Abstract
In healthy individuals, caffeine intake may improve performance on cognitive tests. Obstructive sleep apnea (OSA) is a disorder that has been associated with impaired cognitive function. In this study, we investigated whether increased caffeine intake in untreated patients with OSA is linked to better cognitive performance. Forty-five untreated OSA patients underwent baseline polysomnography after completing a survey of 24-h caffeine intake. Participants completed a battery of neuropsychological tests, then demographically corrected T scores and a global deficit score (GDS) were calculated on these tests. Partial correlation analysis was performed to compare daily caffeine intake with GDS, after controlling for body mass index (BMI) and sleep apnea severity. Analysis of covariance was done to examine differences in daily caffeine intake between cognitively impaired (GDS≥0.5) and non-impaired (GDS<0.5) individuals. Seven out of the 45 subjects met the criteria (GDS≥0.5) for cognitive impairment. There was a significant inverse association between caffeine intake and the GDS, both when controlling for BMI (r=−0.331, p=0.04) and when controlling for BMI and apnea severity (r=−0.500, p=0.002); those with less impairment consumed more caffeine. Analysis of covariance demonstrated that cognitively impaired individuals consumed one-sixth as much caffeine as non-impaired individuals (p<0.05). In patients with moderately severe OSA, higher average daily caffeine intake was associated with less cognitive impairment.
Keywords: Obstructive sleep apnea, Neuropsychology, Caffeine, Cognitive performance
Introduction
Caffeine is an adenosine receptor antagonist with known psychostimulant properties. Caffeine's ability to improve cognitive performance has been demonstrated in a variety of subjects and in a variety of settings [1]. Diverse aspects of cognitive function, including simple reaction time, digit vigilance speed, numeric working memory reaction time and sentence verification accuracy, improve in both habitual and non-habitual caffeine consumers after caffeine intake [2, 3]. Moderate doses of caffeine can improve cognitive functions, such as vigilance, learning, memory, and mood state in not only sleep-deprived individuals, [4, 5] but also in the non-sleep deprived [3]. While some have wondered whether caffeine's cognitive effects may attenuate after repeated ingestion, [6] others have demonstrated that habitual consumers of caffeine do not develop tolerance to its beneficial cognitive effects over time [7].
Obstructive sleep apnea (OSA) is a disorder of repetitive airway obstruction, with associated nocturnal oxyhemoglobin desaturations that not only results in daytime somnolence, but may also result in significant cognitive impairment [8-16]. OSA has been associated with global intellectual dysfunction and deficits in vigilance, concentration, alertness, executive and motor function, and short- and long-term memory [16-17]. Furthermore, greater cognitive impairment in OSA has been often, [18-22] but not always, [16] found to be associated with worse OSA severity (as measured by the apnea hypopnea index (AHI) or by the degree of nocturnal oxyhemoglobin desaturation).
In a previous study, our laboratory reported that patients with OSA reported daily caffeine intake approximately three times higher than that of non-apneics (295 vs 103 mg, P=0.010) [23], likely in an attempt to stay alert during the day. In the present study, we investigated whether daily caffeine intake in sleep apnea patients was related to cognitive performance. We hypothesized that that those patients taking higher doses of caffeine would have better cognitive performance. To do this, we first examined the correlation between daily caffeine intake and a global measure of cognitive performance. Then, we examined differences in caffeine intake between cognitively impaired and non-impaired apneics, based on summary scores derived from a battery of cognitive tests.
Materials and methods
Participants
Men and women between the age of 30 to 65 years with a history of snoring, gasping awakenings, daytime sleepiness, or other symptoms suggestive of sleep apnea were recruited by advertisements and referral from previous study participants and local medical practitioners. To limit potential confounding effects from other medical conditions and/or their therapies, we limited enrollment to subjects with weight within 100 to 200% of ideal body weight as determined by Metropolitan Life tables [24]. Participants were excluded if they were pregnant, abusing alcohol or illicit drugs, or if they had a history of heart, liver, or renal disease, diabetes, asthma, stroke, psychosis, major mood disorders, narcolepsy, significant head trauma or other neurologic disorders, or were currently using prescription medications except antihypertensives. Those who were using antihypertensives had their medications tapered off slowly with a 3-week drug washout period and were included as participants as long as their blood pressure did not exceed 180/110 mmHg. All qualified participants were screened for sleep apnea with an unattended overnight home sleep recording system study (Stardust; Respironics, Marietta, GA). Participants with an AHI>15 were deemed eligible to participate in the study.
The project was approved by the University of California, San Diego, Human Subjects Committee and written informed consent was obtained from the participants before participation in the study.
Experimental design
Participants completed surveys of their subjective daytime sleepiness and of their daily caffeine intake (both described below) during an initial daytime screening visit. During their subsequent admission to the General Clinical Research Center (GCRC) Gillin Laboratory of Sleep and Chronobiology (GLSC) (including the day of neuropsychologic testing), participants were allowed unrestricted access to caffeinated beverages from 6 A.M. to 6 P.M. and access to a wide variety of other menu options.
Daytime sleepiness
Participants rated their subjective daytime sleepiness with the Epworth Sleepiness Scale (ESS). The ESS is an eight-item questionnaire that asks patients to rate how likely they are to fall asleep in different situations, from 0 (not at all likely to fall asleep) to 3 (very likely to fall asleep), yielding a score of 0 (minimum) to 24 (maximum) [25].
Caffeine intake
Participants were given a survey that incorporates type of beverage, medication, and/or food product, the average caffeine content per serving of each of these items, and number of servings per day to determine total daily caffeine intake [26].
Sleep recordings
Sleep was monitored on the GCRC GLSC with the Grass Heritage digital polysomnograph (Model PSG36−2, Astro-Med, West Warwick, RI). Central and occipital electroencephalogram, bilateral electro-oculogram, submental and tibialis anterior electromyogram, electrocardiogram, body position, nasal airflow using a nasal canula/pressure transducer, and oronasal airflow using a thermistor were assessed. Respiratory effort was measured using chest and abdominal piezo-electric belts. Oxyhemoglobin saturation (SpO2) was monitored using a pulse oximeter (Biox 3740; Ohmeda: Louisville, Colorado). For each patient, the percentage of total sleep time with SpO2<90% was calculated as a measure of nocturnal oxyhemoglobin desaturation. Apneas were defined as decrements in airflow 90% from baseline for a period of 10 s. Hypopneas were defined as decrements in airflow 50% but <90% from baseline for a period of 10 s. The number of apneas and hypopneas per hour of sleep were calculated to obtain the AHI. Sleep records were manually scored according to the criteria of Rechtshaffen and Kales [27].
Neuropsychological tests
At 1 P.M. on the day after the overnight polysomnography, subjects were given the following battery of tests: Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Symbol, [28] Digit Span, [28] Letter-Number Sequencing, [28] Symbol Search [28]; Brief Visuospatial Memory Test [29]; Hopkins Verbal Learning Test-Revised [30]; Trail Making A/B [31]; Digit Vigilance [32]; Stroop Color-Word [33]; and Controlled Oral Word Association Test (COWAT) [34]. These tests produced 15 subscale scores per subject and assessed the following cognitive domains: speed of information processing (Digit Symbol, Symbol Search, Digit Vigilance Time, Trail Making A, Stroop color); attention and working memory (Letter-Number Sequencing, Digit Span, Digit Vigilance Errors); executive functions (Trail Making B, Digit Symbol, Symbol Search, Letter-Number Sequencing, Stroop Color-Word Interference Ratio); alertness and sustained attention (Digit Vigilance); verbal learning and memory (Hopkins Verbal Learning Test-Revised); verbal short-term memory and working memory (Digit Span, Letter-Number Sequencing); visuospatial memory (Brief Visuospatial Memory Test-Revised); psychomotor performance (reaction times on tests); and verbal fluency (COWAT). Tests were administered by the same research personnel and required approximately 60 min to complete.
Data analyses
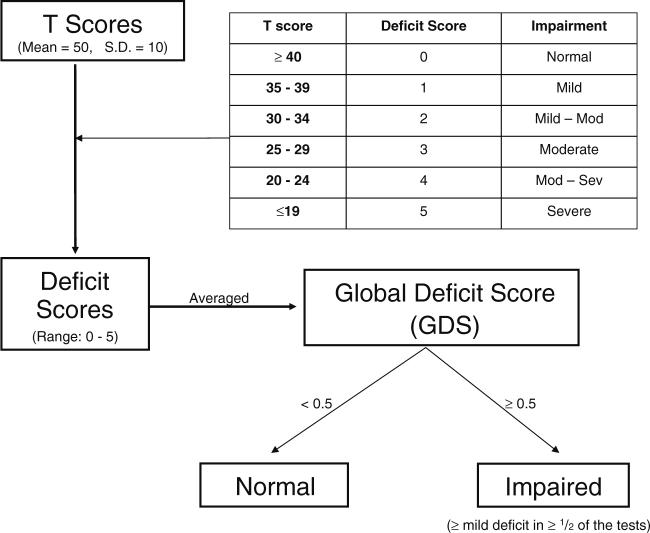
Raw scores were calculated for each neuropsychological subtest. To investigate how many OSA patients had neuropsychological impairment, and in what domains, T scores were calculated for the 15 neuropsychological subtests, using normative data (controlling for ethnicity, gender, age and education, as appropriate) [35, 36]. Higher T scores indicate better performance. As illustrated in Fig. 1, each of the 15 T scores was then converted into a deficit score ranging from 0 to 5 (with 5 representing severe cognitive deficit), and the average of those scores was calculated as the global deficit score (GDS). As described in previous literature that validated this methodology, Global Deficit Scores≥0.5 are defined “impaired”, and GDS<0.5 as non-impaired [37]. In other words, to meet criteria for “impaired”, a subject had to demonstrate, on average, at least mild deficits (e.g., deficit score of ≥1, representing a T score<40) on at least one-half of the 15 neuropsychological subtests.
Fig 1.
Flowchart representation of neuropsychological test data analysis. Each of the 15 individual tests scores are converted into norms-adjusted T scores. Each T score is then converted into a deficit score from 0−5 (where 0 = normal (or no deficit), and 5 = severe deficit). The 15 deficit scores for each patient are then averaged, to obtain the patient's Global Deficit Score (GDS). GDS≥0.5 is defined as “impaired” and GDS<0.5 as non-impaired
Statistical analyses were performed using SPSS statistical software (SPSS for Windows 12.0: SPSS; Chicago). Partial correlation analysis was performed to compare GDS vs daily caffeine intake. Because similar levels of caffeine intake might have different effects on people of different body sizes, we controlled for BMI. In addition, as cognitive performance has been previously linked to apnea severity and degree of nocturnal hypoxemia, we also controlled for AHI and percentage of total sleep time with SpO2<90% (PTST<90). Differences in caffeine intake between impaired and non-impaired individuals were examined using analysis of covariance. Statistics were considered significant at p<0.05.
Results
Forty-five OSA patients completed the study. On average, participants were middle-aged (age 47±10 years), obese (BMI=32±6 kg/m2), had significant daytime somnolence (Epworth Sleepiness Scale=12±5), and suffered from severe sleep apnea (AHI=63±31 events/h, and PTST<90=8.6±13.6%) (all presented as mean±SD). Average daily caffeine intake was 156±214 mg (equivalent to one to two 6-oz cups of coffee). Seven of the 45 met the criterion (GDS≥0.5) for cognitive impairment. Although there was a trend (not statistically significant) for the cognitively impaired to have greater PTST<90 vs non-impaired subjects (Table 1), there were no significant differences between the two groups in terms of age, years of education, BMI, Epworth Sleepiness Scale, or apnea severity.
Table 1.
Comparison of Epworth Sleepiness Scale, polysomnographic, and demographic variables between cognitively impaired subjects and non-impaired subjects
| Variable | Cognitively impaired (n=7) | Non-impaired (n=35) | P value |
|---|---|---|---|
| Age (years) | 48.57±11.73 | 47.26±9.87 | 0.76 |
| BMI (kg/m2) | 34.40±4.98 | 31.31±6.14 | 0.22 |
| Years of education | 14.00±2.31 | 14.66±1.81 | 0.40 |
| Epworth Sleepiness Scale | 12.50±2.74 | 11.89±5.72 | 0.80 |
| Apnea hypopnea index (events/h) | 71.64±28.45 | 61.85±32.12 | 0.46 |
| PTST<90 | 15.10±22.10 | 7.62±14.40 | 0.26 |
| TST (min) | 336.36±30.78 | 351.93±50.00 | 0.43 |
Data are resented as mean±SD
TST Total sleep time, PTST<90 percentage f TST with oxyhemoglobin saturation <90%, BMI body mass index
Partial correlation analysis revealed a significant inverse association between caffeine intake and the GDS, both when controlling for BMI (r=−0.332, p=0.04) and when controlling for BMI, AHI and PTST<90 (r=−0.500, p=0.002). There was no significant correlation between caffeine intake and any of the 15 individual subtest scores. Analysis of covariance demonstrated a marked difference in daily caffeine intake between impaired and non-impaired individuals (Fig. 2).
Fig 2.
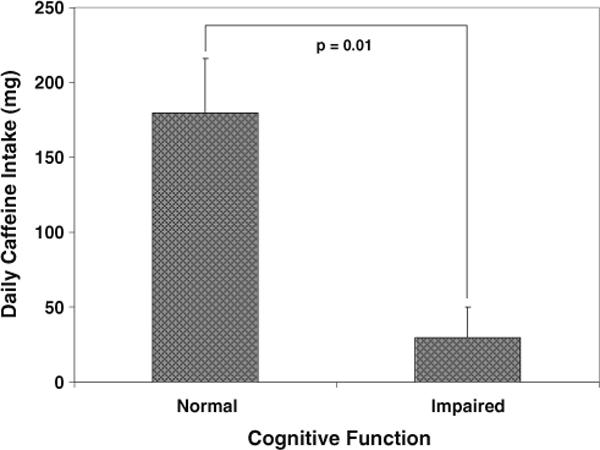
Differences in average daily caffeine intake between cognitively impaired (GDS≥0.5) and non-impaired (GDS<0.5) individuals with OSA. Bars depict mean+SE of the mean
Discussion
Our results demonstrate that the association between greater caffeine intake and enhanced cognitive function—previously established in normal subjects—also exists in patients with obstructive sleep apnea. Caffeine intake was positively associated with a composite measure of cognitive performance, both when controlled for body mass (BMI) and (even more strongly) when also controlled for apnea severity (AHI and PTST<90). Cognitively impaired patients with obstructive sleep apnea consumed one-sixth as much caffeine as non-impaired apneics.
Obstructive sleep apnea, a disorder characterized by repeated episodes of upper airway obstruction during sleep, is not only associated with intermittent hypoxemia, transient arousals, disruption of sleep, and excessive daytime sleepiness, but also with impairment in a number of neuropsychological domains. OSA patients may display deficits in global intellectual function [9, 18, 38, 39]. In a number of studies, OSA patients demonstrate impaired attention and concentration, [9, 18, 38, 39] diminished vigilance, [38-40] reduced short- and long-term memory [8, 9, 38, 39, 41, 42], and impaired executive function skills [19, 38, 39]. The severity of these deficits may correlate with the severity of the OSA, as measured by AHI [19, 43, 44]. Although some of the cognitive deficits seen in sleep apnea patients reverse after continuous positive airway pressure therapy, [12, 13, 45] other deficits (particularly those in executive function and memory) may persist, [12, 45] suggesting the possibility that long-term cerebral dysfunction exists in patients with OSA.
Caffeine intake in normal subjects has long been associated with improved neuropsychological function. By blocking receptors of the inhibitory neurotransmitter adenosine, caffeine is thought to enhance neuronal activity throughout the central nervous system. In doses as low as 12.5 mg, caffeine improves simple reaction time [7]. Caffeine use results in higher speed of encoding new information and vigilance performance in normal subjects [46]. It increases alertness and improves performance on cognitive vigilance, sustained response, tracking and digit detection, speed of encoding new stimuli, and simple and choice reaction tasks, and dual tasks involving tracking and target detection [47]. Caffeine can also improve performance on simulated driving tasks [48].
In our study, greater caffeine intake in sleep apnea patients was associated with higher composite scores on a battery of neuropsychological tests that encompassed several important areas of cognition: speed of information processing, attention and working memory, executive functions (e.g., cognitive set shifting, inhibition, and selective attention), alertness and sustained attention, verbal learning and memory, verbal short-term memory and working memory, visuospatial memory, and psychomotor performance. We did not find significant associations between caffeine intake and performance on any of the individual neuropsychological test domains, but this may reflect our limited sample size.
A potential limitation of our study is that we did not control for daytime sleepiness. Some might argue that sleepy individuals may be more likely to perform poorly on neuropsychological tests and also more likely to consume more caffeine. Review of our data revealed, however, that the Epworth Sleepiness Scale (ESS) score had only a weak, insignificant correlation with GDS (r=0.027, p=0.86) and with caffeine intake (r=0.199, p=0.20). Thus, the significant correlation between GDS and caffeine intake would still be preserved had we controlled for ESS in addition to the other factors listed (r=−0.532, p=0.001). Another potential limitation might be that only 7 out of 45 subjects were considered “cognitively impaired”. While some might expect a greater frequency of cognitive impairment in a group of individuals with severe obstructive sleep apnea, it is also important to note that to meet the study criteria for “cognitively impaired”, subjects had to score more than 1 SD below the mean on at least half of the 15 subscales tested. Thus, the GDS≥0.5 criterion is likely to include only those with significant impairment in a variety of different measures and miss those with milder (but perhaps still clinically significant) impairment. A third limitation of our study was the use of a questionnaire to assess typical daily caffeine intake (rather than direct measurement). While we gave subjects unfettered access to food and drink (including caffeinated beverages) and instructed participants to maintain their usual pattern consumption on the day of cognitive testing, it is possible that they consumed more (or less) than their usual amount. We did not ask about duration of caffeine consumption, changes in consumption patterns over time, or inquire why subjects consumed the amount of caffeine that they did. Therefore, our cross-sectional study design does not allow us to establish causality (e.g., whether caffeine intake in sleep apnea patients results in better cognitive test performance, or conversely, whether sleep apnea patients who perform better on cognitive tests also happen to consume more caffeine). In normal subjects, the effects of caffeine intake are in part dependent on the person's consumption patterns: habitual consumers of caffeine demonstrate greater positive effects of caffeine intake on cognitive function than non-habitual consumers [2, 7]. Thus, while some might be tempted to use the associations reported in our study to hypothesize that caffeine intake may improve cognitive function in patients with sleep apnea, it is likely that a more complex relationship between the two variables exists.
In summary, our data suggest that there is a positive association between caffeine intake and a global measure of cognitive function in patients with obstructive sleep apnea. Previous studies have demonstrated that cognitive deficits which exist in OSA patients do not entirely reverse with CPAP therapy [12, 45] or do not improve beyond that observed in OSA patients on placebo [16, 22]. Some have described the potential benefits of medications like modafenil and armodafenil on continued daytime sleepiness and residual cognitive dysfunction in OSA patients already undergoing treatment with CPAP [49, 50]. Perhaps, the results from this study can serve as a springboard for future research into the potential role that caffeine, a readily available nonprescription medication, may have in improving neuropsychological function in OSA and other disorders that are associated with fatigue, sleepiness, and cognitive dysfunction.
Acknowledgements
This research was supported by grants: HL 44915, RR0827, and AG08415 from National Institute of Health, USA.
Contributor Information
Daniel Norman, St. John's Medical Plaza Sleep Disorders Center, Santa Monica, CA, USA e-mail: dnorman@stjohnsleep.com.
Wayne A. Bardwell, Department of Psychiatry, University of California San Diego, La Jolla, CA, USA
Jose S. Loredo, Department of Pulmonary and Critical Care Medicine, University of California San Diego, La Jolla, CA, USA
References
- 1.Rees K, Allen D, Lader M. The influences of age and caffeine on psychomotor and cognitive function. Psychopharmacol. 1999;145(2):181–8. doi: 10.1007/s002130051047. [DOI] [PubMed] [Google Scholar]
- 2.Haskell CF, Kennedy DO, Wesnes KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacol. 2005;179(4):813–25. doi: 10.1007/s00213-004-2104-3. [DOI] [PubMed] [Google Scholar]
- 3.Hewlett P, Smith A. Acute effects of caffeine in volunteers with different patterns of regular consumption. Hum Psychopharmacol. 2006;21(3):167–80. doi: 10.1002/hup.752. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land. Psychopharmacology. 2002;164(3):250–61. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LC, Spinweber CL, Gomez SA, Matteson LT. Daytime sleepiness, performance, mood, nocturnal sleep: the effect of benzodiazepine and caffeine on their relationship. Sleep. 1990;13(2):121–35. doi: 10.1093/sleep/13.2.121. [DOI] [PubMed] [Google Scholar]
- 6.Judelson DA, Armstrong LE, Sokmen B, Roti MW, Casa DJ, Kellogg MD. Effect of chronic caffeine intake on choice reaction time, mood, and visual vigilance. Physiol Behav. 2005;85(5):629–34. doi: 10.1016/j.physbeh.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Smit HJ, Rogers PJ. Effects of low doses of caffeine on cognitive performance, mood and thirst in low and higher caffeine consumers. Psychopharmacol. 2000;152(2):167–73. doi: 10.1007/s002130000506. [DOI] [PubMed] [Google Scholar]
- 8.Roehrs T, Merrion M, Pedrosi B, Stepanski E, Zorick F, Roth T. Neuropsychological function in obstructive sleep apnea syndrome (OSAS) compared to chronic obstructive pulmonary disease (COPD). Sleep. 1995;18:382–88. doi: 10.1093/sleep/18.5.382. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 10.Kotterba S, Widdig W, Duscha C, Rasche K. Event related potentials and neuro-psychological studies in sleep apnea patients. Pneumologie. 1997;51(Suppl3):712–5. [PubMed] [Google Scholar]
- 11.Kim H, Young T, Matthews C, Weber S, Woodward A, Palta M. Sleep-disordered breathing and neuropsychological deficits. Am J Respir Crit Care Med. 1997;156:1813–9. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 12.Bedard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP). J Clin Exp Neuropsychol. 1993;15:330–41. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- 13.Lojander J, Kajaste S, Maasilta P, Partinen M. Cognitive function and treatment of obstructive sleep apnea syndrome. J Sleep Res. 1999;8:71–6. doi: 10.1046/j.1365-2869.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Chugh DK, Weaver TE, Dinges DF. Psychomotor vigilance performance in sleep apnea patients compared to patients presenting with snoring without apnea. Sleep. 1998;21(Suppl):159. [Google Scholar]
- 15.Findley L, Unverzagt M, Guchu R, Febrizio M, Buckner J, Suratt P. Vigilance and automobile accidents in patients with sleep apnea or narcolepsy. Chest. 1995;108:619–24. doi: 10.1378/chest.108.3.619. [DOI] [PubMed] [Google Scholar]
- 16.Lim W, Bardwell WA, Loredo JS, Kim E, Ancoli-Israel S, Morgan EE, Heaton RK, Dimsdale JE. Effects of two-weeks continuous positive airway pressure treatment vs. oxygen supplementation on neuropsychological functioning in patients with obstructive sleep apnea. Clin Sleep Med. 2007;3(4):380–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24(2):249–59. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 18.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med. 1992;152:538–41. [PubMed] [Google Scholar]
- 19.Naegele B, Thouvard V, Pepin J, Levy P. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- 20.Yesavage J, Bliwise D, Guilleminault C, Carskadon M, Dement W. Intellectual deficit and sleep-related respiratory disturbance in the elderly. Sleep. 1985;8:30–3. doi: 10.1093/sleep/8.1.30. [DOI] [PubMed] [Google Scholar]
- 21.Jennum PJ, Sjil A. Cognitive symptoms in persons with snoring and sleep apnea. Ugeskr Laeger. 1995;157:6252–6. [PubMed] [Google Scholar]
- 22.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: A placebo-controlled study. Psychosomatic Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Bardwell WA, Ziegler MG, Ancoli-Israel S, Berry CC, Nelesen RA, Durning A, Dimsdale JE. Does caffeine confound relationships among adrenergic tone, blood pressure and sleep apnoea? J Sleep Res. 2000;9(3):269–72. doi: 10.1046/j.1365-2869.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 24.Metropolitan Life Foundation . 1983 Metropolitan height and weight tables. Vol. 64. Stat Bull Metropolitan Insur Co; 1983. pp. 3–9. [PubMed] [Google Scholar]
- 25.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;12:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 26.Hale C, Davis RC. The do-it-yourself caffeine audit. J Nutr Educ. 1986;18:122A. [Google Scholar]
- 27.Rechtshaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. National Institute of Health Publication 204: US Government Printing Office; Washington, DC: 1968. [Google Scholar]
- 28.Wechsler D. Wechsler adult intelligence scale—revised manual. Psychological Corp; New York, NY: 1981. [Google Scholar]
- 29.Benedict RHB. BVMT-R Recognition stimulus booklet. Psychological Assessment Resources; Lutz, FL: 1997. [Google Scholar]
- 30.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 31.Boll TJ. The halstead-reitan neuropsychological battery. In: Fikov SB, Boll TJ, editors. Handbook of clinical neuropsychology. Wiley; New York: 1981. pp. 577–607. [Google Scholar]
- 32.Lewis RF. Digit vigilance test. Psychological Assessment Resources; Lutz, FL: 1995. [Google Scholar]
- 33.Golden CJ. A manual for clinical and experimental uses. Stoelting Co; Chicago, IL: 1978. [Google Scholar]
- 34.Lezak MD. Neuropsychological assessment. Oxford University Press; New York, NY: 1995. [Google Scholar]
- 35.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded halstead-reitan battery: Demographically adjusted neuropsychological norms for African and Caucasian adults. Psychological Assessment Resources; Lutz, FL: 2004. [Google Scholar]
- 36.Heaton RK, Taylor MJ, Manly J. Demographic effects and use of demographically corrected norms on the WAIS-III and WMS-III. In: Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, Prifitera A, editors. Ledbetter MF. clinical interpretation of the WAIS-III and WMS-III. Academic; San Diego, CA: 2003. [Google Scholar]
- 37.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. HNRC Group Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exper Neuropsychol. 2004;26:307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 38.Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsycho-logical deficits. J Clin Exp Neuropsychol. 1991;13:950–64. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 39.Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90:686–90. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- 40.Nykamp K, Rosenthal L, Guido P, Roehrs T, Rice FM, Syron ML, Helmus T, Roth T. The effects of sleepiness on performance among patients with OSA. Sleep Research. 1997;26:450. [Google Scholar]
- 41.Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24:93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- 42.Telakivi T, Kajaste S, Partinen M, Koskenvuo M, Salmi T, Kaprio J. Cognitive function in middle-aged snorers and controls: role of excessive daytime somnolence and sleep-related hypoxic events. Sleep. 1988;11:454–62. [PubMed] [Google Scholar]
- 43.Telakivi T, Kajaste S, Partinen M, Brander P, Nyholm A. Cognitive function in obstructive sleep apnea. Sleep. 1993;16:S74–5. [PubMed] [Google Scholar]
- 44.Kingshott RN, Engleman HM, Deary IJ, Douglas NJ. Does arousal frequency predict daytime function? Eur Respir J. 1998;12(6):1264–70. doi: 10.1183/09031936.98.12061264. [DOI] [PubMed] [Google Scholar]
- 45.Naegele B, Pepin JL, Levy P, Bonnet C, Pellat J, Feuerstein C. Cognitive executive dysfunction in patients with obstructive sleep apnea syndrome (OSAS) after CPAP treatment. Sleep. 1998;21:392–7. doi: 10.1093/sleep/21.4.392. [DOI] [PubMed] [Google Scholar]
- 46.Smith A, Sutherland D, Christopher G. Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. J Psychopharmacol. 2005;19(6):620–6. doi: 10.1177/0269881105056534. [DOI] [PubMed] [Google Scholar]
- 47.Brice CF, Smith AP. Effects of caffeine on mood and performance: a study of realistic consumption. Psychopharmacol. 2002;164(2):188–92. doi: 10.1007/s00213-002-1175-2. [DOI] [PubMed] [Google Scholar]
- 48.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40(9):1243–55. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 49.Hirshkowitz M, Black JE, Wesnes K, Niebler G, Arora S, Roth T. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101(3):616–27. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4(5):393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]