Abstract
Background: To compare the efficacy of oral and intravenous administration of iron supplements for treating postpartum anemia.
Methods: One hundred and four anemic postpartum women were studied prospectively. The criteria for the diagnosis of anemia were Hb <8 gr/dl and ferritine < 10μg/dl. They were randomised into two groups. Group A constisted of 78 women who received i.v. a total amount of 300 mg iron sucrose in three days. Group B consisted of 26 women, who received orally 800 mg iron proteinsuccinylate daily for four weeks.
Results: At the end of the study, in group A the increase of Hb mean level was 4.6 gr/dl and of ferritin mean level was 105 mg/L. In group B the increase in hemoglobin mean level was 2.3 gr/dl and ferritin mean level was 68 mg/L. There was significant difference in the increase of hemoglobin level (p= 0.0001) and also in the increase in ferritin level (p= 0.0004) between the two groups.
Conclusion: Intravenous administration of iron sucrose seems to be safe and it helps postpartum women to recover early from anemia.
Keywords: pregnancy, anemia, iron sucrose, iron proteinsuccinylate
Iron deficiency during pregnancy and postpartum could be due to insufficient absorption and to increased needs resulting to chronic iron deficiency and anemia1. The human body does not have a mechanism of getting rid of extra iron amount and the mechanism of iron absorption plays a crucial role in iron homeostasis2. During pregnancy the needs for iron are increased due to the fetus, the placenta and the increased volume of maternal erythrocytes. Women in the reproductive age frequently have anemia and iron deficiency due to menstrual loss. Frequently these women are already anemic by the time they get pregnant3,4.
During the period of pregnancy the extra needs on iron are about 1000mg in total and they should be replaced in order to avoid severe anemia. The usual management is the replacement of iron by oral supplementations. Blood transfusion is the last resort used only in very severe cases of anemia with symptomatic patients5,6. The oral therapy though is time consuming and probably not enough in severe cases of anemia. On the other hand blood transfusion although it can promptly and reliably treat anemia, entails a lot of dangers like cross reactions and viral infections. In order to avoid these side effects intravenous administration of iron sucrose could be a convenient and reliable solution.
Materials and methods
In our prospective study, one hundred and four postpartum women with severe iron deficiency anemia were randomised to receive i.v. or oral iron treatment. Seventy seven of them had a normal vaginal delivery and the rest 27 had a caesarean section. The women were divided randomly in two groups. Demographic data were not matched. Group A consisted of 78 women and group B of 26 women. All the caesarean sections were performed by a consultant obstetrician and were elective. The normal deliveries were all performed by senior midwifes. The estimated blood loss was between 500-800 mls for the sections and not significant in all cases of normal deliveries. From the study we excluded cases with a diagnosis of placenta previa, placenta abruption, pre-eclampsia and clotting disorders. All women who participated were lactating postpartum and had amenorrhea during the study period. Also, after laboratory investigations and hematology assistance all other types of anemia hereditary or acquired were excluded from the study. The main criteria in order to administer intravenous iron was hemoglobin levels < 8 gr/L and ferittin levels < 10 μ/L. In group A we used for intravenous infusion 100 mg iron sucrose daily, diluted in NaCl 0.9%, for three days. The administration was performed slowly within two hours, in order to avoid any adverse reactions. Group B was the control group and we used oral supplementation of iron proteinsuccinylate 800 mg daily for one month. The treatment for group B lasted for 28 days.
One week after the initiation of the treatment we measured hemoglobin, ferritine, SGOT, SGPT blood levels and proteinuria of both groups. The same analysis was performed four weeks later.
For statistical analysis of the results between the two groups we used the unpaired graph-pad t-test.
Results
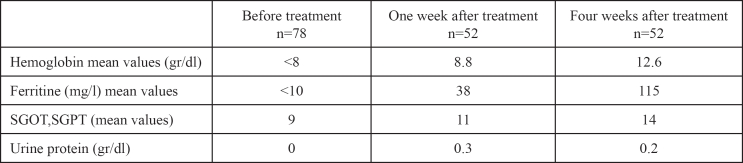
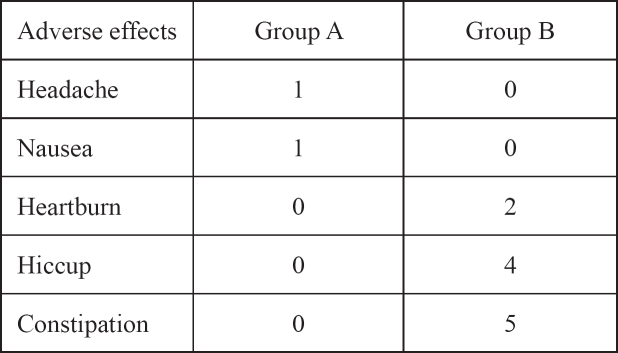
Fifty two out of seventy eight women (66%) from the group A attended the outpatient one month later and the rest 26 (34%) failed to attend. In group B only 20 out of 26 (77%) women kept their follow up appointments. One week after the initiation of the treatment in the group A the mean values of hemoglobin and ferritine blood levels were 8.8 gr/dl and 38 μg/l correspondingly. Four weeks later, the mean values of hemoglobin were 12.6 gr/dl and of ferritine 115 μg/l (Table 1). In group B the increase in hemoglobin and ferritine levels were significantly lower. Hemoglobin mean levels were 8.1 gr/dl and 10.3 gr/dl in the first and fourth week respectively. As for ferritin levels the mean values after the first week of treatment were 19 μg/l and after the fourth week 78 μg/l (Table 2). No abnormal blood results have been detected concerning liver function enzymes and there was no proteinuria. The adverse effects from the iron treatment were mild but more prominent in group B mostly constipation and bloatin (Table3). There was significant difference in the increase of hemoglobin levels p= 0.0001 (group A CI: 12.04-13.15, SD: 0.44, mean Hb increase 4.6gr/dl and group B CI: 9.71-10.88, SD: 0.47, mean Hb increase 2.3 gr/dl)) and also in the increase in ferritin levels p=0.0004 between the two groups (group A CI: 101-128, SD: 11.1, mean ferritin increase 105 mg/L and group B CI:66-89, SD: 9, mean ferritin increase 68 mg/L).
Table 1: Group A; mean values of blood results before and after intravenous iron treatment.
Table 2: Group B; mean values of blood results before and after oral iron treatment.
Table 3: Adverse effects of iron treatment.
Discussion
Menstrual blood loss, pregnancy and delivery are the main causes of anemia in young women. About 30% of anemic women have iron deficiency anemia with hemoglobin levels bellow 10 gr/dl, and about 10% of anemic women had hemoglobin levels bellow 8gr/dl. In about 5% of all deliveries the postpartum hemorrhage is significantly high, more than 1000 mls8. In the past, blood transfusion was the only available method in order to treat severe anemic cases. Blood transfusion is a reliable method with excellent results in the treatment of anemia but also with high risk for transmition of viral infections (HIV, HCV, HBs, CMV) and serious transfusion cross reactions9.
Administration of oral iron supplementations is not sufficiently enough in order to reverse anemia promptly, due to the limited absorbsion, the gastrointestinal symptoms and the poor compliance for long treatment of the patients. Treatments with recombinant human erythropoietin could be useful for cases of chronic inflammation when erythropoiesis is reduced. In cases of iron deficiency anemia the combination of iron supplementations and erythropoietin is not preventing iron loss and is not increasing the endogenous erythropoiesis. On the contrary high iron levels in plasma circulation after simultaneous intravenous administration of iron and erythropoietin, is essential for stimulation of erythropoiesis10.
Intravenous iron treatment is indicated for patients with poor compliance in oral supplementations, in cases with poor iron absorption (bowel operations, or diseases), in patients with severe renal impairment, and in postpartum hemorrhage11,12. Recent evidence suggest that iron sucrose can be detected in high levels in the liver circulation and marrow within 5 minutes after intravenous administration. The time interval is 5 to 6 hours and the renal metabolism is minimal, less than 5% of the total dose. These data lead to the conclusion that iron sucrose is metabolically available in only a few hours after administration. This way iron is engaged exclusively from the reticulate liver cells, transferrin and apoferritin in the marrow and spleen. Then it is quickly metabolized and it is available for erythropoiesis and inversion of anemia13.
In our study after four weeks and with a total dose of i.v. 300 mg of iron sucrose there was complete reversal of anemic status in all women of group A. In group B there was improvement of anemia which was not as significant as in group A, though.
It is already known that intravenous administration of excessive dose of iron might cause liver necrosis, renal, suprarenal and pulmonary damage. The presence of iron sucrose in the plasma circulation is associated with absence of any undesirable effect to the patients. This absence of side effects is partly due to the lower allergenic effect of the sucrose complex because of the very slow release of elementary iron from the complex13,14. Also the accumulation of iron-sucrose in organic parenchyma is much lower compared to iron-dextrans and iron-gluconate13,14. In addition, incorporation into the bone marrow for erythropoiesis is faster than other complexes13,15. Iron sucrose is quite safe for the liver in daily doses of 100 mg, in comparison with other iron supplementations. Rare anaphylactic reactions because of the use of iron sucrose have been reported in about 0.002% of cases14.
Conclusion
From our study we conclude that i.v. iron sucrose compared to oral iron administration is more efficient in treating postpartum anemia without any toxic effects and with minimal anaphylactic reactions.
References
- 1.Momen AK, Meshari A, Nuaim L, et al. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Reprod Biol. 1996;69:121–124. doi: 10.1016/0301-2115(95)02538-3. [DOI] [PubMed] [Google Scholar]
- 2.Ragip A, Unlubilgin E, Kanderim O, Yalvac S, Cakir L, Haberal A. Intranenous versus oral iron treatment of anemia in pregnancy:a randomized trial. Obst Gynecol. 2005;106:1335–1340. doi: 10.1097/01.AOG.0000185260.82466.b4. [DOI] [PubMed] [Google Scholar]
- 3.Breymann C. Modern therapy concepts of severe anemia in pregnancy and postpartum. Prevention and management of anemia in pregnancy and postpartum hemorrhage. Zurich, Switzerland: Schellenberg Druck AC; 1998. pp. 107–121. [Google Scholar]
- 4.Danielson B, Geisser P, Schneider W. Iron therapy with special emphasis on intravenous administration. 1SBN3. 1996;85819:223–226. [Google Scholar]
- 5.Milman N, Bergholt T, Byg K, Eriksen L, Graudal N. Iron status and iron balance during pregnancy, a critical reappraisal of iron supplementation. Acta Obstet Gynecol Scand. 1999;78:749–757. [PubMed] [Google Scholar]
- 6.Mahomed K. Iron and folate supplementation in pregnancy. In The Cochrane Library. 2002. Oxford: Update Software. [DOI] [PubMed]
- 7.Broche DE, Gay C, Armand-Branger S, Grangeasse L, Terzibachian J. Acute postpartum anaemia. Clinical practice and interest of intravenous iron. Gynecol Obstet Fert. 2004;32:613–619. doi: 10.1016/j.gyobfe.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Bhandal N, Russell R. Intravenous versus oral iron therapy for postpartum anaemia. BJOG. 2006;113:1248–1252. doi: 10.1111/j.1471-0528.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 9.Bashiri A, Burstein E, Sheiner E, Mazor M. Anemia during pregnancy and treatment with intravenous iron: review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003;110:2–7. doi: 10.1016/s0301-2115(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 10.Messer J, Escande B, Matis J. Erythropoietin and iron in the anemia of prematurity. TATM. 1:15–17. [Google Scholar]
- 11.Breymann C. Treatment of iron deficiency anaemia in pregnancy and postpartum with special focus on intravenous iron sucrose complex. J Med Assoc Thai. 2005;88:108–109. [PubMed] [Google Scholar]
- 12.Gravier A, Descargues G, Marpeau L. Comment eviter les transfusions dans le post-partum interet d' une supplementation martiale par voie intraveineuse. J Gynecol Obstet Biol Reprod. 1999;28:77–78. [PubMed] [Google Scholar]
- 13.Petewusnyk G, Huch R, Huch A, Breymann C. Parenteral iron therapy in obstetrics: 8 years experience with iron-sucrose complex. Br J Nutr. 2002;88:3–10. doi: 10.1079/BJNBJN2002577. [DOI] [PubMed] [Google Scholar]
- 14.Bailie G, Clark J, Lane CE, Lane PL. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443–1449. doi: 10.1093/ndt/gfh820. [DOI] [PubMed] [Google Scholar]
- 15.Krafft A, Perewusnyk G, Hanseler E, et al. Effect of post partum iron supplementation on red cell and iron parameters in nonanaemic irondeficient women: a randomized placebo-controlled study. BJOG. 2005;112:445–450. doi: 10.1111/j.1471-0528.2005.00301.x. [DOI] [PubMed] [Google Scholar]