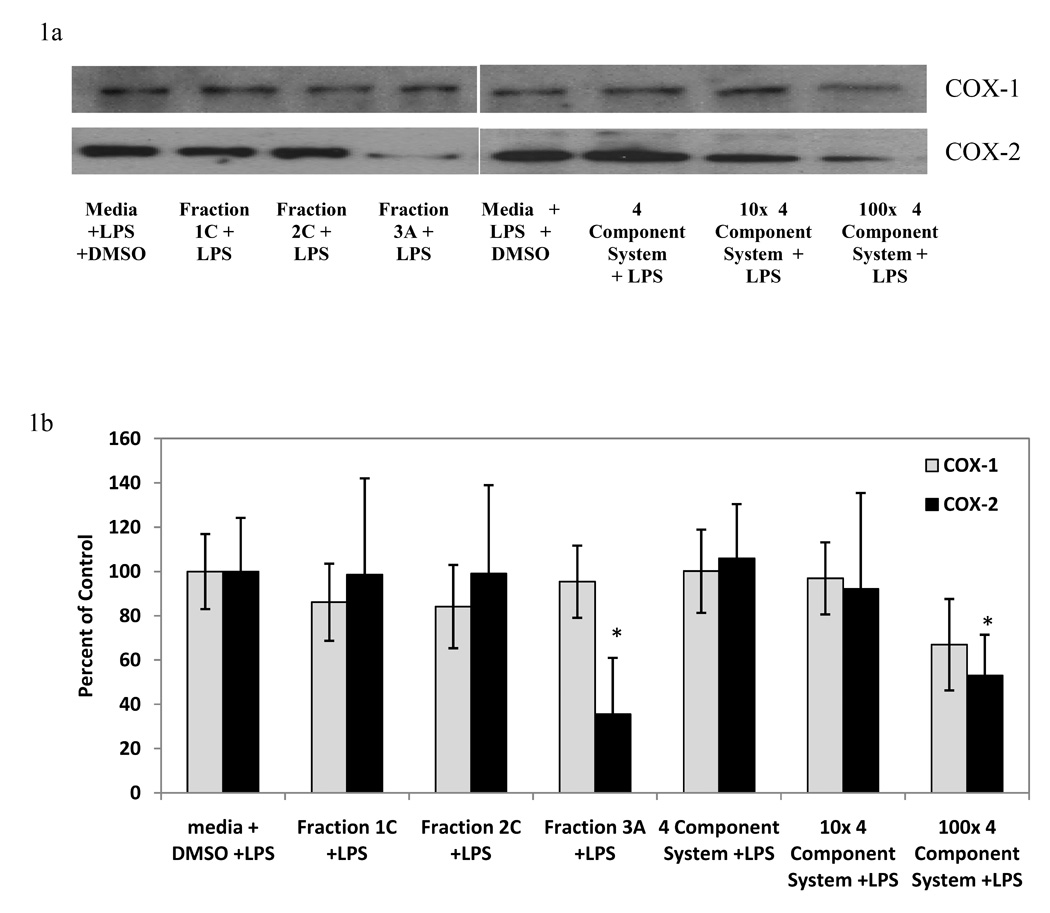
Representative western blots (Figure 1a) and semi-quantitative representation (Figure 1b) of the effect of light-activated Hp fractions (10 µg/ml) and 4 component systems on LPS-induced COX-1 and COX-2 protein levels in RAW 264.7 mouse macrophages. The 4 component system is composed of: 0.07 µM quercetin, 0.08 µM amentoflavone, 0.2 µM chlorogenic acid, 0.03 µM pseudohypericin. Data is represented as mean percent of media + DMSO + LPS control ± standard error. n=4 for each. Treatments without LPS did not significantly affect either COX-1 or COX-2 protein as compared to media + DMSO control (average 98 ± 12% of control). LPS increased the expression of COX-2 protein (29 ± 16 % of control for media + DMSO, 100 ± 17 for media + LPS + DMSO) but not COX-1 protein (100 ± 15 % of control for media + DMSO). Dark treatments did not significantly affect LPS-induced COX-1 or COX-2 protein levels (average 99 ±15% of control). Quercetin (100 µM) used as a positive control for reduction in LPS-induced COX-2 protein (27 ± 22 % of control). Quercetin did not affect LPS-induced COX-1 protein (103 ± 18% of control). * p-value < 0.05 as compared to media + LPS + DMSO control.
Figure 2.
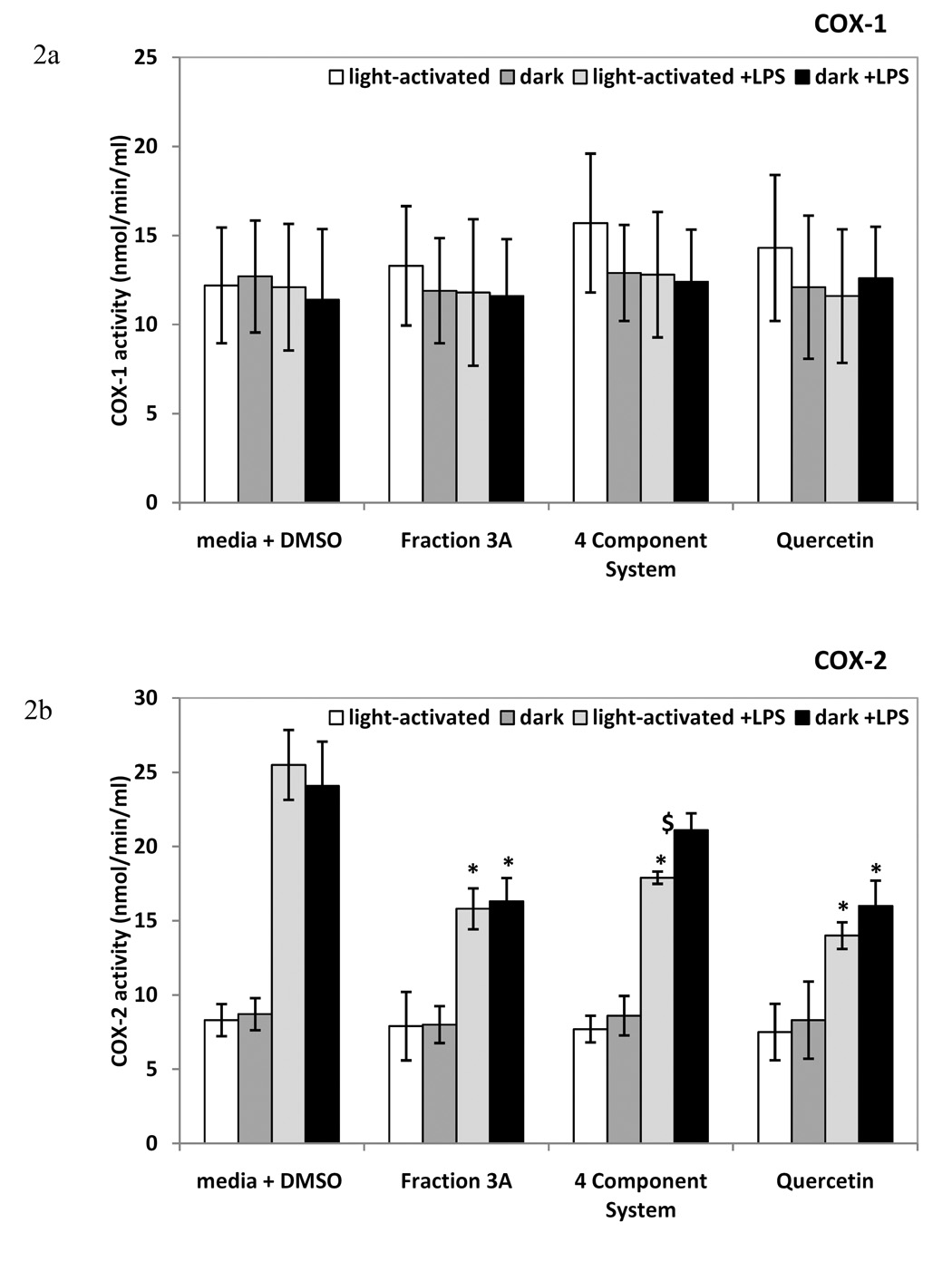
The effect of fraction 3A and 4 component system on enzyme activity of COX-1 (Figure 2a) and COX-2 (Figure 2b) in RAW 264.7 mouse macrophages. Q=0.07 µM quercetin, A= 0.08 µM amentoflavone, CA= 0.2 µM chlorogenic acid, PH= 0.03 µM pseudohypericin. Data is presented as mean COX-1 or COX-2 activity ± standard error (nmol/min/ml). n=4 for each. Quercetin (25 µM) was used as positive control. * p-value < 0.05 as compared to media + LPS + DMSO control. $ significant difference between light-activated and dark treatments.
Consistent with the lack of induction of COX-1 protein, no change in COX-1 activity was detected with fraction 3A or the 4 component system (Figure 2a). In contrast, fraction 3A significantly decreased COX-2 activity as compared to media + LPS + DMSO control in both light-activated and dark conditions, whereas the 4 component system significantly decreased COX-2 activity only in light-activated conditions (Figure 2b). The positive control, 25 µM quercetin, significantly reduced COX-2 activity (Figure 2b). COX-2 activity was similar among treatments without LPS (Figure 2b).
To further assess the breadth of anti-inflammatory capabilities of fraction 3A and to compare the activity of the Hp fraction with the activity of the 4 component system, we examined cPLA2 activity and lipoxygenase inhibition, as well as TNF-α and IL-10 production in the RAW 264.7 macrophage cells. The positive control, 25 µM quercetin, significantly reduced LPS-induced cPLA2 and lipoxygenase activity (Figures 3a and 3b). Fraction 3A decreased LPS-induced cPLA2 activity as compared to the control in both light-activated and dark conditions, but the 4 component system significantly decreased LPS-induced cPLA2 activity only in light-activated conditions (Figure 3a). The light-activated 4 component system displayed similar lipoxygenase inhibitory activity as fraction 3A, and there was no significant difference between light-activated and dark conditions for either treatment (Figure 3b). Fraction 3A did not reduce the pro-inflammatory cytokine TNF-α at either 8 or 24 hours, nor did the 4 component system at 8 hours (Figures 4a and 4b). The 4 component system significantly reduced TNF-α at 24 hours in the light (Figure 4b). The levels of the anti-inflammatory cytokine IL-10 were reduced by fraction 3A at both 8 and 24 hours in light-activated and dark conditions (Figure 5a and 5b). Only the light-activated 4 component system did not significantly inhibit the anti-inflammatory cytokine IL-10 at 8 and 24 hours, although the level of IL-10 was not sustained at the level of the media + LPS + DMSO control. The complexity of this data suggests that perhaps the 4 component system and fraction 3A affect prostaglandin biosynthesis pathways in similar ways, but not the production of IL-10 and TNF-α, two cytokines important in inflammation.
Figure 3.
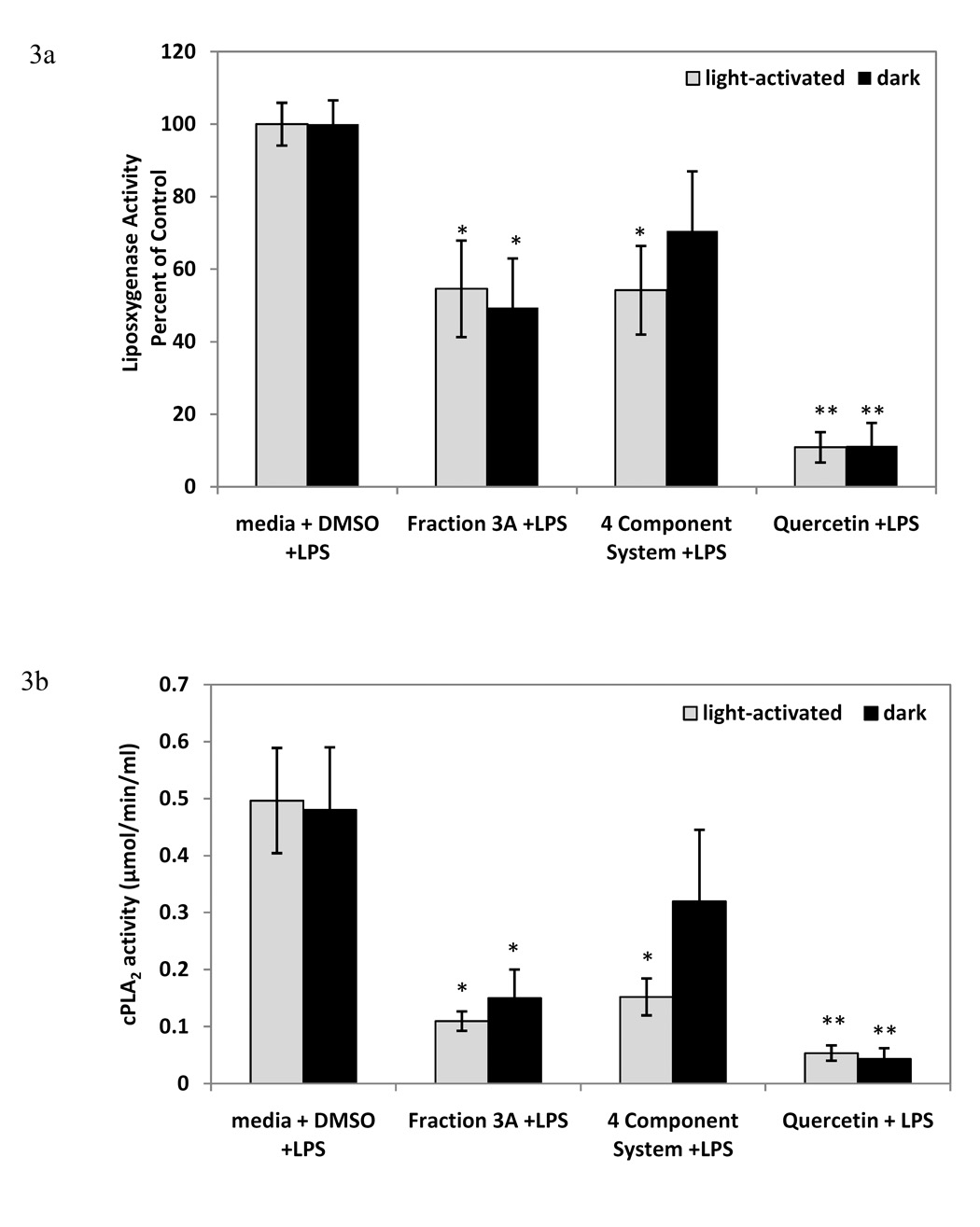
Inhibition of LPS-induced cPLA2 (Figure 3a) and lipoxygenase (Figure 3b) enzyme activity by fraction 3A and 4 component system in RAW 264.7 mouse macrophages (mean cPLA2 activity in µmol/min/ml ± standard error, and mean lipoxygenase activity ± standard error as percent of media + LPS + DMSO control). n=4-6 for each. Q=0.07 µM quercetin, A= 0.08 µM amentoflavone, CA= 0.2 µM chlorogenic acid, PH= 0.03 µM pseudohypericin. Quercetin (25 µM) was used as positive control for cPLA2 and lipoxygenase. * p-value < 0.05 as compared to media + LPS + DMSO control. ** p-value < 0.001 as compared to media + LPS + DMSO control. $ significant difference between light-activated and dark treatments.
Figure 4.
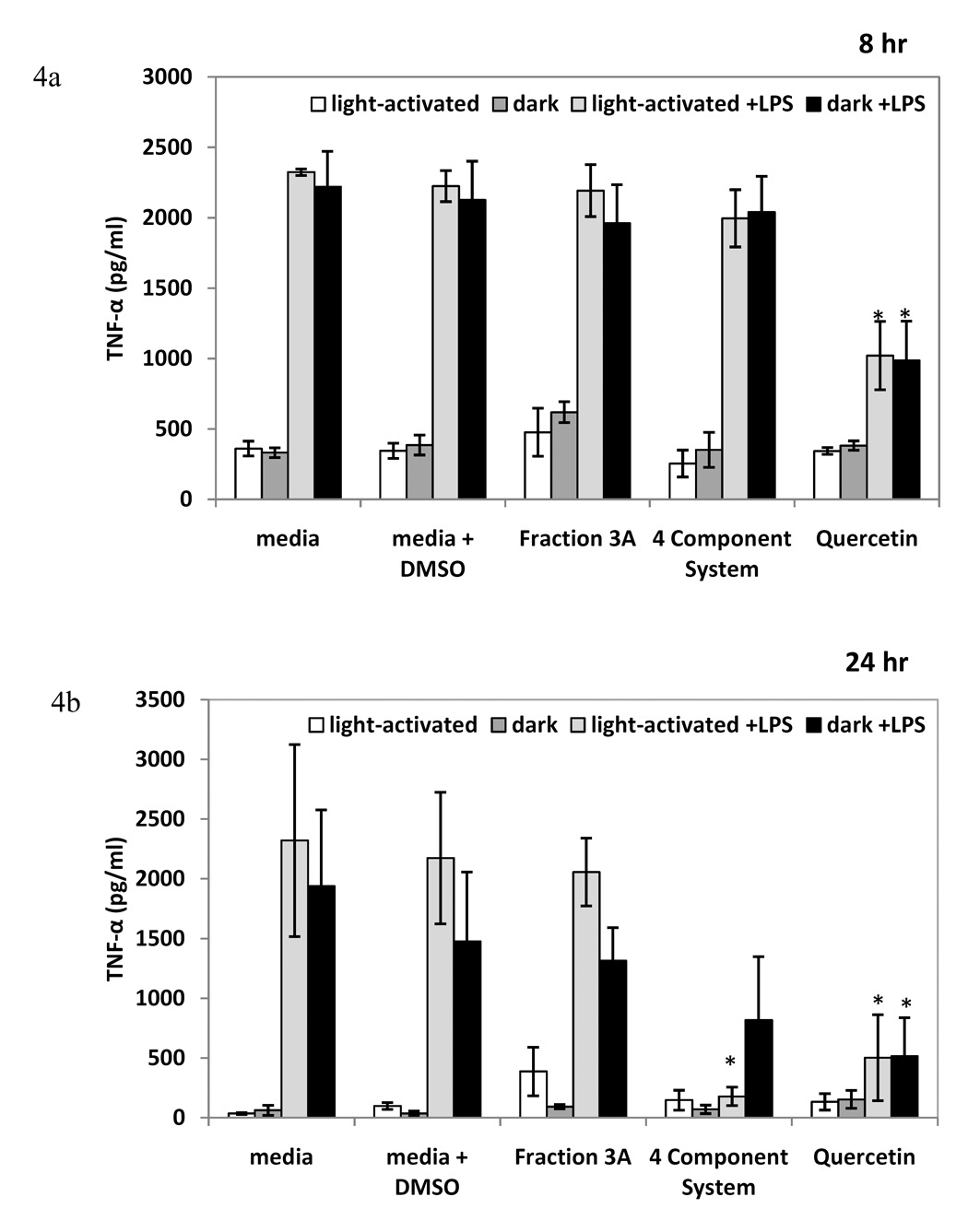
The effect of fraction 3A and 4 component system on TNF-α production at 8 hours (Figure 4a) and 24 hours (Figure 4b) of treatment in RAW 264.7 mouse macrophages (mean level in pg/ml ± standard error). n=3 for each. Q=0.07 µM quercetin, A= 0.08 µM amentoflavone, CA= 0.2 µM chlorogenic acid, PH= 0.03 µM pseudohypericin. Quercetin (25 µM) used as positive control. * p-value < 0.05 as compared to media + LPS + DMSO control.
Figure 5.
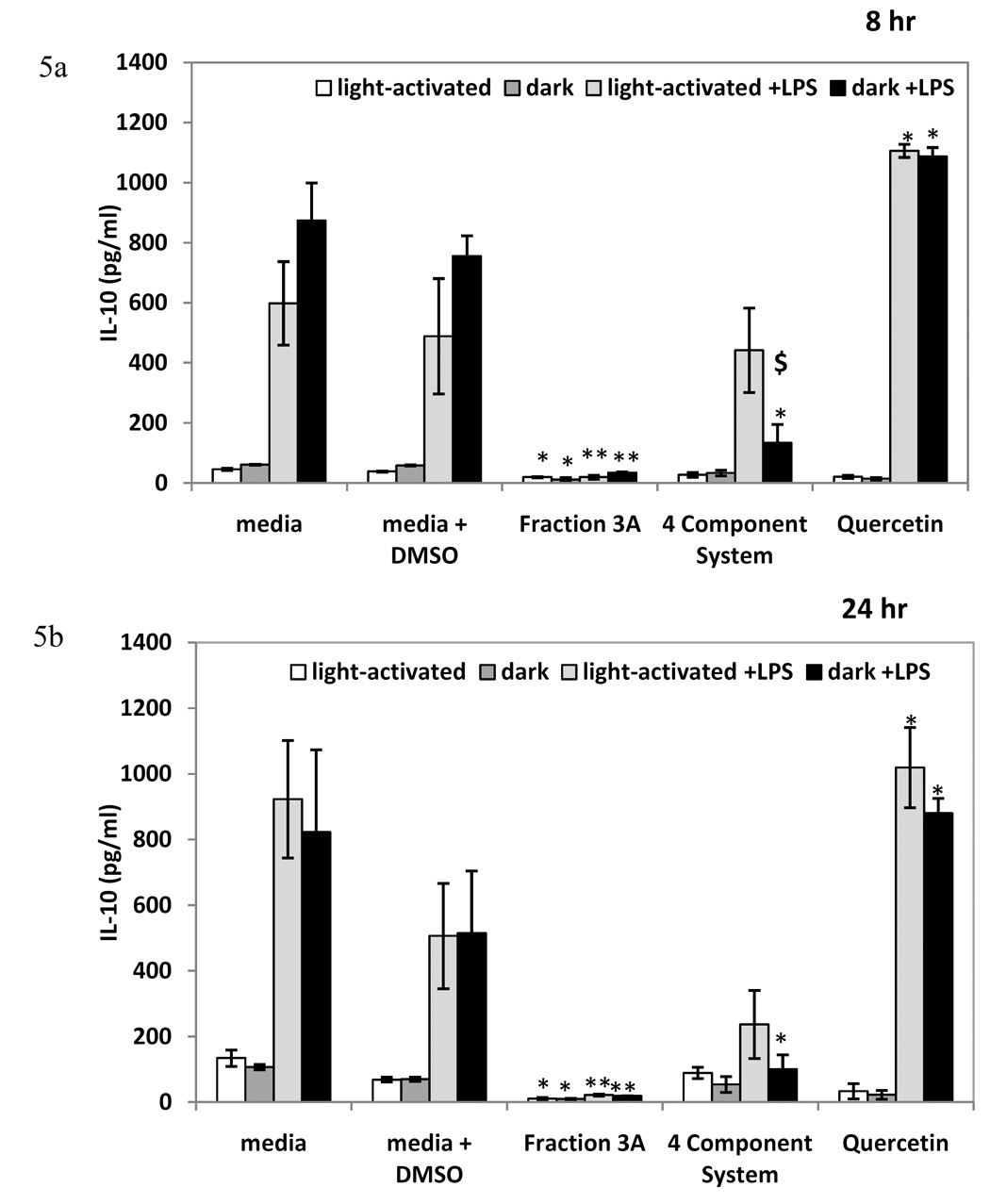
The effect of fraction 3A and 4 component system on IL-10 levels (mean level in pg/ml ± standard error) of RAW 264.7 mouse macrophages treated for 8 hours (Figure 5a) and 24 (Figure 5b) hours. n=3 for each. Q=0.07 µM quercetin, A= 0.08 µM amentoflavone, CA= 0.2 µM chlorogenic acid, PH= 0.03 µM pseudohypericin. Quercetin (25 µM) used as positive control. * p-value < 0.05 as compared to media + LPS + DMSO control. ** p-value < 0.001 as compared to media + LPS + DMSO control. $ significant difference between light-activated and dark treatments.
The most intriguing observations from these experiments are that the combination of chlorogenic acid, amentoflavone, quercetin, and pseudohypericin, at their respective concentrations in fraction 3A, explained the light-activated inhibition of LPS-induced PGE2 production by fraction 3A and that pseudohypericin was necessary for the activity of 4 component system. However, 1 µM light-activated pure pseudohypericin was required to significantly reduce PGE2 (Hammer et al., 2007) and pure pseudohypericin at 0.03 µM did not reduce PGE2. In previous studies, greater than 5 µM quercetin and 10 µM amentoflavone were required to significantly reduce PGE2, and chlorogenic acid up to 40 µM did not reduce PGE2 by itself (Hammer et al, 2007). Since one or more of these constituents were needed in addition to pseudohypericin in combination experiments to effectively reduce PGE2, we postulated that the synergistic interactions among these constituents were important in the RAW 264.7 macrophages and that pseudohypericin was necessary, but not sufficient for the light-activated anti-inflammatory activity. Notably, the 4 component system did not explain the activity of fraction 3A in the dark.
Synergistic interactions have previously been described for the anti-depressant activities of constituents present in Hp extracts, although light conditions were not controlled. In the forced swimming test model of anti-depressant activity, a fraction of procyanidins was not active alone, but was significantly active when pseudohypericin and hypericin were added (Butterweck et al., 1998). Interestingly, procyanidins increased the water solubility of hypericin up to 400 fold (Juergenliemk, 2003a). When the flavonoid rutin, which was inactive in the forced swimming test alone, was combined with inactive Hp extracts, there was a strong anti-depressant effect (Noeldner and Schotz, 2002). The present report is perhaps the first identification of interactions of constituents in Hp necessary for an anti-inflammatory activity of an Hp extract.
The reduction by Hp of PGE2 and COX-2 protein levels confirms that the eicosanoid pathway may be an important pathway for the anti-inflammatory activity of Hp. The Hp fraction 3A and the 4 component system inhibited cPLA2 activity, which could limit the amount of arachidonic acid available to the COX-2 enzyme. Both the Hp fraction and 4 component system also inhibited lipoxygenase activity. Limiting arachidonic acid would also limit the availability of the substrate to the lipoxygenase enzymes. Future studies could explore if products of lipoxygenases such as lipoxins or leukotrienes are also affected by these treatments.
The light-activated 4 component system reduced the pro-inflammatory cytokine TNF-α at 24 hours and the 4 component system in the dark treatment condition inhibited the anti-inflammatory cytokine IL-10 at both 8 and 24 hours. The Hp fraction inhibited IL-10 production at both time points examined, but not TNF-α production. Since TNF-α would be produced early in the inflammatory process and perhaps at the same time as PGE2, select bioactive constituents may act in the early phases of inflammation. Therefore, Hp or select constituents may decrease pro-inflammatory mediators, but not increase mediators involved in suppressing inflammation at later stages. Also, the light-activated 4 component system did not significantly inhibit IL-10, but the IL-10 level was not sustained at the level of the control, suggesting that the bioactive constituents may impact pro-inflammatory mediators more than anti-inflammatory mediators. However, the modulation of cytokines and other mediators in inflammation is complex.
Data on the bioavailability of constituents shown to be responsible for a given bioactivity is also critical for predicting in vivo effects and the synergistic interactions of the constituents might be important for bioavailability. Murota et al. (2000) showed that quercetin glucosides were capable of passing through the Caco-2 epithelial cell monolayer, but their efficiency was lower than the aglycone quercetin. The bioavailability of pseudohypericin might be increased by the presence of the flavonoid quercetin and/or biflavonoid amentoflavone, since the oral bioavailability of hypericin, which has a structure very similar to pseudohypericin, was increased by 34% with the addition of the flavonoid hyperoside in rats (Butterweck et al., 2003). Hyperoside increased the water solubility of hypericin by 58% in vitro using the octanol/water partition coefficient (Juergenliemk, 2003a). Further, a metabolite of orally ingested quercetin, miquelianin, was able to cross small intestine and central nervous system barriers in vitro (Juergenliemk et al., 2003b), suggesting that quercetin metabolites might not only enhance bioavailability of other compounds, but might have considerable bioactivity alone.
Besides increased bioavailability, other plausible explanations to consider are that compounds from the 4 component system may alter the production of reactive oxygen species (ROS), reduce the light-activation of pseudohypericin, or affect electron transport, all processes which may affect the light-activated cytotoxicity of hypericin or pseudohypericin. Data concerning these processes are very limited for pseudohypericin, however, the light-activation and subsequent effects of hypericin have been well documented. There is also data available concerning other compounds found in Hp. Quercetin has been shown to be a strong singlet oxygen quencher and have anti-oxidant properties (Tournaire et al., 1993; Korkina and Afanasev, 1997). Quercetin (10 µM) had a significant protective effect against cytotoxicity of 10 µM hypericin in HL-60 promyelocytic cells, most likely by reducing ROS (Mirossay et al., 2001). Chlorogenic acid (10 µM) attenuated the cytotoxicity of 20 µM hypericin in HaCat human keratinocytes (Schmitt et al, 2007b). Couladis et al. (2002) tested an Hypericum triquetrifolium Turra extract for anti-oxidant activity. Interestingly, four constituents were identified that were present within the extract; quercetin, rutin, chlorogenic acid, and amentoflavone, and each constituent possessed anti-oxidant activity. The antioxidant activity of amentoflavone was similar to the α-tocopherol positive control, whereas the other constituents possessed less anti-oxidant activity. It is plausible that quercetin, chlorogenic acid, and amentoflavone may play a role in lessening ROS damage from pseudohypericin. Perhaps a combination of enhanced bioavailability and other mechanisms like decreased ROS production or reduced light-activation or electron transfer may aid the synergistic interactions of constituents to produce a 4 component system with comparable light-activated anti-inflammatory activity to the Hp fraction.
7) Conclusions and Concluding Remarks
An anti-inflammatory bioactivity-guided fractionation of an Hp extract led to the identification of four constituents (chlorogenic acid, amentoflavone, quercetin, and pseudohypericin) that in concert explained the reduction in LPS-induced PGE2 of an Hp subfraction in light-activated conditions. Pseudohypericin was necessary but not sufficient for the reduction in LPS-induced PGE2. The data presented here and current literature supports that the Hp fraction exerts effects on COX-2 and upstream mediators. These data highlight the possibility that unknown and/or unidentified compounds contribute significantly to the activity of fraction 3A in the dark. These experiments verify the need for more data on the synergistic interactions of constituents present in botanical extracts and their interactive roles in bioactivity.
8) Experimental
General Experimental procedures
Cell culture
RAW 264.7 mouse macrophages were purchased from the American Type Culture Collections (ATCC; Manassas, VA) and cultured as previously described (Hammer et al., 2007). Treatments for the PGE2 and cell viability assays were performed as previously described (Hammer et al., 2007). The assays were always performed in both light-activated and dark conditions, because the naphthodianthrones present within Hp extracts display well-described light-activated properties. Details on the light-activation and dark treatments were previously published in Schmitt et al. (2006a).
PGE2 and Cytotoxicity Assays
Samples were assayed with a Prostaglandin E2 EIA kit (GE Biosciences, Piscataway, NJ) or CellTiter96® Aqueous One Solution cell proliferation assay (Promega Corporation, Madison, WI) as previously described (Hammer et al., 2007).
COX activity assay
The COX activity assay was used according to manufacturer’s instructions (Cayman Chemicals; Ann Arbor, MI). The kit measures the peroxidase activity of cyclooxygenase colorimetrically by addition of arachidonic acid and monitoring the appearance of oxidized N’, N’, N’, N’-tetramethyl-p-phenylenediamine (TMPD) at 590 nm. Quercetin (25 µM) was used as a positive control to demonstrate inhibition of COX-2 activity because Al-Fayez showed the 50% inhibitory dose of quercetin was 5 µM (Al-Fayez et al., 2006).
cPLA2 and Lipoxygenase Inhibitory Assays
Cells were plated in petri dishes and allowed to attach for 24 hours. Cells were treated with or without fraction or constituent and with or without LPS for 8 hours and further processed as described by the manufacturer. Activity was measured using the cPLA2 assay kit or the lipoxygenase inhibitor screening assay kit (both Cayman Chemical Company; Ann Arbor, MI). Quercetin (25 µM) was used as a positive control to demonstrate inhibition of cPLA2 and lipoxygenase because Lindahl and Tagesson (1993) showed quercetin less than 100 µM inhibited cPLA2 activity and Deng et al. (2007) showed that quercetin inhibited 50% of 5-LO and 15-LO at 5.9 and 0.52 µM, respectively.
TNF-α and IL-10 Assays
Cells were treated as previously described (Hammer et al., 2007) and supernatants were collected on ice and frozen at −70° C until assayed using a TNF-α and IL-10 mouse ELISA plate (BD Biosciences Pharmingen, San Diego, CA) with methods described by the manufacturer and similar to Senchina et al. (2007). Quercetin (25 µM) was used as a positive control to demonstrate inhibition of TNF-α and increase in IL-10 because Comalada et al. (2006) showed that quercetin decreased TNF-α production (50% inhibitory dose was 20 µM) and 25 quercetin increased IL-10 production in bone marrow-derived macrophages.
Western Blotting
After an 8 hour treatment, cells were rinsed twice with cold 1X phosphate buffered saline (PBS). Lysis buffer (50 mM Tris-hydrochloride, 2 mM ethylenediamine tetraacetic acid, 2 mM ethylene glycol tetraacetic acid, 150 mM sodium chloride, 2 mM phenylmethanesulphonylfluoride, 25 mM leupeptin, 10 mM aprotinin, 10 mM sodium fluoride, 10 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 0.5% Triton X-100) was added to the dishes on ice and the cells were dissociated from the plate by scraping. The lysate was centrifuged at 4° C, and the supernatant was removed. The protein concentration in each lysate was determined using the bicinchonic acid and copper sulfate protein assay (Sigma; St. Louis, MO). Western blot separation and detection was used as previously described (Przybyszewski et al., 2001). COX-1 and COX-2 rabbit polyclonal antibodies (Santa Cruz Biotechnology; Santa Cruz, CA) were diluted 1:1000 in 5% milk Tris buffered saline with 0.5% Tween-20. Semi-quantitative representation was achieved by using the ImageQuaNT program. Three replicates of each treatment were analyzed on separate blots. Blots were normalized for consistency by using a repeat control present on each blot. Quercetin (100 µM) was used as a positive control to demonstrate reduction in COX-2 protein level because Raso et al. (2002) showed 50 µM quercetin decreased COX-2 protein in J774A.1 macrophages. Quercetin was not shown on the blots to facilitate comparisons among the graphs, however; the values for quercetin are given in the figure legend.
Compound Identification and Quantification using LC-MS-UV Analysis
An Agilent Technologies 100 Ion Trap Liquid Chromatography- Electron Spray Ionization-Mass Spectrometer, with a coupled UV absorption detector (LC-MS-UV) was used for quantification of compounds, as previously described (Hammer et al., 2007). Specifically, ten compounds were identified based on the availability of standards and identification in a previous publication (Hammer et al., 2007). Compounds identified were: chlorogenic acid, rutin, hyperoside, isoquercitrin, quercitrin, quercetin, amentoflavone, pseudohypericin, hypericin, and hyperforin (Juergenliemk and Nahrstedt, 2001; Williams et al., 2006). Each standard was run on the LC-MS. The peaks from the Hp fractions were confirmed by evaluating the retention time and mass spectra of each peak with the retention time and mass spectra for the standard of interest. Stock solutions of each extract or subfraction were: 20 mg/ml for extract, 10 mg/ml for fraction 1C, 40 mg/ml for fraction 2C, 10 mg/ml for fraction 3A, and 0.7 mg/ml for fraction 4F.
Statistical Analysis
The COX activity, cPLA2, lipoxygenase, TNF-α, IL-10, and protein data were logarithmically transformed to eliminate unequal variances and skewed distribution and an F-protected two-way ANOVA was used followed by a Tukey-Kramer test for multiple comparisons for all samples (Snedecor and Cochran, 1989). The PGE2, cytotoxicity, light versus dark treatments, and LC-MS-UV data were analyzed as previously described (Hammer et al., 2007). P-values < 0.05 were considered statistically significant.
Documentation of plants
One accession of Hp, the commercial variety Common, was provided by the North Central Regional Plant Introduction Station (NCRPIS; Ames, IA). Plant material was harvested in July 2004 from plants cultivated on site and processed as previously described (Schmitt et al., 2006). Dried Hp plant material (108 grams; aerial parts) was extracted by Soxhlet extraction for 6 hours with 95% ethanol and yielded 38 grams of dry residue. Two grams of dry residue was dissolved in 10 mls dimethylsulfoxide (DMSO) (Sigma; St. Louis, MO) for the PGE2 screening of the original extract.
For fractionation, the residue from an ethanol extract of Hp (36 g) was dissolved in 10% aqueous ethanol (1100 mL) and extracted with hexanes (300 mL). After the ethanol solution was extracted with CHCl3 (500 mL), all three fractions (1A, 1B, 1C) were concentrated in vacuo. Samples of the resulting residues were tested for activity, and only the CHCl3 fraction (1C) displayed significant reduction in PGE2.
The residue from fraction 1C (3.1 g) was dissolved in a minimum volume of CHCl3 and further purified by normal phase column chromatography on silica gel. Silica gel was chosen as a support for column chromatography to maximize the separation efficiency, even though the potential for non-reversible adsorption was recognized. Elution with a solvent series consisting of CHCl3 (375 mL), a 1:1 mixture of CHCl3:CH3CN (475 mL), CH3CN (450 mL), and finally a 1:1 mixture of CH3CN:MeOH (500 mL) afforded four fractions (2A, 132 mg; 2B, 1.01 mg; 2C, 158 mg; and 2D, 951 mg; respectively) for a total recovery of 73%. After concentration in vacuo, bioassays of the four fractions identified fraction 2C as the most active and 2C was further purified by column chromatography on silica gel. A solvent step gradient from 1:1 CH3CN:CHCl3 to 1:1 MeOH:CH3CN afforded 6 fractions, (3A, 1:1 CH3CN:CHCl3, 70 mL, 35.4 mg; 3B, 1:1 CH3CN:CHCl3, 70 mL, 61.5 mg; 3C, 1.5:1 CH3CN:CHCl3, 135 mL, 16.1 mg; 3D, 3:1 CH3CN:CHCl3, 100 mL, then 100% CH3CN, 100 mL, 5.2 mg; 3E, 1:9 MeOH:CH3CN, 100 mL, then 1:5 MeOH:CH3CN, 100 mL, 21.6 mg; and finally 3F, 1:1 MeOH:CH3CN, 100 mL, 7.7 mg, respectively, representing 99% recovery). The fraction that displayed the most significant activity, fraction 3A, was finally purified by column chromatography on silica gel. This fraction (34 mg) was placed on a column of silica gel (0.5 by 14 cm) and eluted with a step gradient from 10% CH3CN:CHCl3 to 100% MeOH to give the final 7 fractions 4A–G (4A, 1:9 CH3CN:CHCl3, 100 mL, 6.2 mg; 4B, 1:9 CH3CN:CHCl3, 75 mL, 8.7 mg; 4C, 1:3 CH3CN:CHCl3, 75 mL, 12.6 mg; 4D, 1:1 CH3CN:CHCl3, 75 mL, then 3:1 CH3CN:CHCl3, 75 mL, 4.9 mg; 4E, 1:5 CH3CN:CHCl3, 20 mL, 3.8 mg; 4F, 1:5 MeOH:CH3CN, 50 mL, 1.4 mg; and 4G, 100% MeOH, 100 mL, 3.4 mg). Endotoxin levels of the extracts were assayed as previously described (Hammer et al., 2007) to confirm that endotoxin present in the extracts did not affect PGE2 levels. The range of endotoxin levels present was 0.000003 to 0.0001 endotoxin units/milliliter (EU/ml). Endotoxin up to 5 EU/ml did not significantly increase the RAW 264.7 cells’ production of PGE2 in the assay (Hammer et al., 2007).
Acknowledgement
The authors would like to thank members of the Iowa Center for Research on Botanical Dietary Supplements. This publication was made possible by grant number 9P50AT004155-06 from the National Center for Complementary and Alternative Medicine (NCCAM) and Office of Dietary Supplements (ODS), National Institutes of Health (NIH) and P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and ODS, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCCAM, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Aggarwal BB. Tumour necrosis factor receptor associated signaling molecules and their role in the activation of apoptosis: JNK and NF-kappa β. Ann Rheum. Dis. 2000;59:6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Fayez M, Cai H, Tunstall R, Steward WP, Gescher AJ. Differential modulation of cyclooxygenase-mediated prostaglandin production by the putative cancer chemopreventive flavonoids tricin, apigenin, and quercetin. Cancer Chemother. Pharmacol. 2006;58(6):816–825. doi: 10.1007/s00280-006-0228-3. [DOI] [PubMed] [Google Scholar]
- Bilia AR, Gallori S, Vincieri FF. St. John’s wort and depression: Efficacy, safety and tolerability-an update. Life Sci. 2002;70:3077–3096. doi: 10.1016/s0024-3205(02)01566-7. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Petereit F, Winterhoff H, Nahrstedt A. Solubilized hypericin and pseudohypericin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Med. 1998;64:291–294. doi: 10.1055/s-2006-957437. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Lieflander-Wulf U, Winterhoff H, Nahrstedt A. Plasma levels of hypericin in presence of procyanidin B2 and hyperoside: a pharmacokinetic study in rats. Planta Med. 2003;69:189–192. doi: 10.1055/s-2003-38495. [DOI] [PubMed] [Google Scholar]
- Calamia KT. Current and future use of anti-TNF agents in the treatment of autoimmune, inflammatory disorders. Adv. Exp. Med. Biol. 2003;528:545–549. doi: 10.1007/0-306-48382-3_110. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Kraus GA. Photosensitization is required for inactiation of equine infectious anemia virus by hypericin. Photochem. Photobiol. 1991;53:169–174. doi: 10.1111/j.1751-1097.1991.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Comalada M, Ballester I, Bailon E, Sierra S, Xaus J, Galvez J, Sanchez de Medina F, Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem. Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Couladis M, Baziou P, Verykokidou E, Loukis A. Antioxidant activity of polyphenols from Hypericum triquetrifolium Turra. Phytother. Res. 2002;16:769–770. doi: 10.1002/ptr.1062. [DOI] [PubMed] [Google Scholar]
- Deng S, Palu AK, West BJ, Su CX, Zhou B-N, Jensen JC. Lipoxygenase inhibitory constituents of the fruits of Noni (Morinda citrifolia) collected in Tahiti. J. Nat. Prod. 2007;70:859–862. doi: 10.1021/np0605539. [DOI] [PubMed] [Google Scholar]
- de Waal-Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 inhibits cytokine synthesis by human monocytes: an auto-regulatory role of Il-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- Hammer KDP, Hillwig ML, Solco AKS, Dixon PM, Delate K, Murphy PA, Wurtele ES, Birt DF. Inhibition of PGE2 production by anti-inflammatory Hypericum perforatum extracts and constituents in RAW 264.7 mouse macrophage cells. J. Agric. Food Chem. 2007;55:7323–7331. doi: 10.1021/jf0710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergenliemk G, Nahrstedt A. Phenolic compounds from Hypericum perforatum. Planta Med. 2001;68:88–91. doi: 10.1055/s-2002-20053. [DOI] [PubMed] [Google Scholar]
- Juergenliemk G, Nahrstedt A. Dissolution, solubility and cooperativity of phenolic compounds from Hypericum perforaum L. in aqueous systems. Pharmazie. 2003a;58:200–203. [PubMed] [Google Scholar]
- Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla H-J, Nahrstedt A. In vitro studies indicate that miquelianin (quercetin 3-O-β-d-glucuropyranoside) is able to reach the CNS from the small intestine. Planta Med. 2003b;69:1013–1017. doi: 10.1055/s-2003-45148. [DOI] [PubMed] [Google Scholar]
- Korkina LG, Afanasev IB. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol. 1997;38:151–163. doi: 10.1016/s1054-3589(08)60983-7. [DOI] [PubMed] [Google Scholar]
- Lindahl M, Tagesson C. Selective inhibition of group II phospholipase A2 by quercetin. Inflammation. 1993;17:573–582. doi: 10.1007/BF00914195. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Walsh DT, Levi G, Perry VH. In vivo expression of cyclooxygenase-2 in rat brain following intraparenchymal injection of bacterial endotoxin and inflammatory cytokines. J. Neuropathol. Exp. Neurol. 1999;58:1184. doi: 10.1097/00005072-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Miroassay A, Onderkova H, Miroassay L, Sarissky M, Mojzis J. The effect of quercetin on light-induced cytotoxicity of hypericin. Physiol. Res. 2001;50:635–647. [PubMed] [Google Scholar]
- Murota K, Shimizu S, Chujo H, Moon J-H, Terao J. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line Caco-2. Biochem. Biophys. 2000;384:391–397. doi: 10.1006/abbi.2000.2123. [DOI] [PubMed] [Google Scholar]
- Niiro H, Otsuka T, Tanabe T, Hara S, Kuga S, Nemoto Y, Tanaka Y, Nakashima H, Kitajima S, Abe M, et al. Inhibition of interleukin-10 of inducible cyclooxygenase expression in lipopolysaccharide-stimulated monocytes: its underlying mechanism in comparison with interleukin-4. Blood. 1995;85:3736–3745. [PubMed] [Google Scholar]
- Noeldner M, Schotz K. Rutin is essential for the antidepressant activity of Hypericum perforatum extracts in the forced swimming test. Planta Med. 2002;68:577–580. doi: 10.1055/s-2002-32908. [DOI] [PubMed] [Google Scholar]
- O’Sullivan MG, Huggins EM, Meade EA, DeWitt DL, McCall CE. Lipopolysaccharide induces prostaglandin H synthase-2 in alveolar macrophages. Biochem. Biophys. Res. Commun. 1992;187:1123–1127. doi: 10.1016/0006-291x(92)91313-f. [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp.Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyszewski J, Yaktine AL, Duysen E, Blackwood D, Wang W, Au A, Birt DF. Inhibition of phorbol ester-induced AP-1-DNA binding, c-Jun protein and c-jun mRNA by dietary energy restriction is reversed by adrenalectomy in SENCAR mouse epidermis. Carcinogenesis. 2001;22:1421–1427. doi: 10.1093/carcin/22.9.1421. [DOI] [PubMed] [Google Scholar]
- Raso GM, Pacilio M, Di Carlo G, Esposito E, Pinto L, Meli R. In-vivo and in-vitro anti-inflammatory effects of Echinacea purpurea and Hypericum perforatum. J. Pharm. Pharmacol. 2002;54(10):1379–1383. doi: 10.1211/002235702760345464. [DOI] [PubMed] [Google Scholar]
- Schmitt LA, Liu Y, Murphy PA, Birt DF. Evaluation of the light-sensitive cytotoxicity of Hypericum perforatum extracts, fractions, and pure compounds. J Agric. Food Chem. 2006;54:2681–2890. doi: 10.1021/jf052344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LA, Liu Y, Murphy PA, Petrich JW, Dixon PM, Birt DF. Reduction in hypericin-induced phototoxicity by Hypericum perforatum extracts and pure compounds. J. Photochem. Photobiol. B. 2006;85(2):118–130. doi: 10.1016/j.jphotobiol.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchina DS, McCann DA, Asp JM, Johnson JA, Cunnick JE, Kaiser MS, Kohut ML. Changes in immunomodulatory properties of Echinacea spp. Root infusions and tinctures stored at 4° C for four days. Clin. Chim Acta. 2005;355:67–82. doi: 10.1016/j.cccn.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Edition 8. Ames, Iowa: Iowa State University Press; 1989. [Google Scholar]
- Spinella M. The importance of pharmacological synergy in psychoactive herbal medicines. Alt. Med. Rev. 2002;7(2):130–137. [PubMed] [Google Scholar]
- Tournaire C, Croux S, Maurette MT, Beck I, Hocquaux M, Braun AM, Oliveros E. Antioxidant activity of flavonoids: efficiency of singlet oxygen quenching. J. Photochem. Photobiol. B. 1993;19:205–215. doi: 10.1016/1011-1344(93)87086-3. [DOI] [PubMed] [Google Scholar]
- Williams FB, Sander LC, Wise SA, Girard J. Development and evaluation of methods for determination of naphthodianthrones and flavonoids in St. John’s wort. J Chrom A. 2006;1115:93–102. doi: 10.1016/j.chroma.2006.02.078. [DOI] [PubMed] [Google Scholar]


