Abstract
Non-alcoholic fatty liver disease (NAFLD) is a clinicopathologic entity increasingly recognized as a major health burden in developed countries. It includes a spectrum of liver damage ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), advanced fibrosis, and rarely, progression to cirrhosis. Recent studies emphasize the role of insulin resistance, oxidative stress and subsequent lipid peroxidation, proinflammatory cytokines, adipokines and mitochondrial dysfunction in the development and progression of NAFLD. Furthermore, accumulating evidence supports an association between NAFLD and metabolic syndrome. Although the data are mainly epidemiological, the pathogenesis of NAFLD and metabolic syndrome seems to have common pathophysiological mechanisms, with focus on insulin resistance as a key factor. This review summarizes the current knowledge on the epidemiology, pathophysiology and diagnosis of both NAFLD and metabolic syndrome and the findings that strongly support the association of nonalcoholic fatty liver disease as a possible component in the cluster of metabolic syndrome.
Keywords: non alcoholic fatty liver disease, non alcoholic steatohepatitis, metabolic syndrome
Non alcoholic fatty liver disease (NAFLD) is the most common liver disease since its prevalence is estimated to be 20-30% in general population of Western countries1. NAFLD occurs as a histological spectrum of disease and includes the subtypes of simple steatosis and nonalcoholic steatohepatitis (NASH). It was thought to be a benign condition but is now increasingly recognized as a major cause of liver-related morbidity and mortality. Studies introduced that NAFLD may progress to cirrhosis, liver failure, and hepatocellular carcinoma2. It has been shown that NAFLD is strongly associated to the features of metabolic syndrome. Insulin resistance is a key pathogenic factor in both NAFLD and metabolic syndrome. Available data from clinical, experimental and epidemiological studies indicate that NAFLD may be the hepatic manifestation of metabolic syndrome3.
Metabolic syndrome
Definition – Diagnosis
The metabolic syndrome is a clustering of risk factors that greatly increases an individual's probability for developing atherosclerotic cardiovascular disease (ASCVD), type 2 diabetes mellitus and chronic kidney disease. The predominant underlying risk factors appear to be abdominal obesity, atherogenic dyslipidaemia, hypertension, elevated plasma glucose, a prothrombotic state, and a proinflammatory state4–7.
Several organizations have attempted to formulate the definition of metabolic syndrome and used simple criteria for its diagnosis, but it is beyond the scope of the present review to delineate the published definitions8–12. In 2001 the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) introduced simple clinical criteria which are widely adopted since they are simple to use in clinical practice and since a large number of studies evaluated their reliability13. In 2005 the American Heart Association (AHA) and the National Heart Lung and Blood Institute (NHLBI) updated the ATP III criteria with minor modifications5. Thus, the metabolic syndrome is identified by the presence of three or more of the following components: abdominal obesity (waist circumference >102 cm in men, >88 cm in women), elevated triglycerides (>150 mg/dl or on drug treatment for elevated triglycerides), reduced HDL-C level (<40 mg/dl in men, <50 mg/ dl in women or on drug treatment for reduced HDL-C ), hypertension (systolic blood pressure >130 mmHg or diastolic blood pressure >85 mm Hg or on antihypertensive drug treatment) and impaired fasting glucose (100 - 125 mg/dl or on antidiabetic drug treatment)5.
The lack of a standard definition makes comparisons between studies difficult. Depending on the definition used, estimates of the prevalence of the metabolic syndrome will differ. However, studies comparing the definitions reported that all definitions will identify persons at increased risk for diabetes, cardiovascular disease, and all-cause mortality7,10. Data indicate that the metabolic syndrome prevalence varies widely across populations. According to the National Health and Examination Survey (NHANES) III (1988-1994) and the NHANES 1999-2000, the age-adjusted prevalence of the MS was 24.1% and 27% respectively14. In Greece during 2003 the MetS-Greece study was carried out and the age- adjusted prevalence of the metabolic syndrome in the general population was 23.6%. This was similar in men (24.2%) and women (22.8%), but the prevalence increased with age in both sexes. This can be attributed to a similar age-related trend in each of the components of the metabolic syndrome. Most individuals with MS had three components of the syndrome (61%), 29% had four and 10% had all five components.
The most common abnormalities were abdominal obesity (72%) and arterial hypertension (66%). The prevalence of elevated glucose (including patients with diabetes mellitus) was unexpectedly high (53%). The prevalence of elevated triglyceride levels among subjects with MS was 62% and that of low HDL-C levels was 54%15.
Pathophysiology
Insulin resistance and compensatory hyperinsulinaemia have central etiologic roles in the development of MS16. Evidence from animal models as well as human studies have identified hepatic very low density lipoprotein (VLDL) overproduction as a critical underlying factor in the development of hypertriglyceridaemia, one of the main features of MS. The reduction of HDL-cholesterol is a consequence of changes in HDL composition and metabolism17. Obesity has been also described as the central causative component in the development of the MS. In both muscle cells and adipocytes of obese individuals, insulin binding to its receptor, receptor phosphorylation, tyrosine kinase activity, and phosphorylation of IRSs are reduced. Increased adipose energy storage in obesity results in increased FFA flux to other tissues and increased triglyceride storage in these tissues, which promote insulin resistance and other adverse effects18.
Accumulated visceral adipose tissue produces and secretes a number of adipocytokines, such as TNF-α and IL-6, which induce development of hypertension19. Individuals with MS have significantly higher rate of sodium and water reabsorption at the proximal tubular level20. Insulin promotes endothelin-1 (ET-1) production from endothelial cells as well as ET-1 action in vascular smooth muscle cells, causing vasoconstriction and increased smooth muscle cells proliferation21,22. Insulin also increases the activity of the sympathetic nervous system23.
Chronic inflammation is frequently associated with the MS and the main inflammatory mediators are adipocytokines and FFAs. Proinflammatory cytokines that have been associated with MS include CRP, TNF-α, IL-6 and others and they result in more insulin resistance and lipolysis of adipose tissue triglyceride stores, in enhanced hepatic glucose and VLDL production. Cytokines and FFA also increase the production of fibrinogen and plasminogen activator inhibitor-1 (PAI-1) by the liver that complements the overproduction of PAI-1 by adipose tissue, so inducing pro-thrombotic state24.
Non Alcoholic Fatty Liver Disease
Definition
NAFLD is a clinicopathologic entity with wide histological spectrum which includes simple steatosis and steatohepatitis (NASH). Steatosis is determined by estimating the proportion of hepatocytes containing fat droplets. The suggested lower threshold is 5% of hepatocytes. Features of steatohepatitis include hepatocellular injury (ballooning, apoptosis/necrosis, Mallory's hyaline, giant mitochondria), inflammation and fibrosis (perisinusoidal, pericellular)25,26. There are different suggestions on the level of alcohol consumption that can reliably distinguish between alcoholic fatty liver and NAFLD. Many centres accept that the maximum allowable level of alcohol intake for definition of NAFLD is 2 standard drinks a day (140 g ethanol/week) for men, and one standard drink a day (70 g ethanol/week) for women27.
NAFLD may be categorized as primary or secondary depending on the underlying pathogenesis. Primary NAFLD is associated with insulin resistance and metabolic syndrome. Other conditions associated with NAFLD are total parenteral nutrition, acute starvation, abdominal surgery (e.g. extensive small bowel resection, biliopancreatic diversion, and jejunal bypass), use of several drugs (e.g. amiodarone, tamoxifen, glucocorticoids, synthetic estrogens, diltiazem, aspirin, methotrexate, highly active antiretroviral therapy). It is also associated with hepatitis C, HIV and metabolic disorders i.e. hypobetalipoproteinemia, lipodystrophy, hypopituitarism, hypothalamic obesity, Weber-Christian syndrome, acute fatty liver of pregnancy, Reyes syndrome and Mauriac syndrome28,29. Studies indicate that occupational exposure to organic solvents may play a role in the development of NAFLD30 and that women with polycystic ovary syndrome may have an increased prevalence of non alcoholic fatty liver disease31.
Epidemiology – Natural History
Attempts to screen for NAFLD in the general population have been limited by the low accuracy of non-invasive tools and self-reported ethanol ingestion histories32. Despite the limitations studies suggest that 20-30% of individuals in Western countries have NAFLD1. NAFLD may represent the majority of unexplained cases of aminotrasferase elevation33. The prevalence increases from 16.5% in lean persons to 75% in obese persons34. The disease is reported in all age groups, even in paediatric patients in which is strongly associated with obesity35. The prevalence of NAFLD also increases with age and the highest prevalence is in those between 40 and 49 years old. Whereas in older studies NAFLD was more frequent in women, the opposite was found in recent series. However, these studies are biased by their selection criteria and especially by the use of an elevated ALT as an entry criterion. The Dionysos study support that gender is not a risk factor for NAFLD in general population1,36. Studies in USA report that the frequency of NAFLD varies significantly with ethnicity (45% in Hispanics, 33% in whites, 24% in blacks)37. Obesity is present in the majority of individuals with NAFLD2. It is an independent risk factor and it is strongly associated with the progression of the disease1,38. The role of central adiposity seems crucial. Visceral fat is an important source of triglycerides leading to steatosis. This probably explains the presence of generally lean, centrally obese individuals with NAFLD 3,39. The prevalence increases in subjects with impaired glucose tolerance (43%) and in subjects with newly diagnosed diabetes mellitus (62%)40. In a prospective study of 100 patients with type 2 diabetes mellitus the incidence of NAFLD was 49%, confirming that diabetes mellitus is a strong independent risk factor of NAFLD41. Insulin resistance and hyperinsulinemia are the laboratory findings most closely associated with the presence of NAFLD in a large series of patients, even in lean subjects with normal glucose tolerance1,3,42. Hypertension and especially systolic hypertension is also an independent predictor of NAFLD43.
Despite being common and potentially serious, the natural history of NAFLD remains poorly clarified. Mortality is significantly increased among patients with NAFLD compared with the expected mortality of the general population of same age and sex. Liver-related death is a leading cause of mortality, although the absolute risk is low2. Patients with simple steatosis have a relatively benign liver-related prognosis with 1.5% developing cirrhosis and 1% dying from liver-related causes over one to two decades44. In contrast, 30-50% of individuals with steatohepatitis will develop fibrosis, 15% will develop cirrhosis and 3% will progress to terminal liver failure3,38. Studies report that older age, obesity, and presence of diabetes mellitus are independent predictors of severe hepatic fibrosis in patients with nonalcoholic steatohepatitis (NASH)45,46. An important observation is that many patients with cryptogenic cirrhosis have had misdiagnosed NAFLD. This is due to the decrease of histological evidence of hepatic steatosis as the disease progresses to cirrhosis2. Finally NAFLD may account for a substantial proportion of cases of hepatocellular carcinoma (HCC). A study reports that NAFLD accounts for at least 13% of the cases of HCC47.
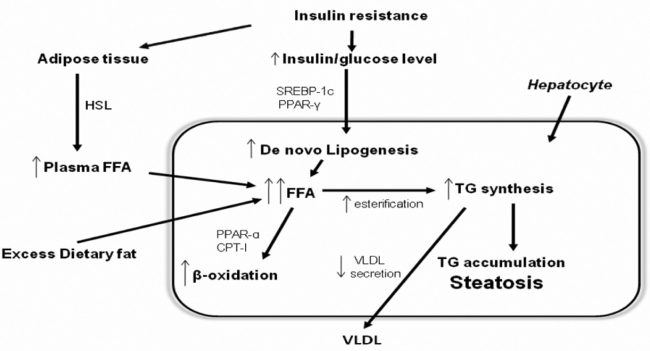
Pathophysiology (Figure 1)
Figure 1: Development of nonalcoholic hepatic steatosis. In adipose tissue, insulin resistance decreases the inhibitory action of insulin on hormone sensitive lipase (HSL), thus enhancing triglyceride (TG) lipolysis and free fatty acid (FFA) release. The results are increased circulating levels of FFAs, which are then taken up by the liver. Dietary fatty acids enter the liver through spillover into the plasma NEFA pool and through the uptake of intestinally derived chylomicron remnants. In hepatocytes hyperinsulinemia increases the 'de novo' synthesis of fatty acids. Hepatic TG synthesis is driven by the increased hepatocyte FFA content and favoured by insulin-mediated upregulation of lipogenic enzymes, such as peroxisome proliferator-activated receptor gamma (PPAR-γ) and sterol regulatory element binding protein 1 (SREBP-1). The large pool of FFAs activates mitochondrial fatty acid β-oxidation by activation of PPAR-α and enchanced CPT-I. Meanwhile, TG export via very-low-density lipoproteins (VLDL) may be inhibited by decreased synthesis of apolipoprotein B (apo B) or reduced incorporation of TG with apo B by microsomal triglyceride transfer protein (MTP). (abbrevations: HSL: hormone-sensitive lipase, TG: triglyceride, FFA: free fatty acid, PPAR-: peroxisome proliferator-activated receptor gamma, SREBP-1: sterol regulatory element binding protein 1, PPAR-α: peroxisome proliferator-activated receptor a, CPT-I: carnitine palmitoyltransferase I, VLDL: very-low-density lipoproteins, apo B: apolipoprotein B, MTP: microsomal triglyceride transfer protein).
Pathophysiology of NAFLD still has not been completely clarified. Nevertheless, insulin resistance plays a fundamental role in the pathogenesis of fatty liver. Insulin resistance may be defined as a condition in which a) higher than normal insulin concentrations are needed to achieve normal metabolic responses or b) normal insulin concentrations fail to achieve a normal metabolic response48. Insulin is a pleiotropic hormone which has diverse functions including stimulation of nutrient transport into cells, regulation of gene expression, modification of enzymatic activity and regulation of energy homeostasis49. Insulin binds to its receptor in the plasma insulin-dependent cell membrane, resulting in phosphorylation of the receptor and activation by phosphorylation in tyrosine of insulin receptor substrate proteins (IRS proteins). IRS proteins are linked to the activation of two main signaling pathways: the phosphatidylinositol 3-kinase (PI3K)–AKT/protein kinase B (PKB) pathway, which is responsible for most of the metabolic actions of insulin, and the Ras–mitogen-activated protein kinase (MAPK) pathway, which regulates expression of some genes and cooperates with the PI3K pathway to control cell growth and differentiation. AKT/PKB is a serine/ threonine kinase that activates the translocation of the glucose transporter (GLUT4) to the plasma membrane, thus facilitating cell glucose uptake. Another target of AKT/PKB is glycogen synthase kinase-3 (GSK3). Phosphorylation of GSK3 decreases its activity towards glycogen synthase, which leads to increased glycogen synthesis. AKT/PKB also regulates the expression of gluconeogenic and lipogenic enzymes by controlling the activity of the winged helix or forkhead (FOX) class of transcription factors. AKT/PKB effectively activates the mTOR pathway which regulates protein synthesis. A number of acquired factors including oxidative stress, FFAs, TNFα, and -as intracellular mediators- ceramide, IKKβ (inhibitor of κβ kinase) NFkB (nuclear factor kappa B), PKC-θ (Protein Kinase C-θ), JNK1 (Jun N-Terminal Kinase-1) cytochrome CYP2E1 and SOCS (suppressors of cytokine signaling ) have been implicated in altering the above described insulin signaling pathways, resulting in insulin resistance50,51.
The most widely accepted model to explain the development of NAFLD and the progression from simple steatosis to NASH is the "two-hit hypothesis". The "first hit" is the accumulation of lipids in the hepatocytes and insulin resistance is the key pathogenic factor for the development of hepatic steatosis .The "second hit" leads to hepatocyte injury, inflammation and fibrosis. Factors initiating the second hit are oxidative stress and subsequent lipid peroxidation, proinflammatory cytokines, adipokines and mitochondrial dysfunction52.
Liver fat accumulation results from a disturbance in the balance between supply, formation, consumption and hepatic oxidation or disposal of triglycerides. The potential sources of lipids contributing to fatty liver include plasma nonesterified fatty acid (NEFA) pool from adipose tissue, newly made fatty acids within the liver through de novo lipogenesis (DNL) and dietary fatty acids, which can enter the liver by two means: through spillover into the plasma NEFA pool and through the uptake of intestinally derived chylomicron remnants. Nearly 60-80% of liver triacylglycerol is derived from circulating free fatty acids (FFA). Insulin resistance decreases the inhibitory effects of insulin on peripheral lipolysis, increasing the availability of free fatty acids (FFAs). Twenty five percent of liver triacylglycerol is derived from increased de novo lipogenesis (DNL)53. DNL is mediated by enzymes under the transcriptional regulation of sterol regulatory element binding protein-1c (SREBP-1c), which is upregulated by insulin and is likely to be activated by hyperinsulinaemia. DNL is also increased by activation of the cannabinoid receptors on hepatocytes. Activation of these pathways has been implicated in the pathogenesis of hepatic steatosis54. Dietary fat intake is responsible for 15% of FFA supply to the liver. FFA and monoglycerides in intestine are absorbed and packaged into triglycerides in intestinal epithelial cells. Then they are secreted in chylomicrons, which release FFA to adipose. Lipids are normally exported from the liver in very-low density lipoproteins (VLDL), which are formed by microsomal triglyceride transfer protein (MTP) incorporating triglyceride into apolipoprotein B (apo B). A reduction in MTP activity and apo B synthesis and secretion may impair hepatic lipid export and favour hepatic triglyceride accumulation55.
Recent findings in animal models suggest that triglyceride accumulation in liver may be hepato-protective rather than being hepatotoxic since it decreases the free fatty acids accumulation in hepatocytes, their peroxidation and oxidative stress56. However, according to the "two-hit hypothesis" steatosis increases the vulnerability of the liver to "second hit", oxidative stress. Disturbance in the prooxidant/antioxidant balance constitutes oxidative stress. The consequences of oxidative stress are lipid peroxidation, cell degeneration and necrosis, apoptosis, proinflammatory cytokine expression, liver stellate cell activation and fibrogenesis. Multiple possible sources of oxidative stress have been identified and include mitochondrial dysfunction, hepatic cytochrome CYP2E1, β-oxidation by peroxisomes in mitochondria and recruited inflammatory cells57–59.
The mechanisms responsible for increased mitochondrial β-oxidation in NASH are poorly understood. The first mechanism is the increased hepatic uptake and synthesis of free fatty acids in liver60. The large pool of FFAs, especially from de novo lipogenesis, activates hepatic PPAR-a (peroxisome proliferator activated receptor) which in turn activates the expression of genes involved in fatty acid β-oxidation61. Another mechanism is the enhanced CPT-I (carnitine palmitoyltranferase) which increases the entry of long-chain fatty acids into mitochondria62. It is still unclear whether leptin activates fatty acid oxidation through AMPK and PPAR-a activation63. The mitochondrial abnormalities associated with NAFLD include ultrastructural lesions, depletion of mitochondrial DNA (mtDNA), impaired activity of respiratory chain complexes, and impaired mitochondrial β-oxidation. Mitochondria are big and swelled, scarce in number, and the mitochondrial matrix has paracrystalline inclusions. Mechanisms which explain this dysfunction found in NAFLD include excessive reactive oxygen species (ROS) production, increased TNF-α expression, and altered PGC-1 (peroxisome proliferator-activated receptor-γ coactivator) expression64. Recent animal experiments and preliminary human studies support that increased expression and activity of cytochrome P450 2E1 (CYP2E1), a microsomal fatty acid oxidase that generates reactive oxygen species, may play a role in pathogenesis of NASH65,66. ROS trigger lipid peroxidation which in turn initiates release of malindialdehyde (MDA) and 4- hydroxynonenal (HNE). MDA and HNE bind to hepatocyte proteins initiating a potentially harmful immune response, form of Mallory hyaline, or activate hepatic stellate cells promoting collagen synthesis and stimulate neutrophil chemotaxis67. Oxidative stress also activates transcriptional factor NFkB which in turn increase production of proinflammatory cytokines promoting hepatocyte injury and apoptosis, neutrophil chemotaxis, and hepatic stellate cell activation29,68.
Initially, hepatic iron was believed to be a determinant factor in the development of NASH. However, recent studies report that iron burden and HFE mutations do not contribute significantly to hepatic fibrosis in the majority of patients with NAFLD69.
Another potential contributor to hepatic steatosis and inflammation is bacterial overgrowth. It has been proposed that bacterial overgrowth increases endogenous production of toxins such as ethanol or bacterial lipopolysaccharide that promote the production of reactive oxygen species by hepatocytes and liver macrophages. This proposed mechanism is supported by studies from animal studies but remains untested in patients70,71.
NAFLD is strongly associated with obesity but body fat distribution appears to play a more important role in the pathogenesis of NAFLD. Excess of intraabdominal fat in particular may be a key determinant in the pathogenesis of NAFLD, because of its strong association with insulin resistance and possibly as a source of FFAs72. A study in individuals with normal weight showed that accumulation of intra-abdominal fat correlates with insulin resistance73. Furthermore visceral adiposity is positively correlated with clinical manifestations associated with insulin resistance, including type 2 diabetes, dyslipidaemia and MS. Visceral fat is also known to release greater quantities of adipokines and excess FFAs into the portal vein, exposing the liver to higher FFA concentrations74,75. Although most studies have found intraabdominal fat to be more strongly associated with whole-body insulin resistance, subcutaneous fat is also important in NAFLD. Subcutaneous fat, because of its greater overall mass, contributes more than intraabdominal fat to circulating FFAs. Fifty nine percent of the triacylglycerol that accumulates in the liver in subjects with NAFLD is derived from circulating FFAs and most of this is from nonsplanchnic sources53. In lean healthy subjects during the post-absorptive phase, only about 15% of the systemic FFA flux is accounted for by visceral fat, whereas 75% is derived from the upper body and only 10% from the lower extremities76.
Adipose tissue includes adipocytes, mesenchymal cells, vascular endothelial cells and macrophages. It is now recognized that it is not simply a storage depot for excess energy but rather an endocrine organ that secretes adipokines77. Adiponectin is a hormone secreted by adipocytes. It is found in relatively high circulating levels in plasma but is decreased in patients with NAFLD and in clinical manifestations associated with insulin resistance such as MS and type 2 diabetes mellitus78. It regulates energy homeostasis and glucose and lipid metabolism. Adiponectin increases phosphorylation and activity of AMPK which in turn stimulates phosphorylation of acetyl coenzyme A carboxylase (ACC), fatty-acid oxidation, glucose uptake and lactate production in myocytes79. It has also anti-inflammatory effect since it suppresses the hepatic production and plasma concentrations of TNF-α. In a study treatment with adiponectin decreased hepatomegaly, steatosis and alanine aminotransferase abnormality80. Leptin is released into the circulation by mature adipocytes in response to changes in body fat mass and nutritional status. It has varied metabolic effects with the most significant of these being related to body weight and energy expenditure81. Leptin acts as an insulin sensitizing hormone and reduces lipid content of myocytes, hepatocytes and pancreatic β-cells. In obese NAFLD patients, leptin levels are elevated and are directly correlated with the severity of steatosis82. The presence of hepatic steatosis despite the presence of hyper-leptinemia suggests the development of leptin resistance83. In animal models leptin also has been shown to play an important pro-fibrogenic role84.
In humans the major source of resistin is probably from the peripheral blood macrophages and adipocytes85. In animal models resistin regulates hepatic glucose and lipid metabolism and act as a mediator of hepatic insulin resistance. The role of resistin in humans remains uncertain. Resistin levels are increased in patients with NAFLD and levels are related to the histological severity of the disease86,87. TNF-a, IL-6 and other cytokines are synthesized and secreted by adipocytes, stromavascular cells and endotoxin-activated macrophages. The factors regulating cytokine release within adipose tissue include catecholamines, lipopolysaccarides, but also the size of the fatty cells. TNF-α and IL-6 inhibit lipoprotein lipase, and TNF-α additionally stimulates hormone-sensitive lipase and induces uncoupling protein expression. TNF- α also downregulates insulin-stimulated glucose uptake via effects on GLUT-4, insulin receptor autophosphorylation and IRS-1. TNF-α is increased in obese and diabetic individuals while weight loss decreases its levels. Furthermore, TNF-a decreases the production and secretion of leptin by adipocytes. No study has demonstrated whether TNF-α, via the portal vein reaches the liver and causes hepatic injury, but it is certain that TNF-a is an autocrine and paracrine regulator. The local production of TNF-α by Kupffer cells has been proposed to play a key role in the pathogenesis of NASH. On the other hand, IL-6 appears to be released systemically by adipose tissue and act on the hypothalamus and liver. IL-6 promotes hepatic insulin resistance, has pro-inflammatory properties and causes activation of Kupffer cells resulting in fibrogenesis. It also enhances hepatic C-reactive protein synthesis. In paracrine fashion, it decreases adiponectin secretion from the surrounding adipocytes, inhibits lipoprotein lipase (LPL) on endothelial cells and activates lipolysis59,88. IL-6 and TNF-α further promote insulin resistance by increasing hepatic suppressors of cytokine signaling (SOCS) expression. Over expression of SOCS-1 and SOCS-3 in liver causes insulin resistance and an increase in the key regulator of fatty acid synthesis in liver, SREBP-1c89. Accumulating data report that the renin-angiotensin aldosterom system (RAAS) is a major modulator of insulin resistance. Clinical studies have shown that treatment with angiotensin II antagonists improve insulin sensitivity and liver function tests in patients with NAFLD90.
The fact that prevalence of NAFLD varies among different racial groups, the common observation of considerable variation in the severity of liver disease among individuals with NAFLD and similar risk factors and the observation that several very rare human monogenic disorders are associated with the development of fatty liver disease suggest that genetic factors affect the development of NAFLD. At the present time, only few small studies examine potential genes which are related to the pathogenetic mechanisms of NAFLD. The data show that multiple changes in gene expression characterize simple steatosis. All of these studies are interesting and provocative but will require further validation in larger populations before their true significance is known91.
Symptoms, laboratory abnormalities, diagnosis
Most patients with NAFLD are asymptomatic. The disease is discovered incidentally during routine laboratory examination in subjects treated with hypolipidaemic drugs and during sonographic examination for suspected gallstone disease. When present, clinical symptoms are nonspecific and unreliable in assessing disease severity. The most common signs and symptoms are fatigue and right upper quadrant discomfort. During physical examination there are no pathognomic signs and the most common abnormalities are obesity and hepatomegaly, which has been reported in up to 50% of subjects. A smaller fraction of patients experience symptoms indicative of more serious liver disease such as ascites, jaundice and liver encephalopathy58,92–94.
The primary laboratory abnormality is the elevated serum AST and ALT levels. However, liver aminotransferase levels are seldom higher than 3 or 4 times the upper limit of normal. The ALT levels are higher than the AST levels in most instances, but the AST level may occasionally be higher than the ALT level, especially in the presence of cirrhosis. A reversal of the ALT/AST ratio to more than 1 had been reported to predict the presence of more advanced fibrosis45. However, many individuals with the entire histological spectrum of NAFLD have normal ALT values95. Changes of aminotransferases do not parallel changes in fibrosis stage. It has been reported that improvement in aminotransferase levels appears to indicate improvement in steatosis and inflammation but not fibrosis96. Alkaline phosphatase [ALP] and gamma-glutamyltranspeptidase [GGT] can also be elevated in NAFLD. Studies have described NAFLD patients that present with isolated elevation of alkaline phosphatase and normal serum aminotransferases97. It has been suggested that the normal limits for ALT values should be revised and be lowered. The decrease of the upper limit of normal for ALT level from 40 U/L to 30 U/L in men and from 30 U/L to 19 U/L in women increases the sensitivity for detection of patients with liver injury from 55% to 76% but decreases the specificity from 97% to 88%. Prati et al report that ALT activity is independently related to body mass index (BMI), to laboratory indicators of abnormal lipid or carbohydrate metabolism and to sex. They support that lowering the AST threshold in the evaluation of persons with specific risk factors for fatty liver would allow earlier recognition and appropriate counselling in persons with mild hepatocellular injury. On the other hand, Kaplan believes that the recommendations of Prati would greatly increase the number of asymptomatic patients, their anxiety and the economic cost98,99. Serum auto antibodies, antinuclear antibodies (ANA), smooth muscle antibodies (SMA) and antimitochondrial antibodies (AMA), are present in 23% to 36% of NAFLD patients. High titre of antinuclear antibodies is strongly associated with insulin resistance. But the question of the significance of antibodies remains largely unanswered100–102.
The most commonly used imaging technique in the diagnosis of NAFLD is ultrasonography (US) with remarkable sensitivity but without accurate quantification of the degree of steatosis. The sonographic findings in non-alcoholic fatty liver disease are: 1) diffuse enhancement of near field echo in the hepatic region (stronger than in the kidney and spleen region) and gradual attenuation of the far field echo, 2) unclear display of intra-hepatic lacuna structure, 3) mild to moderate hepatomegaly with a round and blunt border and 4) unclear display of envelop of right liver lobe and diaphragm103. A study introduced that the sensitivity and specificity of ultrasound in patients of any degree of steatosis is 64% and 97% respectively. However, in patients with steatosis >30% the sensitivity and the specificity increase to 89.7% and 100%104. Hamaguchi et al reported that ultrasonography has high sensitivity (91.7%) and specificity (100%) in detecting fatty liver. The limitation of this study is the low body mass index (BMI) of the subjects105. Obesity reduces the sensitivity of ultrasound. In morbidly obese patients, the sensitivity and specificity of ultrasound in diagnosing steatosis was 49.1% and 75%, respectively. This could be related to the lack of objective criteria for the ultrasound diagnosis of steatosis, and probably, technical problems in performing ultrasound in such patients106. The hepatic steatosis can be accurately determined radiologically only when there is moderate or severe (>30%) fatty infiltration of the liver107,108. Other limitations of the method are operator–dependency and inability to distinguish between NASH and other types of NAFLD108,109.
CT imaging can accurately detect and quantify the amount of steatosis in patients. For steatosis > 30%, the sensitivity and specificity are 82% to 93% and 100%, respectively. However, CT scan cannot distinguish simple steatosis from steatohepatitis. There is no difference in diagnostic value between a non-contrast CT scan and a contrast-enhanced one108,110,111.
Magnetic resonance imaging (MRI) is the most accurate available technique in diagnosis and quantification of hepatic steatosis. It is based on the signal differences between fat and water. MRI shows a good correlation with histological examination and the sensitivity and specificity are 100% and 92.3%, respectively111,112. Limitations of the method include expense, inability to use in patients with implantable devices or claustrophobia, and altered values in patients with iron overload93.
Magnetic resonance spectroscopy (MRS) is an alternative, non-invasive method to measure hepatic triglyceride content (HTGC), but has been used only in small research studies. The principle of MRS is based on the differences in resonance frequencies of protons. Szczepaniak et al reported that MRS is sensitive enough to detect small amounts of triglyceride and it is not affected by food intake. Another advantage over the other commonly used imaging modalities is that it is a quantitative rather than qualitative or semi quantitative method. The accuracy and safety of MRS make it an ideal methodology to assess and monitor hepatic steatosis113.
Transient elastography (TE) is a recently developed, non-invasive method designed to predict liver fibrosis, based upon a mechanical wave generated by vibration. The measurement of the wave velocity across the hepatic parenchyma provides an estimate of the liver elasticity, which in turn is a marker of liver fibrosis. It can accurately diagnose advanced liver fibrosis, but its performance in early liver fibrosis is less satisfactory. Its reproducibility is significantly reduced in patients with steatosis and increased BMI. Liver fibrosis also tends to be overestimated by TE when ALT levels are elevated114,115.
A growing number of potential biomarkers are being studied in the search for non-invasive indicators of the extent of disease and prognosis in NAFLD. Markers of the processes that lead to the development and progression of NAFLD are potential targets for study. Oxidative stress is a significant component of the progression of NAFLD. Many studies have measured multiple byproducts of oxidative stress in order to assess the oxidative stress in the liver, but the results are mixed. While some of the markers were found to be useful after further study, others have not been found to correlate with NAFLD. Many questions also remain unanswered regarding the relative importance of each of the oxidation pathways in the liver damage seen in NASH and if measuring these markers in blood or breath would reflect what is happening in the liver116,117. Inflammation has a central role in the development of NAFLD, however, there is no clear marker identified as a predictor of disease. Adiponectin level is reduced in patients with NAFLD and furthermore the level is lower for individuals with NASH than for those with simple steatosis. Thus measurements of adiponectin might be accurate for differentiating between steatosis and steatohepatitis but these findings need to be validated with clinical features, which can affect adiponectin levels in the steatosis and steatohepatitis groups, such as type 2 diabetes mellitus and obesity. In the same study TNF-α was also increased but the level of TNF-α did not correlate with the severity of necroinflammation118,119. On the other hand other studies report that TNF-α levels are higher in patients with NASH as compared to subjects with simple steatosis or with no liver disease. C-reactive protein (CRP) is another inflammatory marker which has A growing number of potential biomarkers are being studied in the search for non-invasive indicators of the extent of disease and prognosis in NAFLD. Markers of the processes that lead to the development and progression of NAFLD are potential targets for study. Oxidative stress is a significant component of the progression of NAFLD. Many studies have measured multiple byproducts of oxidative stress in order to assess the oxidative stress in the liver, but the results are mixed. While some of the markers were found to be useful after further study, others have not been found to correlate with NAFLD. Many questions also remain unanswered regarding the relative importance of each of the oxidation pathways in the liver damage seen in NASH and if measuring these markers in blood or breath would reflect what is happening in the liver116,117. Inflammation has a central role in the development of NAFLD, however, there is no clear marker identified as a predictor of disease. Adiponectin level is reduced in patients with NAFLD and furthermore the level is lower for individuals with NASH than for those with simple steatosis. Thus measurements of adiponectin might be accurate for differentiating between steatosis and steatohepatitis but these findings need to be validated with clinical features, which can affect adiponectin levels in the steatosis and steatohepatitis groups, such as type 2 diabetes mellitus and obesity. In the same study TNF-α was also increased but the level of TNF-α did not correlate with the severity of necroinflammation118,119. On the other hand other studies report that TNF-α levels are higher in patients with NASH as compared to subjects with simple steatosis or with no liver disease. C-reactive protein (CRP) is another inflammatory marker which has mixed results in NAFLD and several studies showed no predictive value. It was found no difference in the mean high sensitive CRP (hsCRP) levels between patients with NASH and those with simple steatosis. However, Yoneda et al. suggested that hsCRP may be a clinical feature that not only distinguishes NASH from simple steatosis but also indicates the severity of hepatic fibrosis in cases of NASH. They reported that hsCRP was significantly elevated in cases of NASH among patients with NASH and hsCRP was significantly elevated in those with advanced fibrosis compared with that in those with mild fibrosis120. Many studies that are in progress test several cytokines such as IL-1, IL-6, CC-chemokine ligand-2 (CCL2) as potential biomarkers for NASH diagnosis.
Hepatic apoptosis plays a significant role in the progression of NAFLD and biomarkers of hepatic apoptosis have been explored as a potential diagnostic tool. Caspase generated cytokeratin-18 (CK-18) is a protein involved in apoptosis and it was found to be higher in patients with NASH. Wieckoska et al. reported that plasma CK-18 fragments were strikingly increased in patients with NAFLD compared to controls and the plasma levels correlated with the expression levels in the liver. These findings suggest that CK-18 is an independent predictor of NASH and it may become a useful and reliable tool in determining histological disease severity in patients with NAFLD. A large multicenter prospective validation study is in progress93,94,121.
Several panel markers have been created, using combinations of clinical and biochemical parameters in order to generate clinical models of fibrosis. One of the early scoring systems developed is called BAAT score and combines four clinical variables: body mass index (>28 kg/m2), age (>50 years), ALT (>2x normal), serum triglycerides (>150 mg/dl). This scoring system showed good positive predictive value (PPV) in determining advanced fibrosis but it was not reliable identifying mild to moderate disease122. Fibro Test combines 5 biochemical markers including 2-macroglobulin, apolipoprotein A1, haptoglobulin, total bilirubin, and GGT. Ratziu et al showed that Fibro Test is a simple that and non-invasive quantitative estimate of liver fibrosis reliably predicts advanced fibrosis. In advanced fibrosis negative predictive value (NPV) was 90% and positive predictive value (PPV) was 70%. But in moderate fibrosis NPV and PPV were 70% and 74% respectively. Gilbert's syndrome, acute inflammation and high serum apoA1 concentration due to high serum HDL-cholesterol were the most frequent causes of Fibro Test failures123. The NAFLD fibrosis scoring system uses six commonly measured parameters including age, hyperglycaemia, BMI, platelet count, albumin level, and AST/ALT ratio. Study in 733 individuals showed that the scoring system can predict absence or presence of fibrosis in 90% of patients with NAFLD and liver biopsy would have been avoided in 75%124. The Original European Liver Fibrosis (OELF) test consists of age, tissue inhibitor of matrix metalloproteinase 1(TIMP 1), hyaluronic acid, and N-terminal propeptide of type III collagen (P3NP). The algorithm detected fibrosis with sensitivity 90% and ruled out fibrosis with negative predictive value 92% for significant fibrosis125. A modified algorithm which does not contain age, Enhanced Liver fibrosis panel (ELF), was validated in an independent cohort of patients with NAFLD. It was reported that EFL panel has good diagnostic accuracy comparable to the accuracy of OEFL panel and the addition of simple markers to the panel improved diagnostic performance126. Many studies suggest that single markers or a combination of markers may be used for noninvasive diagnosis and staging of NAFLD. However, they have not been prospectively validated in different populations and they have not been evaluated for their utility in monitoring disease activity or progression. In addition, an important limitation of these serumbased biomarker panels is that they may reflect fibrotic pathogenetic processes occurring in other organs. To overcome this limitation, several groups have validated genomic and proteomic techniques in studying non-alcoholic fatty liver disease with results that cannot be introduced in clinical practice, yet127. Liver biopsy remains the most appropriate tool for diagnosis of NAFLD and the evaluation of histological features. Histological features have been recognized as useful in determining the risk of progression to more advanced liver disease. However, there have been arguments and hesitation to perform liver biopsy in individuals with suspected NAFLD. This hesitation is result of the invasive nature of a biopsy, the potential risk of bleeding and death, the cost of a biopsy, the lack of effective medical treatment for patients with NAFLD. In addition there is still no international consensus regarding the histopathological criteria that would firmly define non-alcoholic steatohepatitis and differentiate between NAFLD entities. Consequently, there have been no widely accepted guidelines and the decision to perform liver biopsy remains highly individual. However, some factors can help to identify the NASH patient expected to derive the most benefit from liver biopsy. Age older than 45 years, presence of obesity, type 2 diabetes mellitus or other risk factors of metabolic syndrome and an SGOT/SGPT ratio greater than one are associated with advanced liver fibrosis and they are indications of liver biopsy128,129.
Metabolic syndrome and non-alcoholic fatty liver
Recent studies have pointed that NAFLD, in its whole spectrum ranging from pure fatty liver to non-alcoholic steatohepatitis (NASH), might represent another feature of MS. Pathophysiologic considerations, clinical associations, and laboratory investigations support that insulin resistance and hyperinsulinaemia have a central role in pathogenesis of both MS and non-alcoholic fatty liver. Studies concluded that NAFLD, in the presence of normoglycaemia and normal or moderately increased body weight, is characterized by clinical and laboratory data similar to those found in diabetes and obesity such as impaired insulin sensitivity and abnormalities in lipid metabolism3. Ninty percent of individuals with NAFLD have at least one risk factor of MS, and 33% have all the features of MS. Study concluded that liver fat content is significantly increased in subjects with the MS as compared with those without the syndrome, independently of age, gender, and body mass index130. In 304 NAFLD patients without diabetes mellitus the prevalence of metabolic syndrome increased from 18% in normal weight individuals to 67% in obese individuals. The presence of multiple metabolic disorders such as diabetes mellitus, obesity, dyslipidaemia and hypertension is associated with a potentially progressive, severe liver disease131,132. Obesity is found in 30-100% of subjects with NAFLD. In obese persons steatosis is 4.6 fold higher than in normal weight persons34,128. Prevalence of type 2 diabetes mellitus in NAFLD patients ranges from 10% to 75%. A study reported that type 2 diabetes mellitus was found in 33% of individuals with NAFLD 133. Another study in Japanese population showed that prevalence of NAFLD increased to 43% in individuals with impaired fasting glucose and 62% in individuals with type 2 diabetes mellitus40. Hyperlipidaemia and specifically high levels of triglycerides and low levels of HDL-cholesterol are strongly associated with NAFLD. Hypertriglyceridemia and low HDL-cholesterol level are present in 64% and 30-42% of NAFLD patients respectively131. In 55 nonobese, non-diabetic patients with primary hypertension the prevalence of fatty liver was more than twofold than in the control group134. Recent studies have pointed that NAFLD is strongly associated with increased risk of cardiovascular disease. There is an independent association among hepatic steatosis and carotid atherosclerotic plaques and endothelial dysfunction. This relationship remains statistically significant after adjustment for risk factors of MS135,136.
In a prospective observational study of 4401 apparently healthy individuals Hamagushi et al found that the MS is a strong risk factor for nonalcoholic fatty liver disease. Participants with the MS have a 4 to 11 times higher risk for future nonalcoholic fatty liver disease. In addition, if nonalcoholic fatty liver disease and the MS coexist, disease regression is less likely105. Furthermore Hsiao et al demonstrated that the presence of severe fatty liver correlated significantly with the prevalence and degree of hypertension, abnormal glucose and triglyceride metabolism137.
Conclusion
Non alcoholic fatty liver is the object of significant scientific and clinical interest which is going to increase in the following years. Epidemiological studies demonstrate that NAFLD and MS are emerging as major problems of public health. The targets of future investigations are to clarify the pathogenesis and to establish effective treatment in both NAFLD and MS. Several studies are in progress and a few of them have provided encouraging results.
References
- 1.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, Sauver J, St, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/ National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Reisin E, Alpert MA. Definition of the metabolic syndrome: current proposals and controversies. Am J Med Sci. 2005;330:269–272. doi: 10.1097/00000441-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds K, He J. Epidemiology of the metabolic syndrome. Am J Med Sci. 2005;330:273–279. doi: 10.1097/00000441-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Athyros VG, Ganotakis ES, Elisaf MS, Liberopoulos EN, Goudevenos IA, Karagiannis A. Prevalence of vascular disease in metabolic syndrome using three proposed definitions. Int J Cardiol 25. 2007;117:204–210. doi: 10.1016/j.ijcard.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 11.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 12.Worldwide definition of the metabolic syndrome. Federation ID. 2005 http://www.idf.org/webdata/docs/IDF_Meta-syndrome_definition.pdf[cited] [Google Scholar]
- 13.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 17. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 15.Athyros VG, Bouloukos VI, Pehlivanidis AN, et al. The prevalence of the metabolic syndrome in Greece: the MetS-Greece Multicentre Study. Diabetes Obes Metab. 2005;7:397–405. doi: 10.1111/j.1463-1326.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 16.Reaven G. Why a cluster is truly a cluster: insulin resistance and cardiovascular disease. Clin Chem. 2008;54:785–787. doi: 10.1373/clinchem.2008.105254. [DOI] [PubMed] [Google Scholar]
- 17.Kashyap SR, Defronzo RA. The insulin resistance syndrome: physiological considerations. Diab Vasc Dis Res. 2007;4:13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- 18.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–33. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 20.Strazzullo P, Barbato A, Galletti F, et al. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J Hypertens. 2006;24:1633–1639. doi: 10.1097/01.hjh.0000239300.48130.07. [DOI] [PubMed] [Google Scholar]
- 21.Sarafidis PA, Bakris GL. Review: Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2007;92:379–385. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 22.Sowers JR, Frohlich ED. Insulin and insulin resistance: impact on blood pressure and cardiovascular disease. Med Clin North Am. 2004;88:63–82. doi: 10.1016/s0025-7125(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 23.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 25.Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450–465. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 28.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Duvnjak M, Lerotic I, Barsic N, et al. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol 14. 2007;13:4539–4550. doi: 10.3748/wjg.v13.i34.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundqvist G, Flodin U, Axelson O. A case-control study of fatty liver disease and organic solvent exposure. Am J Ind Med. 1999;35:132–136. doi: 10.1002/(sici)1097-0274(199902)35:2<132::aid-ajim4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic Fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741–1747. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 32.Lidofsky SD. Nonalcoholic fatty liver disease: diagnosis and relation to metabolic syndrome and approach to treatment. Curr Diab Rep. 2008;8:25–30. doi: 10.1007/s11892-008-0006-1. [DOI] [PubMed] [Google Scholar]
- 33.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 34.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 35.Baldridge AD, Perez-Atayde AR, Graeme-Cook F, Higgins L, Lavine JE. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr. 1995;127:700–704. doi: 10.1016/s0022-3476(95)70156-7. [DOI] [PubMed] [Google Scholar]
- 36.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 37.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 38.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 39.Marchesini G, Forlani G. NASH: from liver diseases to metabolic disorders and back to clinical hepatology. Hepatology. 2002;35:497–499. doi: 10.1053/jhep.2002.31551. [DOI] [PubMed] [Google Scholar]
- 40.Jimba S, Nakagami T, Takahashi M, et al. Prevalence of nonalcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 41.Gupte P, Amarapurkar D, Agal S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 42.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 43.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 44.Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129–1133. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- 45.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 46.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 47.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 48.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 49.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(3 Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 51.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 52.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:46–53. doi: 10.1038/ncpgasthep0084. [DOI] [PubMed] [Google Scholar]
- 55.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295–300. doi: 10.1097/MOL.0b013e3282ff5e55. [DOI] [PubMed] [Google Scholar]
- 57.Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD) J Clin Gastroenterol. 2006;40:930–935. doi: 10.1097/01.mcg.0000212608.59090.08. [DOI] [PubMed] [Google Scholar]
- 58.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 59.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 61.Memon RA, Tecott LH, Nonogaki K, et al. Up-regulation of peroxisome proliferator-activated receptors (PPAR-a) and PPAR-g messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-gresponsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;41:4021–4031. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- 62.Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B. Metabolic syndrome without obesity: hepatic overexpression of 11-b-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci. 2004;101:7088–7093. doi: 10.1073/pnas.0305524101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006 Feb;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology Mar. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 66.Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 67.Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 68.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bugianesi E, Manzini P, D'Antico S, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 71.Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumenliver interactions and possible role for probiotics. J Hepatol. 2003;38:681–687. doi: 10.1016/s0168-8278(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 72.Eguchi Y, Eguchi T, Mizuta T, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–469. doi: 10.1007/s00535-006-1790-5. [DOI] [PubMed] [Google Scholar]
- 73.Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185–192. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen MD, Johnson CM. Contribution of leg and splanchnic free fatty acid (FFA) kinetics to postabsorptive FFA flux in men and women. Metabolism. 1996;45:662–666. doi: 10.1016/s0026-0495(96)90040-2. [DOI] [PubMed] [Google Scholar]
- 77.Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320–327. doi: 10.1097/MOG.0b013e3282fbccf2. [DOI] [PubMed] [Google Scholar]
- 78.Pagano C, Soardo G, Esposito W, et al. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113–118. doi: 10.1530/eje.1.01821. [DOI] [PubMed] [Google Scholar]
- 79.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 80.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56(2 Pt 2):s38–s46. doi: 10.1111/j.1753-4887.1998.tb01685.x. discussion s54-75. [DOI] [PubMed] [Google Scholar]
- 82.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 83.Chitturi S, Farrell G, Frost L, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403–409. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
- 84.Honda H, Ikejima K, Hirose M, et al. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002;36:12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- 85.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 86.Banerjee RR, Lazar MA. Resistin: molecular history and prognosis. J Mol Med. 2003;81:218–226. doi: 10.1007/s00109-003-0428-9. [DOI] [PubMed] [Google Scholar]
- 87.Pagano C, Soardo G, Pilon C, et al. Increased serum resistin in nonalcoholic fatty liver disease is related to liver disease severity and not to insulin resistance. J Clin Endocrinol Metab. 2006;91:1081–1086. doi: 10.1210/jc.2005-1056. [DOI] [PubMed] [Google Scholar]
- 88.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 89.Sanal MG. The blind men see the elephant-the many faces of fatty liver disease. World J Gastroenterol 14. 2008;14:831–844. doi: 10.3748/wjg.14.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–198. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 91.Merriman RB, Aouizerat BE, Bass NM. Genetic influences in nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(3 Suppl 1):S30–S33. doi: 10.1097/01.mcg.0000168643.16074.19. [DOI] [PubMed] [Google Scholar]
- 92.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 93.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 94.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 95.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 96.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 97.Pantsari MW, Harrison SA. Nonalcoholic fatty liver disease presenting with an isolated elevated alkaline phosphatase. J Clin Gastroenterol. 2006;40:633–635. doi: 10.1097/00004836-200608000-00015. [DOI] [PubMed] [Google Scholar]
- 98.Kaplan MM. Alanine aminotransferase levels: whats normal? Ann Intern Med. 2002;137:49–51. doi: 10.7326/0003-4819-137-1-200207020-00012. [DOI] [PubMed] [Google Scholar]
- 99.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 100.Adams LA, Talwalkar JA. Diagnostic evaluation of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(3 Suppl 1):S34–S38. doi: 10.1097/01.mcg.0000168642.38945.f1. [DOI] [PubMed] [Google Scholar]
- 101.Brunt EM. Pathology of fatty liver disease. Mod Pathol. 2007;20(Suppl 1):S40–48. doi: 10.1038/modpathol.3800680. [DOI] [PubMed] [Google Scholar]
- 102.Loria P, Lonardo A, Leonardi F, et al. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. 2003;48:2173–2181. doi: 10.1023/b:ddas.0000004522.36120.08. [DOI] [PubMed] [Google Scholar]
- 103.Zeng MD, Fan JG, Lu LG, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 104.Palmentieri B, de Sio I, La Mura V, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–489. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 105.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 106.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14:635–637. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 107.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 108.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 109.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am J Gastroenterol. 2007;102:2716–2717. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 110.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 111.Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: An overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol 28. 2008;14:2474–2486. doi: 10.3748/wjg.14.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karcaaltincaba M, Akhan O. Imaging of hepatic steatosis and fatty sparing. Eur J Radiol. 2007;61:33–43. doi: 10.1016/j.ejrad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 114.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong GL, Wong VW, Choi PC, et al. Assessment of Fibrosis by Transient Elastography Compared With Liver Biopsy and Morphometry in Chronic Liver Diseases. Clin Gastroenterol Hepatol. 2008 doi: 10.1016/j.cgh.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 116.Bonnefont-Rousselot D, Ratziu V, Giral P, Charlotte F, Beucler I, Poynard T. Blood oxidative stress markers are unreliable markers of hepatic steatosis. Aliment Pharmacol Ther. 2006;23:91–98. doi: 10.1111/j.1365-2036.2006.02719.x. [DOI] [PubMed] [Google Scholar]
- 117.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 118.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 119.Musso G, Gambino R, Biroli G, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Betacell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438–2446. doi: 10.1111/j.1572-0241.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 120.Yoneda M, Mawatari H, Fujita K, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 121.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 122.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 123.Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 126.Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 127.Baranova A, Younossi ZM. The future is around the corner: Noninvasive diagnosis of progressive nonalcoholic steatohepatitis. Hepatology. 2008;47:373–375. doi: 10.1002/hep.22140. [DOI] [PubMed] [Google Scholar]
- 128.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 129.Gaidos JK, Hillner BE, Sanyal AJ. A decision analysis study of the value of a liver biopsy in nonalcoholic steatohepatitis. Liver Int. 2008;28:650–658. doi: 10.1111/j.1478-3231.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 130.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 131.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 132.Marchesini G, Marzocchi R. Metabolic syndrome and NASH. Clin Liver Dis. 2007;11:105–117. doi: 10.1016/j.cld.2007.02.013. ix. [DOI] [PubMed] [Google Scholar]
- 133.Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353–358. doi: 10.1016/s0261-5614(99)80015-6. [DOI] [PubMed] [Google Scholar]
- 134.Donati G, Stagni B, Piscaglia F, et al. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut. 2004;53:1020–1023. doi: 10.1136/gut.2003.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 136.Volzke H, Robinson DM, Kleine V, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol 28. 2005;11:1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsiao PJ, Kuo KK, Shin SJ, et al. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. J Gastroenterol Hepatol. 2007;22:2118–2123. doi: 10.1111/j.1440-1746.2006.04698.x. [DOI] [PubMed] [Google Scholar]