Abstract
Enzymes play a crucial role in the progression of colorectal cancer and the development of metastases. They facilitate malignant cell invasion through the degradation of the extracellular matrix, the rupture of the basement membrane and the derangement of cell-cell adhesion. Furthermore, they promote tumour cell migration and support the evolution of metastatic lesions in the liver and other organs, through multiple molecular mechanisms, including growth factor release and angiogenesis. Urokinase plasminogen activator system, matrix metalloproteinases, heparanase and autocrine motility factor constitute important enzymatic complexes which assist colorectal cancer growth, with potential clinical applications in the diagnosis and treatment of the disease.
Keywords: colorectal cancer, metastasis, extracellular matrix, urokinase, metalloproteinase, heparanase, autocrine motility factor
Colorectal cancer is one of the commonest malignant neoplasms worldwide, with over 1 million new cases and around half a million cancer-related deaths reported in 20001. It is considered the second most lethal cancer type in the developed world and the main cause of death is the development of liver metastases. Most patients who suffer from the disease, succumb to the effects of distant metastatic lesions rather than the primary carcinoma itself2,3.
The invasion of foreign tissue by malignant cells involves their interaction with the extracellular matrix (ECM) and the basement membranes. The latter in particular, create connective tissue barriers at various steps of the metastatic process: escape from the primary tumour site, intravasation, extravasation, muscular and perineural infiltration. Basement membranes consist of collagen-type IV meshwork, laminin and heparan sulphate proteoglycans (HSPGs) and do not present pores, which would facilitate cell migration. Thus, tumour cells need to degrade these component constituents, in order to promote metastasis; then they are able to reach local vessels, intravasate and create new colonies in distant tissues4,5.
The proteolytic activity of malignant cells involves four classes of proteases: seryl-, thiol-, aspartylproteases and metalloproteinases. In addition to enhanced protease activity, high heparanase levels also correlate with tumour aggressiveness5.
High motility of malignant cells is another necessary feature in the tissue invasion and it appears to be promoted by a variety of compounds such as host and tumour secreted factors, ECM components, growth factors and hyaluronan. Motility stimulated by all these components may be defined as chemokinesis (random cell movements), chemotaxis (directed cell movements in response to concentration gradients of soluble compounds) and aptotaxis (directed cell migration towards insoluble ECM proteins)5,6.
Urokinase plasminogen activator (uPA) system
The uPA system consists of the urokinase-type plasminogen activator (uPA), its receptor (uPAR), the tissuetype plasminogen activator (tPA), the plasminogen (Plg) and plasminogen activator inhibitors 1 and 2 (PAI-1 and PAI-2)7. This biological system is implicated in multiple cell activities, including cytokine release, protease expression, adhesion, chemotaxis, proliferation and neutrophil activation for oxidant production. Consequently, it modulates growth, invasion, inflammation, angiogenesis and metastasis of multiple tumour types8–10.
uPA is a serine protease, which is secreted as an inactive single-chain zymogen named pro-uPA. When bound to uPAR, pro-uPA is cleaved by plasmin into active double-chain uPA that converts plasminogen into plasmin. The latter degrades the extracellular matrix and the basement membrane, acting either directly or through the activation of other proteases, such as pro-metalloproteinases and pro-collagenases. This sequence of actions eventually results in cancer invasion and progression (Figure 1). After hepatic resection, due to colorectal cancer metastases, uPA activity was found to be increased in the remnant parenchyma, leading to liver regeneration through ECM production and hepatocyte proliferation. A 50% resection was observed to be the threshold for intense uPA activity10–12.
Figure 1: The role of urokinase-type plasminogen activator (uPA), uPA receptor (uPAR) and plasmin in the metastatic process. Regulation of extracellular matrix (ECM) degradation, neoangiogenesis, invasion and metastasis by uPA, uPAR and plasmin7,8.
uPAR is a cysteine rich 50-60kDa extracellular glycoprotein without any transmembrane domain8. Normal tissues present little or no uPAR, although exceptions occur: The receptor is expressed in haematopoietic cells, including monocytes, neutrophils, eosinophils, T-lymphocytes, natural killer cells, dendritic cells; also in smooth muscle cells, hepatocytes, fibroblasts and placental cells. It is also over-expressed in various tumour cells, including colon cancer, liver, breast, lung, stomach, ovary, prostate and bone; additionally in several tumour assisting cells, such as endothelial cells, macrophages and fibroblasts7,9,10.
A cohort study including 354 Swedish and 255 Danish patients with rectal cancer, evaluated preoperative plasma levels of soluble uPAR. It was discovered by ELISA that the receptors concentration was significantly higher in Dukes' D patients. The study concluded that uPAR preoperative measurement could be a prognostic parameter, aiding clinical decisions on effective treatment13. Another study on 100 colorectal carcinoma patients, reported that high antigen levels of uPAR in tumour tissue correlated with liver metastases and involvement of lymph nodes14. Similarly, a Danish study, including 591 patients with colorectal cancer, evaluated preoperatively soluble uPAR and indicated a substantial increase in patients with high mortality. It was concluded that uPAR blood test independently predicted survival15. Nowadays gene therapy is practiced towards down regulation of uPAR expression in various types of tumours10.
PAIs are anti-proteases that inhibit uPA and tPA. Both PAI-1 and PAI-2 belong to the serine protease inhibitor superfamily and are involved in cancer growth and metastasis. PAI-1 is the major inhibitor of the uPA system, is synthesised primarily in endothelial cells, modulates the fibrinolytic activity in the vasculature and is widely expressed throughout tumours. PAI-2 is presented in the epidermis, monocytes and macrophages, as well as in the plasma and the placenta of pregnant women. Apart from anti-protease activity, PAI-2 presents anti-viral activity8,14.
Initially, it was expected that PAI-1 would prevent tumour growth through the inhibition of uPA. However, multiple research studies associated this molecule with poor prognosis of multiple types of cancer, increased cancer invasion and neoangiogenesis. Also, experiments where this inhibitor was absent or blocked showed that tumour growth and invasion was reduced8. Bajou et al demonstrated in vivo and in vitro that PAI-1 played a key role in tumour progression, controlled proteolysis and regulated cell migration during angiogenesis. When host cells expressed the inhibitor in normal concentration, angiogenesis was promoted. On the contrary, in the case of host absence of PAI-1, the production by tumour cells could not induce angiogenesis16. Similarly, in vitro studies with smooth muscle cells concluded that PAI-1 inhibited cell attachment. Upon administrating a PAI inhibitor, smooth muscle cell adhesion was restored and angiogenesis was reduced in vivo17. A clinical study on 206 patients with colorectal cancer, evaluated the association of PAI-1 genotypes with the disease prognosis. It was concluded that there was an increase of 4G/4G genotype in Dukes' C and D cancer, although not statistically significant18.
The ability of PAI-1 to modify cell adhesion, regulating attachment and detachment, has been suggested in order to explain its tumour promoting role. This unexpected action appears a significant observation, which is not related to its role as a protease inhibitor19. Specifically, PAI-1 can detach cells from fibronectin and type I collagen. This detachment presupposes the formation of uPA and uPAR complexes on the cell surface and their binding to matrix-engaged integrins. The subsequent binding of PAI-1 to uPA and uPAR promotes the disengagement of integrins from the ECM, thus causing cell detachment19,20. The de-adhesive properties of this inhibitor may be regulated by cytokines, growth factors and hormones, which remain to be precisely investigated19,21.
Matrix metalloproteinases (MMPs)
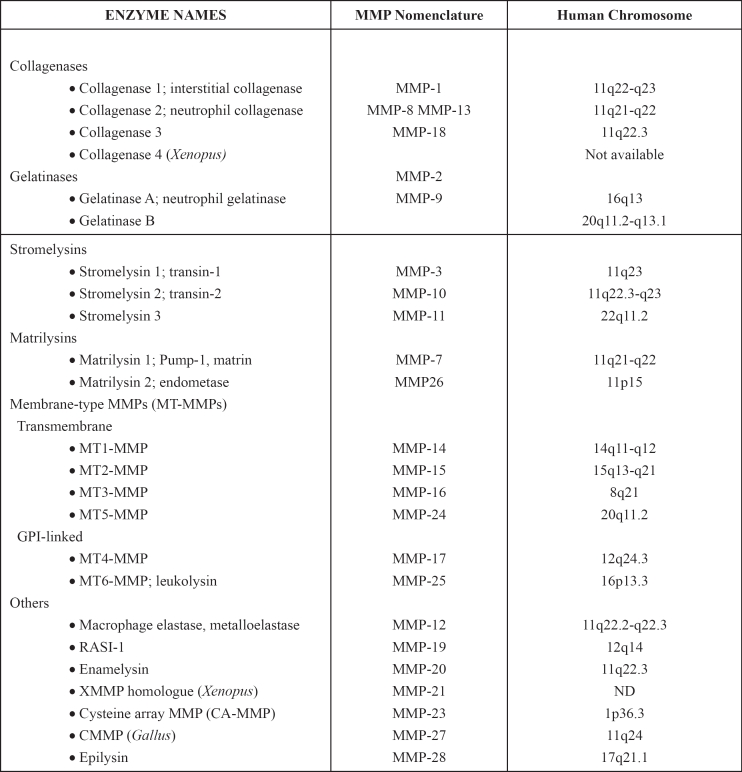
The collagenous structure of the extracellular matrix and basement membrane, as well as the dynamic balance between fibrogenesis and fibrolysis, are very important for cancer progression. MMPs are molecules which control these procedures and are implicated in cancer cell migration. They compose a family of zinc endopeptidases, which degrade extracellular matrix, but also non-matrix proteins. MMPs include more than 24 enzymes, with epilysin (MMP-28) being the newest member of the family22 (Figure 2).
Figure 2: Matrix metalloproteinase 1 (MMP-1)71 and Matrix metalloproteinase 12 (MMP-12)72, members of the matrix metalloproteinase (MMP) family. The catalytic and structural zinc ions are showed light grey, while the structural calcium ions as yellow spheres. The zinc ligands are shown in ball-and-stick representation: His218, His222 and His228 in purple. The catalytic Glu219 is shown in blue. (http://merops.sanger.ac.uk/ "Use us and cite us! Many authors find it useful to include data from MEROPS in their publications, and that is very much what we are here for, but please cite the appropriate publication as well as the URL when you do so").
Their structure, activity and substrate preferences vary considerably and are classified into numerous separate subgroups23 (Table 1). Structurally, MMPs present three common domains: a pre domain (N-terminal signal sequence), a catalytic domain (N-terminal propeptide) and a hemopexin-like domain (C-terminal Hpx). The catalytic domain contains cysteine with the Zn ion and keeps pro-MMP inactive. Metalloproteinases get activated, when the propeptide is removed by another MMP or other protease24.
Table 1: Matrix Metalloproteinases23,73.
Their actions are numerous. They contribute to the wound healing process, morphogenesis and tissue remodelling after injury, like acute myocardial infarction. Additionally, they play a key role in rheumatoid arthritis, neural diseases and cancer growth. Their ability to degrade the ECM and the basement membrane is frequently utilized by tumour cells to invade the tissues24.
The degradative ability of MMPs also facilitates cancer evolution via growth factor release and/or angiogenesis. As they produce ECM-fragments, growth factors such as tumour necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β) and insulin like growth factor (IGF) may be activated leading to tumour progression; concurrently, these factors or the ECM degradation itself, may directly or indirectly promote angiogenesis25. Although, multiple ECM fragments like tumstatin, MMP fragments such as the hemopexin like domain named PEX and plasminogen fragments like angiostatin may inhibit tumour angiogenesis through various mechanisms, including restriction of endothelial cell proliferation or migration26–28.
Cell adhesion plays a crucial role in malignant progression. Specifically, cadherins and catenins promote cell anchorage and any modification in their status may facilitate cell migration. Moreover, several studies have presented a functional link between E-cadherin and MMPs, where E-cadherin suppresses the activity of these proteinases in vivo and in vitro conditions29,30. Miyaki et al using murine experimental models reported that COKFu colon carcinoma cell lines without the expression of E-cadherin showed very low or no activity of a 62kDa gelatinase and subsequent decreased invasion, in comparison with highly E-cadherin-expressing cells. They concluded that E-cadherin not only is involved in cell-cell adhesion, but also regulates proteinase secretion, suppressing tumour invasion and growth31.
ECM includes various types of collagen, but the most common is collagen type IV. Therefore, MMP-2, -9 and -11, which regulate this type of collagen, have been studied for clinical purposes. However, the results were not clear; high levels were recorded in patients with various adenocarcinomas, with or without liver metastases, as well as in patients with liver metastases and resected primary tumour. In this last group, increased expression of MMP-2 was attributed to previous stimulation of stromal cells by the primary tumour32.
Metalloproteinase functions are regulated by endogenous inhibitors such as α2-macroglobulin and specific inhibitors, named tissue inhibitors of metalloproteinases (TIMPs). Pathological conditions occur, when MMPs and TIMPs act independently. Synthesis of MMP inhibitors is an ambitious target for cancer treatment, though with dismal outcomes. Eighty two percent of numerous synthesized matrix metalloproteinase inhibitors (MMPIs) had no clinically significant effect, seven remain in clinical trials and only one (Periostat) has been approved for clinical use by the FDA24,33,34.
Asano et al investigated the expression of multiple MMPs and their inhibitors in 112 colorectal cancer tissue samples and 11 metastatic liver lesions. They indicated a significant higher expression of MMP-1, -3, -7, -9, -10 and -11 in the tumours in comparison with normal tissue. In the metastatic foci they demonstrated lower levels of MMP-1 and -11 and higher of TIMP-1 comparing with primary cancer. They also announced that the lower expression of MMP-15 was an important risk factor for early recurrence of the disease35.
Heparanase
Heparan sulphate proteoglycans (HSPGs) are macromolecules, which consist of a protein core linked with heparin sulphate chains. They can be traced at the cytoplasm, the cell surface or may be secreted into the ECM36. Heparan sulphate (HS) chains present an extensive structural heterogeneity and they are considered the most "information dense" biopolymer in nature37. They may interact with a surprising multiplicity of molecules (>100), such as ECM proteins, growth factors, chemokines, cytokines, morphogens, coagulation factors, enzymes and their inhibitors (Table 2)38,39. The biodiversity of HS and the variety of core proteins are responsible for the multiple biological functions of HSPGs. These macromolecules regulate the stability and insolubility of ECM and therefore contribute to cell adhesion and locomotion. Furthermore, they are present in blood vessels and capillaries interfering in angiogenesis. In general, they control intracellular and extracellular responses and stand at the intersection of numerous signalling pathways38,40.
Table 2: Heparan sulphate binding interactions38,40.
FGF: Fibroblast Growth Factor, HGF: Hepatocyte Growth Factor, VEGF: Vascular Endothelial Growth Factor, TGF-β: Transforming Growth Factor-beta, NCAM: Neural Cell Adhesion Molecule, SOD: Superoxide dismutase, LPL: Lipoprotein Lipase, PN-1: Protease Nexin I, HCII: Heparin Cofactor II, MPI: Mucus Proteinase Inhibitor, HB-EGF: Heparin-Binding egf-like Epithelial Growth Factor, HBPTc: Trypanosoma cruzi Heparin Binding Protein.
Various enzymes may transform the HSPG structure and thus regulate their function: sheddases cleave the proteoglycans from the cell surface, heparanase degrades HS into biologically active fragments and endosulfatases specifically remove 6-O sulphates from HS chains36. The most studied and interesting of these enzymes in cancer research remains heparanase
Heparanase is an endo-β-D-glucuronidase, rare in normal tissue, but frequently expressed in highly metastatic tumours. This enzyme cleaves HS at specific sites, creating fragments of considerable size (5 to 10 kD, 10 to 20 sugar units long), which may be more biologically active than their ancestor heparin sulphate chains36,39.
By cleaving the HS chains, heparan degrades HSPGs and subsequently ECM, facilitating tumour cell locomotion, invasion and metastasis. Additionally, the enzyme releases factors, which are bound with HS chains, mediating cell behaviour. Angiogenic growth factors such as fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF) constitute apparent examples of trapped molecules in the ECM, which are released and activated through this enzymatic action41.
Heparan's increased expression is associated with a high metastatic capacity of various tumour cell lines and tissues including brain, oral cavity, nasopharynx, thyroid, oesophagus, stomach, gall bladder, pancreas, ovary, endometrium, bladder, prostate, multiple myeloma and acute myeloid leukaemia, liver and colon39. Various proposed mechanisms attempt to explain how heparan assists the metastatic process. Degradation of HS chains destroys natural biological barriers and facilitates tumour migration and invasion. Also, the release of HS-bound growth factors, involved in angiogenesis, such as basic fibroblast growth factor (bFGF) and VEGF may assist the creation of neovessels, which supply growing tumours or metastatic foci39,42. The upregulation of cyclooxygenase- 2 through heparanase action progresses neoangiogenesis in oesophageal cancer43, invasive breast cancer44 and signet-ring cell stomach cancer45. Furthermore, the enhancement of syndecan-1 synthesis and shedding from the cell surface, may constitute another mechanism which induces metastasis, as this proteoglycan promotes growth and angiogenesis of tumours36,46.
Heparanase is related to colon cancer progression and metastasis. Friedmann et al studied the expression of heparanase in 16 patients with colon adenocarcinoma and concluded that the most poorly differentiated carcinomas showed the highest expression of the enzyme; high expression was also noted in lung, lymph node, liver metastases, as well as in the accompanying stromal fibroblasts. In contrast, heparanase expression was low in normal tissue47. Immunohistochemical studies by Elkin et al on human colon adenocarcinoma tissue revealed that heparanase was specifically expressed in newly formed capillaries and small blood vessels, while no traces of the enzyme were detected in mature vessels42.
Heparanase inhibitors were used in several studies to inhibit the enzymes activity. Heparin and heparin species containing at least 16 sugar units both at the N and O positions showed specific heparanase inhibition. Also, other sulphated polysaccharides, such as dextran sulphate, carrageenan lambda and laminarin sulphate exercised similar inhibitory action48,49. By preventing heparanase functions, these compounds reduced the incidence of experimental metastases, neoangiogenesis and tumour cell colonisation of normal organs, like liver and lungs49,50.
Today, many new laboratory techniques are developed in order to better observe and understand heparanase biological activities. New ELISA methods and mass spectroscopy are currently used for quantitative and qualitative analysis of the enzyme42,51. Several research groups are testing heparanase inhibitors with the aim of creating an effective antitumour drug. Phosphomannopentaose sulphate (PI-88) is already being evaluated in a phase II clinical trial, in patients with advanced malignancies52. However, due to its multiple natural functions the inhibition of heparanase, apart from tumour growth, affects embryonic development, immune surveillance, anticoagulant activities, inflammation and healing process. This becomes evident by the therapeutic amplitude of PI-88, which includes antiangiogenic, antiviral, antimalarial, antiproliferative and anticoagulant activities. Multiple ongoing studies attempt to answer the existing queries and accurately control the heparanase biological functions42,50,52.
Autocrine motility factor (AMF)
This 55kDa protein was initially isolated from A2058 melanoma cells, as a tumour secreted cytokine that stimulates direct and random migration53. Experiments using molecular cloning and sequencing suggested that AMF is a multifunctional member of the ectoenzyme/exoenzyme family54. This cytokine was identified as the glycolytic enzyme posphoglucose isomerase (PGI)55, as neuroleukin (NLK), inducing growth of embryonic spinal and sensory neurons55,56 and as maturation factor, mediating human myeloid leukaemia cell differentiation57. Additionally, AMF exhibits the function of a sperm antigen-36 and a myofibril-bound serine proteinase inhibitor58.
AMF promotes cell motility via binding to a cell surface receptor named AMFR/gp78. This is a seven-transmembrane glycoprotein of 78 kDa59,60. When AMF reacts with its receptor, the latter is internalised, stimulates a pertussis toxin-sensitive G protein, activates protein kinase C (PKC) and inositol phosphate is produced; the receptor also undergoes phosphorylation. These molecular alterations are implicated in normal and tumour cell locomotion54,58. Multiple studies have revealed that the expression of AMF and its receptor is associated with increased tumour penetrating ability to normal tissues61–63. From the clinical aspect, the presence of this motility factor in urine and serum marks a worse prognosis, indicating progression in gastrointestinal, kidney and breast cancer63, as well as in colorectal cancer58,62. Nakamori et al studied the expression of AMFR in 118 patients who underwent surgical resection of colorectal cancer. They reported that patients with AMFR-positive tumours had significantly poorer survival rate. Among 101 patients who had curative resection, a significant difference in disease free survival was demonstrated among positive and negative tumours, as well64.
Carcinoma-derived AMF promoted angiogenesis, influencing endothelial cells. In vitro experiments with human umbilical vein endothelial cells (HUVECs) demonstrated that AMF induced an intensive expression of AMFR, which was hardly detected in untreated cells. This expression was associated with endothelial cell motility58. Moreover, HUVECs underwent morphogenesis and formed capillary-like tubes, by anastomosing one another. In vivo experiments in mice showed that AMF over-expressing tumour cell were able to provoke the development of new capillary blood vessels, which in turn could be prevented by specific AMF inhibitors58,65,66
Vascular endothelial growth factor (VEGF) is one of the most important angiogenic factors and AMF increases its activity by promoting the expression of Flt1, a transmembrane VEGF receptor on endothelial cells. Flt-1 expression is achieved through the activation of phosphatidylinositol 3'-kinase (PI3K) in endothelial cells through a paracrine way. This angiogenic effect, reported in various in vitro and in vivo experiments with tumour cells, assisted the metastatic process58,67,68.
An anti-apoptotic action has also been attributed to AMF. Transfected mouse fibroblasts with the AMF gene demonstrated an aggressive ability for invasion and apoptotic resistance via Akt/protein kinase B (Akt/PKB)54. Akt/PKB inactivated caspase-9 and BAD, which belong to Bcl-2 family and therefore apoptosis was suppressed54,69. Haga et al also concluded that AMF-transfected cells were apoptosis resistant. They used mitomycin C to induce apoptosis, a chemotherapeutic drug with anticancer action, and showed that AMF over-expressing cells had increased survival to apoptotic signals70.
In general, AMF is a multifuctorial protein, which interferes with tumour growth and metastasis, through multiple mechanisms (Figure 3). Numerous experiments, in vitro and in vivo, with multiple cancer types, including colorectal cancer, revealed some of its tumour regulatory actions. However, many aspects need to be elucidated and current studies are focused on the evaluation of AMF's application in cancer diagnosis and treatment58.
Figure 3: Signal pathways by autocrine motility factor (AMF). The pathways of migration and suppression of apoptosis refer to tumour cells, whereas angiogenesis pathway refers to endothelial cells. Rho-like proteins and Rac1: GTPases. Fms related tyrosine kinase (Flt-1): Vascular endothelial growth factor (VEGF) receptor on endothelial cells. BAD and Caspase-9: apoptotic enzymes. PI3K: phosphatidylinositol 3'-kinase. Akt/PKB: Akt protein kinase B54,58.
Conclusions
Tumours, including colorectal cancer, progress using a variety of enzymatic systems. The functions of these enzymes are multipotent and involve interactions with the extracellular matrix and the basement membrane, tissue remodelling, alterations in the cell adhesion and migration, vessel formation and the regulation of apoptosis. Accumulating clinical and experimental data show that enzymes significantly affect not only the invasion of colorectal cancer cells in the primary site, but also the development of distant metastatic lesions, predominantly in the liver.
Urokinase plasminogen activator, matrix metalloproteinases, heparanase and autocrine motility factor, attract great research interest, due to their enzymatic activity in various stages of colorectal cancer. Clinical studies attempt to exploit current knowledge toward the discovery of effective pharmaceutical factors, which could modulate the activity of these enzymes in favour of patients. However, as they intervene in numerous pathological and physiological functions within the human body, therapeutic applications are slowly developed, because they require time-consuming and cautiously designed clinical trials.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Rothbarth J, van de Velde CJ. Treatment of liver metastases of colorectal cancer. Ann Oncol. 2005;16:144–149. doi: 10.1093/annonc/mdi702. [DOI] [PubMed] [Google Scholar]
- 4.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 5.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 6.Aznavoorian S, Stracke ML, Krutzsch H, Schiffmann E, Liotta LA. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazar AP, Henkin J, Goldfarb RH. The urokinase plasminogen activator system in cancer: implications for tumor angiogenesis and metastasis. Angiogenesis. 1999;3:15–32. doi: 10.1023/a:1009095825561. [DOI] [PubMed] [Google Scholar]
- 8.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Ge Y, Elghetany MT. Urokinase plasminogen activator receptor (CD87): something old, something new. Lab Hematol. 2003;9:67–71. [PubMed] [Google Scholar]
- 10.Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25:33–39. doi: 10.1016/j.tibtech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med Res Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Mangnall D, Smith K, Bird NC, Majeed AW. Early increases inplasminogen activator activity following partial hepatectomy in humans. Comp Hepatol. 2004;3:11. doi: 10.1186/1476-5926-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riisbro R, Christensen IJ, Nielsen HJ, Brunner N, Nilbert M, Fernebro E. Preoperative plasma soluble urokinase plasminogen activator receptor as a prognostic marker in rectal cancer patients. An EORTC-Receptor and Biomarker Group collaboration. Int J Biol Markers. 2005;20:93–102. [PubMed] [Google Scholar]
- 14.Abe J, Urano T, Konno H, et al. Larger and more invasive colorectal carcinoma contains larger amounts of plasminogen activator inhibitor type 1 and its relative ratio over urokinase receptor correlates well with tumor size. Cancer. 1999;86:2602–2611. doi: 10.1002/(sici)1097-0142(19991215)86:12<2602::aid-cncr4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Stephens RW, Nielsen HJ, Christensen IJ, et al. Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. J Natl Cancer Inst. 1999;91:869–874. doi: 10.1093/jnci/91.10.869. [DOI] [PubMed] [Google Scholar]
- 16.Bajou K, Maillard C, Jost M, et al. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23:6986–6990. doi: 10.1038/sj.onc.1207859. [DOI] [PubMed] [Google Scholar]
- 17.Leik CE, Su EJ, Nambi P, Crandall DL, Lawrence DA. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J Thromb Haemost. 2006;4:2710–2715. doi: 10.1111/j.1538-7836.2006.02244.x. [DOI] [PubMed] [Google Scholar]
- 18.Loktionov A, Watson MA, Stebbings WS, Speakman CT, Bingham SA. Plasminogen activator inhibitor-1 gene polymorphism and colorectal cancer risk and prognosis. Cancer Lett. 2003;189:189–196. doi: 10.1016/s0304-3835(02)00556-6. [DOI] [PubMed] [Google Scholar]
- 19.Czekay RP, Loskutoff DJ. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp Biol Med. 2004;229:1090–1096. doi: 10.1177/153537020422901102. [DOI] [PubMed] [Google Scholar]
- 20.Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;60:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci. 2006;119:3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 23.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 24.Ganea E, Trifan M, Laslo AC, Putina G, Cristescu C. Matrix metalloproteinases: useful and deleterious. Biochem Soc Trans. 2007;35:689–691. doi: 10.1042/BST0350689. [DOI] [PubMed] [Google Scholar]
- 25.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Marneros AG, Olsen BR. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol. 2001;20:337–345. doi: 10.1016/s0945-053x(01)00151-2. [DOI] [PubMed] [Google Scholar]
- 27.Gately S, Twardowski P, Stack MS, et al. The mechanism of cancer-mediated conversion of plasminogen to the angiogenesis inhibitor angiostatin. Proc Natl Acad Sci U S A. 1997;94:10868–10872. doi: 10.1073/pnas.94.20.10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 29.Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51:445–457. [PubMed] [Google Scholar]
- 30.Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P. Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol. 2004;49:179–186. doi: 10.1016/j.critrevonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Miyaki M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Konishi M, Takeichi M. Increased cell-substratum adhesion, and decreased gelatinase secretion and cell growth, induced by E-cadherin transfection of human colon carcinoma cells. Oncogene. 1995;11:2547–2552. [PubMed] [Google Scholar]
- 32.Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: A review. J Surg Oncol. 2006;94:68–80. doi: 10.1002/jso.20558. [DOI] [PubMed] [Google Scholar]
- 33.Garvin J. "Research is in my blood": Dr Lorne Golub, developer of Periostat, receives the ADAs Gold Medal Award for Excellence in Dental Research. J Am Dent Assoc. 2006;137:1281–1282. doi: 10.14219/jada.archive.2006.0387. [DOI] [PubMed] [Google Scholar]
- 34.Oba K, Konno H, Tanaka T, et al. Prevention of liver metastasis of human colon cancer by selective matrix metalloproteinase inhibitor MMI-166. Cancer Lett. 2002;175:45–51. doi: 10.1016/s0304-3835(01)00726-1. [DOI] [PubMed] [Google Scholar]
- 35.Asano T, Tada M, Cheng S, et al. Prognostic values of matrix metalloproteinase family expression in human colorectal carcinoma. J Surg Res. 2008;146:32–42. doi: 10.1016/j.jss.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 37.Venkataraman G, Shriver Z, Raman R, Sasisekharan R. Sequencing complex polysaccharides. Science. 1999;286:537–542. doi: 10.1126/science.286.5439.537. [DOI] [PubMed] [Google Scholar]
- 38.David G. Integral membrane heparan sulfate proteoglycans. Faseb J. 1993;7:1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- 39.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 41.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elkin M, Ilan N, Ishai-Michaeli R, et al. Heparanase as mediator of angiogenesis: mode of action. Faseb J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 43.Okawa T, Naomoto Y, Nobuhisa T, et al. Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2. Clin Cancer Res. 2005;11:7995–8005. doi: 10.1158/1078-0432.CCR-05-1103. [DOI] [PubMed] [Google Scholar]
- 44.Imada T, Matsuoka J, Nobuhisa T, et al. COX-2 induction by heparanase in the progression of breast cancer. Int J Mol Med. 2006;17:221–228. [PubMed] [Google Scholar]
- 45.Ohtawa Y, Naomoto Y, Shirakawa Y, et al. The close relationship between heparanase and cyclooxygenase-2 expressions in signet-ring cell carcinoma of the stomach. Hum Pathol. 2006;37:1145–1152. doi: 10.1016/j.humpath.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Macleod V, Miao HQ, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 47.Friedmann Y, Vlodavsky I, Aingorn H, et al. Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma. Evidence for its role in colonic tumorigenesis. Am J Pathol. 2000;157:1167–1175. doi: 10.1016/S0002-9440(10)64632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlodavsky I, Mohsen M, Lider O, et al. Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Invasion Metastasis. 1994;14:290–302. [PubMed] [Google Scholar]
- 49.Miao HQ, Elkin M, Aingorn E, Ishai-Michaeli R, Stein CA, Vlodavsky I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int J Cancer. 1999;83:424–431. doi: 10.1002/(sici)1097-0215(19991029)83:3<424::aid-ijc20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 50.Goldshmidt O, Zcharia E, Abramovitch R, et al. Cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and metastasis. Proc Natl Acad Sci U S A. 2002;99:10031–10036. doi: 10.1073/pnas.152070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafat I, Zcharia E, Nisman B, et al. An ELISA method for the detection and quantification of human heparanase. Biochem Biophys Res Commun. 2006;341:958–963. doi: 10.1016/j.bbrc.2006.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fairweather JK, Hammond E, Johnstone KD, Ferro V. Synthesis and heparanase inhibitory activity of sulfated mannooligosaccharides related to the antiangiogenic agent PI-88. Bioorg Med Chem. 2008;16:699–709. doi: 10.1016/j.bmc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 53.Liotta LA, Mandler R, Murano G, et al. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986;83:3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanagawa T, Funasaka T, Tsutsumi S, Watanabe H, Raz A. Novel roles of the autocrine motility factor/phosphoglucose isomerase in tumor malignancy. Endocr Relat Cancer. 2004;11:749–759. doi: 10.1677/erc.1.00811. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996;56:2960–2963. [PubMed] [Google Scholar]
- 56.Gurney ME, Apatoff BR, Spear GT, et al. Neuroleukin: a lymphokine product of lectin-stimulated T cells. Science. 1986;234:574–581. doi: 10.1126/science.3020690. [DOI] [PubMed] [Google Scholar]
- 57.Xu W, Seiter K, Feldman E, Ahmed T, Chiao JW. The differentiation and maturation mediator for human myeloid leukemia cells shares homology with neuroleukin or phosphoglucose isomerase. Blood. 1996;87:4502–4506. [PubMed] [Google Scholar]
- 58.Funasaka T, Raz A. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev. 2007;26:725–735. doi: 10.1007/s10555-007-9086-7. [DOI] [PubMed] [Google Scholar]
- 59.Silletti S, Watanabe H, Hogan V, Nabi IR, Raz A. Purification of B16-F1 melanoma autocrine motility factor and its receptor. Cancer Res. 1991;51:3507–3511. [PubMed] [Google Scholar]
- 60.Shimizu K, Tani M, Watanabe H, et al. The autocrine motility factor receptor gene encodes a novel type of seven transmembrane protein. FEBS Lett. 1999;456:295–300. doi: 10.1016/s0014-5793(99)00966-7. [DOI] [PubMed] [Google Scholar]
- 61.Nabi IR, Watanabe H, Raz A. Autocrine motility factor and its receptor: role in cell locomotion and metastasis. Cancer Metastasis Rev. 1992;11:5–20. doi: 10.1007/BF00047599. [DOI] [PubMed] [Google Scholar]
- 62.Filella X, Molina R, Jo J, Mas E, Ballesta AM. Serum phosphohexose isomerase activities in patients with colorectal cancer. Tumour Biol. 1991;12:360–367. doi: 10.1159/000217737. [DOI] [PubMed] [Google Scholar]
- 63.Baumann M, Kappl A, Lang T, Brand K, Siegfried W, Paterok E. The diagnostic validity of the serum tumor marker phosphohexose isomerase (PHI) in patients with gastrointestinal, kidney, and breast cancer. Cancer Invest. 1990;8:351–356. doi: 10.3109/07357909009012053. [DOI] [PubMed] [Google Scholar]
- 64.Nakamori S, Watanabe H, Kameyama M, et al. Expression of autocrine motility factor receptor in colorectal cancer as a predictor for disease recurrence. Cancer. 1994;74:1855–1862. doi: 10.1002/1097-0142(19941001)74:7<1855::aid-cncr2820740705>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Abe T, Okamura K, Ono M, et al. Induction of vascular endothelial tubular morphogenesis by human glioma cells. A model system for tumor angiogenesis. J Clin Invest. 1993;92:54–61. doi: 10.1172/JCI116599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Passaniti A, Taylor RM, Pili R, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 67.Kanno S, Oda N, Abe M, et al. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 68.Soker S, Kaefer M, Johnson M, Klagsbrun M, Atala A, Freeman MR. Vascular endothelial growth factor-mediated autocrine stimulation of prostate tumor cells coincides with progression to a malignant phenotype. Am J Pathol. 2001;159:651–659. doi: 10.1016/S0002-9440(10)61736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 70.Haga A, Funasaka T, Niinaka Y, Raz A, Nagase H. Autocrine motility factor signaling induces tumor apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome expression. Int J Cancer. 2003;107:707–714. doi: 10.1002/ijc.11449. [DOI] [PubMed] [Google Scholar]
- 71.Cawston TE. Collagenase 1. 2nd ed. London: Elsevier; 2004. pp. 472–480. [Google Scholar]
- 72.Shapiro SD, Hartzell WO, Senior RM. Macrophage elastase. 2nd ed. London: Elsevier; 2004. pp. 504–544. [Google Scholar]
- 73.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]