Abstract
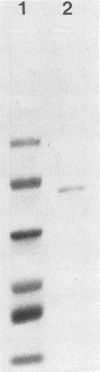
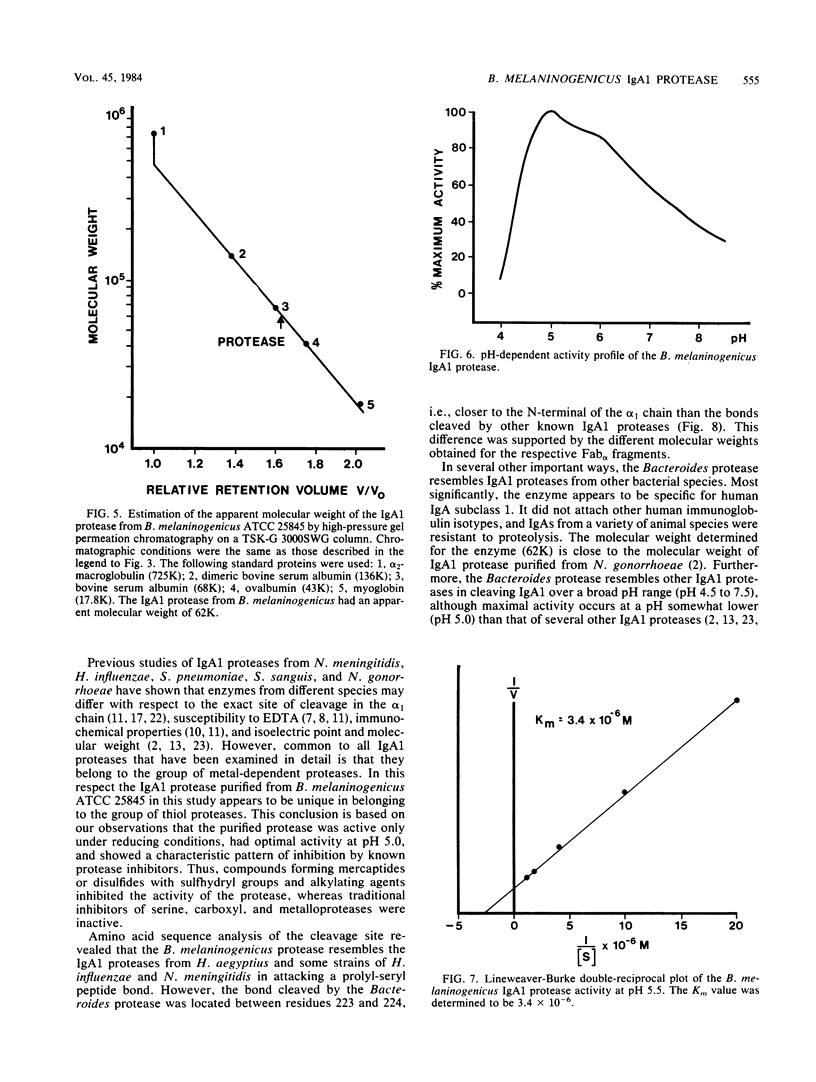
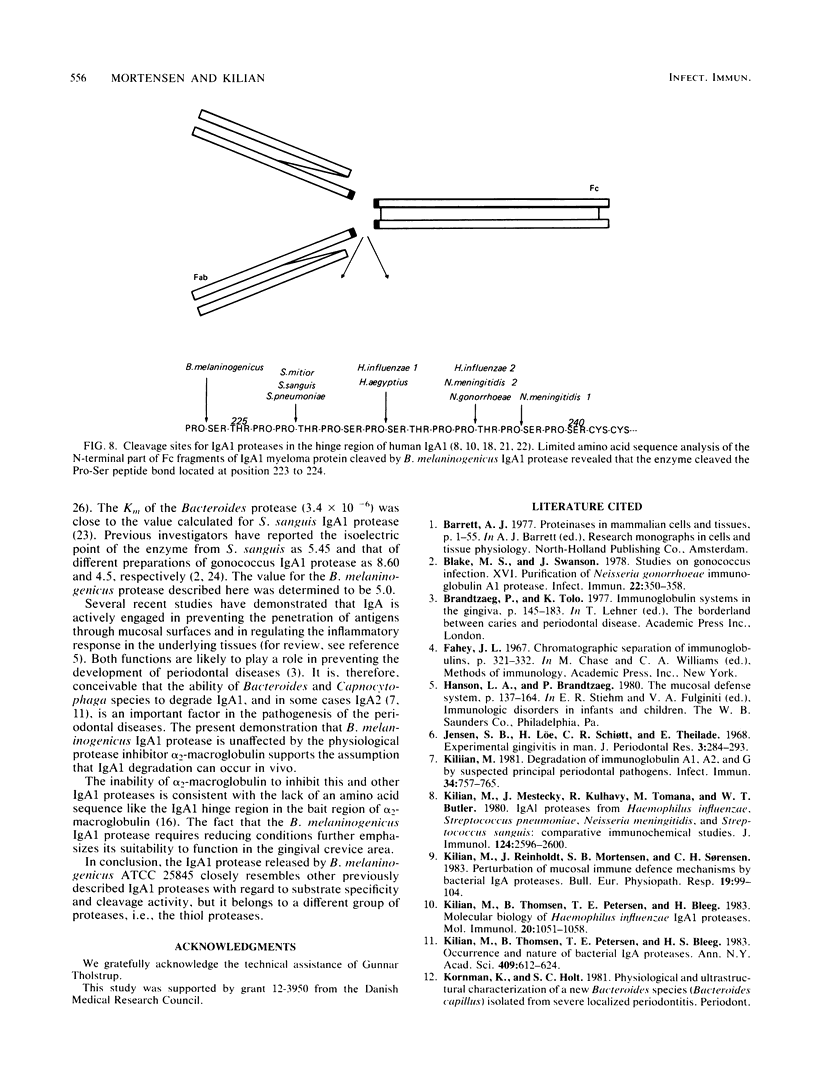
Attention has recently been focused on bacterial proteases with the capacity to cleave immunoglobulin A (IgA proteases) as possible pathogenic factors in bacterial meningitis, gonorrhoea, and destructive periodontal disease. Here, we describe a method for the rapid purification of a specific IgA1 protease from Bacteroides melaninogenicus. The IgA1 protease was purified 6,172-fold with a yield of 9% by ammonium sulfate precipitation, DEAE-ion exchange chromatography, and separation on a preparative TSK-G 3000SWG high-pressure gel permeation chromatography column. The enzyme was specific for human IgA1 and cleaved a prolyl-seryl peptide bond in the hinge region of the alpha 1 chain between residues 223 and 224. The molecular weight of the enzyme was 62,000, the isoelectric point was 5.0, and the Km was 3.4 X 10(-6). The enzyme was active over a broad pH range and had maximal activity at pH 5.0. B. melaninogenicus IgA1 protease was classified as a thiol protease on the basis of its inhibition by traditional protease inhibitors and the fact that it was active only under reducing conditions.
Full text
PDF
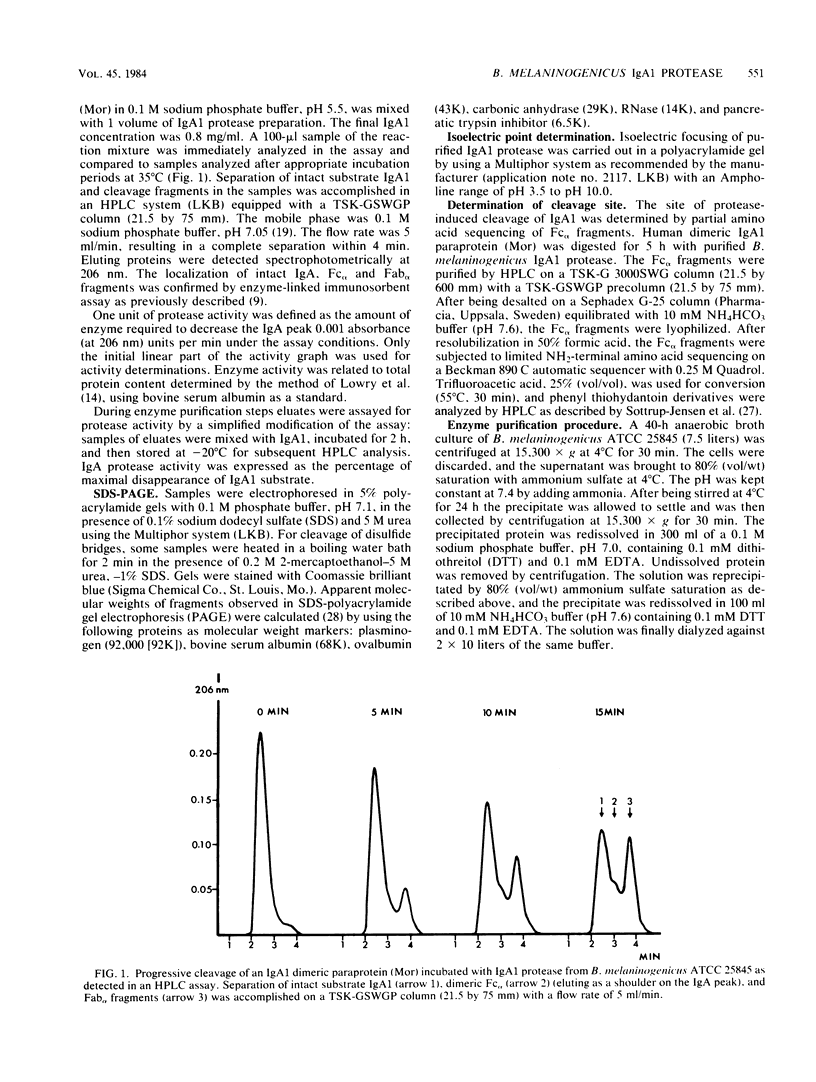
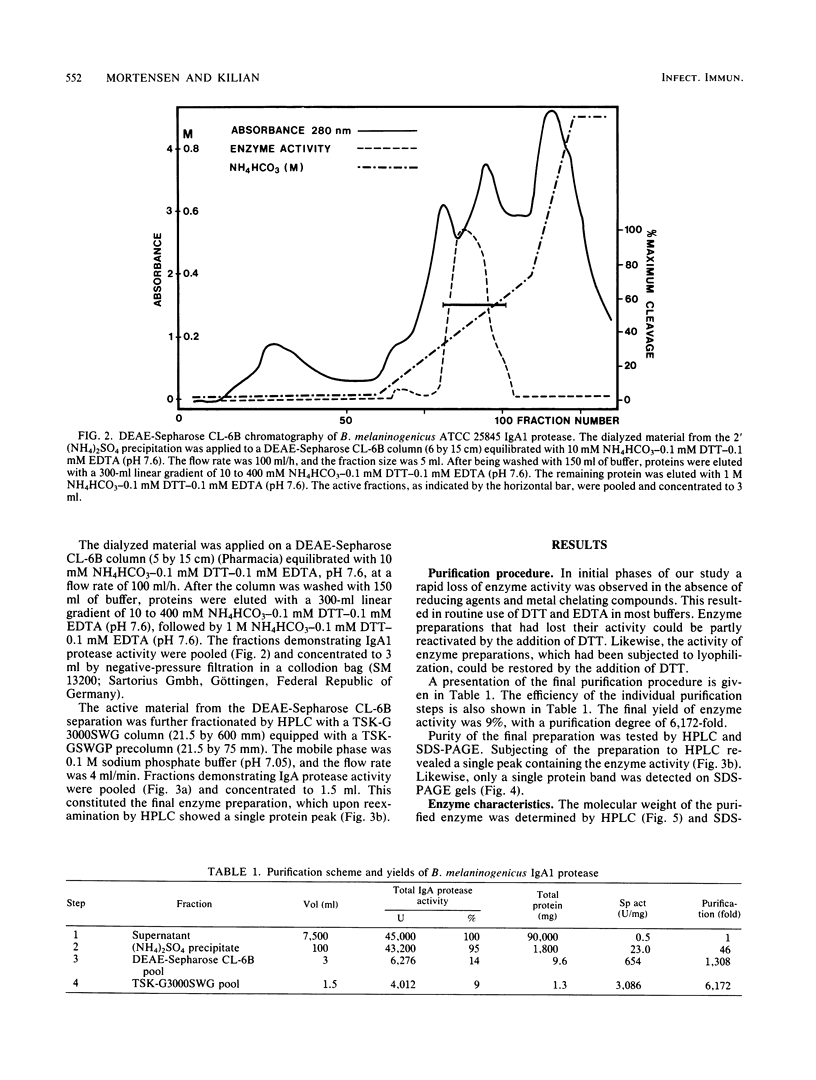
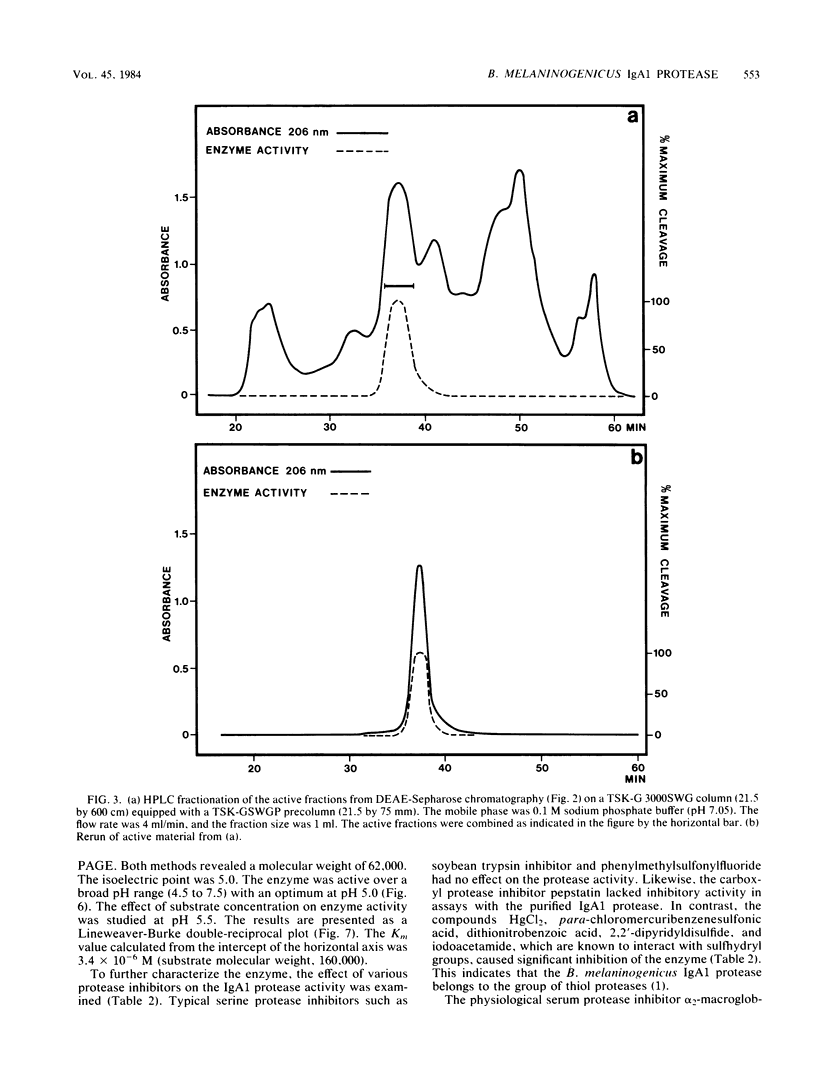


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Swanson J. Studies on gonococcus infection. XVI. Purification of Neisseria gonorrhoeae immunoglobulin A1 protease. Infect Immun. 1978 Nov;22(2):350–358. doi: 10.1128/iai.22.2.350-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. B., Löe H., Schiött C. R., Theliade E. Experimental gingivitis in man. 4. Vancomycin induced changes in bacterial plaque composition as related to development of gingival inflammation. J Periodontal Res. 1968;3(4):284–293. doi: 10.1111/j.1600-0765.1968.tb01934.x. [DOI] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Kilian M., Reinholdt J., Mortensen S. B., Sørensen C. H. Perturbation of mucosal immune defence mechanisms by bacterial IgA proteases. Bull Eur Physiopathol Respir. 1983 Mar-Apr;19(2):99–104. [PubMed] [Google Scholar]
- Kilian M., Thomsen B., Petersen T. E., Bleeg H. S. Occurrence and nature of bacterial IgA proteases. Ann N Y Acad Sci. 1983 Jun 30;409:612–624. doi: 10.1111/j.1749-6632.1983.tb26903.x. [DOI] [PubMed] [Google Scholar]
- Kilian M., Thomsen B., Petersen T. E., Bleeg H. Molecular biology of Haemophilus influenzae IgA1 proteases. Mol Immunol. 1983 Sep;20(9):1051–1058. doi: 10.1016/0161-5890(83)90046-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labib R. S., Calvanico N. J., Tomasi T. B., Jr Studies on extracellular proteases of Streptococcus sanguis. Purification and characterization of a human IgA1 specific protease. Biochim Biophys Acta. 1978 Oct 12;526(2):547–559. doi: 10.1016/0005-2744(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Kulhavy R., Kraus F. W. Studies on human secretory immunoglobulin A. II. Subunit structure. J Immunol. 1972 Mar;108(3):738–747. [PubMed] [Google Scholar]
- Mortensen S. B., Kilian M. A rapid method for the detection and quantitation of IgA protease activity by macrobore gel-permeation chromatography. J Chromatogr. 1984 Jul 27;296:257–262. doi: 10.1016/s0021-9673(01)96419-2. [DOI] [PubMed] [Google Scholar]
- Mortensen S. B., Sottrup-Jensen L., Hansen H. F., Petersen T. E., Magnusson S. Primary and secondary cleavage sites in the bait region of alpha 2-macroglobulin. FEBS Lett. 1981 Dec 7;135(2):295–300. doi: 10.1016/0014-5793(81)80804-6. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Kornfeld S. J., Frangione B., Plaut A. G. Relationship between the specificity of IgA proteases and serotypes in Haemophilus influenzae. J Infect Dis. 1982 Aug;146(2):266–274. doi: 10.1093/infdis/146.2.266. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G., Feldman H. A., Frangione B. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J Exp Med. 1980 Nov 1;152(5):1442–1447. doi: 10.1084/jem.152.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Heller I. Assay and properties of IgA protease of Streptococcus sanguis. Adv Exp Med Biol. 1978;107:489–495. doi: 10.1007/978-1-4684-3369-2_55. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Wistar R., Jr, Capra J. D. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J Clin Invest. 1974 Dec;54(6):1295–1300. doi: 10.1172/JCI107875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Liu Y. S., Low T. L. Primary structure of a human IgA1 immunoglobulin. IV. Streptococcal IgA1 protease, digestion, Fab and Fc fragments, and the complete amino acid sequence of the alpha 1 heavy chain. J Biol Chem. 1979 Apr 25;254(8):2865–2874. [PubMed] [Google Scholar]
- Reinholdt J., Kilian M. A sensitive enzyme-linked immunosorbent assay for IgA protease activity. J Immunol Methods. 1983 Oct 28;63(3):367–376. doi: 10.1016/s0022-1759(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Hansen H. F., Mortensen S. B., Petersen T. E., Magnusson S. Sequence location of the reactive thiol ester in human alpha 2-macroglobulin. FEBS Lett. 1981 Jan 12;123(1):145–148. doi: 10.1016/0014-5793(81)80039-7. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Wierzbicki D. M., Jones C. M., Lønblad P. B., Kristensen T., Mortensen S. B., Petersen T. E., Magnusson S. The primary structure of alpha 2-macroglobulin and localization of a Factor XIIIa cross-linking site. Ann N Y Acad Sci. 1983;421:41–60. doi: 10.1111/j.1749-6632.1983.tb18091.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]